
Abstract
Aims: The aim of the study was to evaluate the safety and efficacy of left atrial appendage occlusion (LAAO) with the AMPLATZER Cardiac Plug (ACP) or Amulet using aspirin alone (ASA) as post-implantation antithrombotic treatment.
Methods and results: This was a single-centre, prospective, non-randomised study on LAAO with the ACP or Amulet in a consecutive cohort (n=110) treated by ASA alone post implantation. The primary outcome was device-related thrombosis, while secondary outcomes were ischaemic stroke or major bleeding. Clinical follow-up was conducted after six weeks and 12 months with TEE and cardiac CT. One hundred and seven patients were included in the analysis. Three patients were excluded due to a mechanical valve prosthesis. CHA2DS2-VASc score was 4.4±1.6 and HAS-BLED 4.1±1.1. Successful implantation was obtained in all patients with a periprocedural complication rate of 4.6%. Median follow-up was 2.3 years, with a total of 265 patient-years. Device-related thrombosis was detected in 2/107 (1.9%) cases. Stroke occurred in 6/107 patients, with an annualised rate of 2.3%, which is a 61% risk reduction compared to the predicted rate. Annual risk of major bleeding was reduced by 57%.
Conclusions: LAAO with the ACP or Amulet was safely performed with ASA monotherapy after implantation without an increased risk of device-related thrombosis or stroke.
Abbreviations
ACP: AMPLATZER Cardiac Plug
AF: atrial fibrillation
ASD: atrial septal defect
DRT: device-related thrombus
EHRA/EAPCI: European Heart Rhythm Association/European Association of Percutaneous Cardiovascular Interventions
ICH: intracranial haemorrhage
LAAO: left atrial appendage occlusion
OAC: oral anticoagulation
TEE: transoesophageal echocardiography
TIA: transient ischaemic attack
Introduction
Transcatheter left atrial appendage occlusion (LAAO) is increasingly used as an alternative to oral anticoagulation (OAC) in atrial fibrillation (AF). In the randomised PROTECT AF trial, with the WATCHMAN® device (Boston Scientific, Marlborough, MA, USA), LAAO was superior to warfarin after four years1. A large observational study in a real-world population supports the evidence for LAAO, showing marked reductions in expected rates of stroke and bleeding in AF patients treated with the AMPLATZER™ Cardiac Plug (ACP; St. Jude Medical, St. Paul, MN, USA)2-4. Current guidelines from the ESC5 and EHRA/EAPCI6 recommend LAAO in AF patients who have a contraindication to OAC or a high bleeding risk. According to data from a large real-world registry, LAAO is currently performed as an alternative to OAC both in patients with and in those without a contraindication to OAC2,7.
The most frequently used devices for LAAO are the WATCHMAN, ACP, and the Amulet™ (St. Jude Medical). Thrombus formation on the surface of the device is one of the adverse events associated with LAAO. This complication is potentially serious, as it may lead to thromboembolic events. In the WATCHMAN PROTECT AF trial, the reported occurrence of device-related thrombus (DRT) was 27/485 (5.6%)8 and in the ASAP trial 6/150 (4%)9. In the European/Canadian ACP registry, DRT occurred in 28/632 (4.4%)2 and in the ACP European post-market Registry 5/204 (2.9%)6. A large single-centre study reported DRT in 16% of cases7, and in small ACP and Amulet case series DRT was reported from 4% to as high as 17.6%10-14.
Post-implantation antithrombotic treatment is used to prevent device thrombosis. This is probably most important in the first six months after LAAO until ingrowing endothelium covers the surface of the device. Antithrombotic regimens after LAAO differ between devices, studies and centres1,2,9,15,16. The recommended antithrombotic protocol for the WATCHMAN device is warfarin and ASA for 45 days, dual antiplatelet therapy for six months and ASA for life1,6,15-18. For the ACP and Amulet devices, dual antiplatelet therapy with clopidogrel and ASA for one to three months and then ASA until at least six months are used by most centres and recommended in the EHRA/EAPCI consensus paper6,16.
Since most patients undergoing LAAO have a high bleeding risk, the post-implant antithrombotic protocol to prevent DRT should be with a bleeding risk as low as possible. The ACP and Amulet are built from the same materials as other devices in the AMPLATZER family of occluders. There is a reported low occurrence of thrombus formation on AMPLATZER™ Septal Occluders (St. Jude Medical)19,20. In our own institution we have used ASA monotherapy for six months following closure of atrial septal defects by AMPLATZER devices. Only one case of device thrombosis in about 400 ASD closures has been observed over a period of 15 years. This led us to use ASA monotherapy for six months after LAAO using the ACP or Amulet. In this paper we report our single-centre experience with the first 110 cases of LAAO using AMPLATZER devices and ASA alone for post-implant antithrombotic therapy.
Methods
This was a single-centre, prospective non-randomised observational study of LAAO with the ACP or Amulet device in patients with AF. Between March 2010 and March 2015, 110 patients consecutively underwent LAAO at Aarhus University Hospital, Denmark. All patients had a baseline clinical examination, transthoracic echocardiography, transoesophageal echocardiography (TEE) and cardiac computed tomography (CT). Indications for LAAO were: 1) intracranial bleeding during OAC; 2) gastrointestinal (GI) bleeding during OAC; 3) genitourinary bleeding during OAC; 4) other spontaneous bleeding; 5) ischaemic stroke despite OAC; 6) cerebral amyloid angiopathy; 7) high bleeding risk and recurrent falls, cerebral aneurysms or liver disease with oesophageal varices. Patients could have more than one indication for LAAO, but only the primary indication was included for analysis.
PROCEDURE AND FOLLOW-UP
Cardiac CT was carried out the day prior to LAAO. LAAO was performed under general anaesthesia guided by TEE. Heparin (initial dose 100 U/kg) was used during the procedure, adjusted to give an active clotting time >250 s. Patients who were not treated with ASA upon admission received 300 mg of ASA the day prior to LAAO followed by ASA 75 mg daily for six months or indefinitely if other indications were present. Transthoracic echocardiography and fluoroscopy were performed the day after implantation to check the device position and exclude pericardial effusion before discharge. Patients were followed up after both six to eight weeks and 12 months with clinical examination, TEE and cardiac CT. Thereafter, follow-up was through systematic reviewing of patient files, clinical follow-up, and through a search in the Danish Civil Registration System21 as well as the Danish National Patient Registry22.
DEFINITIONS AND VARIABLES
Along with demographics, CHA2DS2-VASc23 and HAS-BLED24 scores were calculated at baseline. Baseline CHA2DS2-VASc and HAS-BLED scores were used to calculate the predicted annual risk of stroke or major bleeding events. All antithrombotic therapy prior to LAAO was registered at baseline, and antithrombotic therapy during and after LAAO was registered consecutively during follow-up. Patient-years were calculated from the date of implant to either the date of event (see below), the date of last known status or death.
PRIMARY AND SECONDARY OUTCOMES
The primary outcome was device-related thrombus detected by TEE and/or cardiac CT upon follow-up. Secondary outcomes were ischaemic and haemorrhagic stroke, systemic embolism or major bleeding. Efficacy and safety were tested by comparing actual stroke and bleeding rates at follow-up with the predicted event rates from CHA2DS2-VASc and HAS-BLED. Stroke was defined as an acute episode of focal or global neurological dysfunction caused by vascular injury as a result of ischaemic infarct or haemorrhage25. All patients suspected of stroke were admitted for neurological evaluation and neuroimaging. Patients with a confirmed or suspected stroke underwent TEE during admission to evaluate for device-related thrombus or other cardiac sources of embolism. Peripheral embolism was defined as acute vascular insufficiency or occlusion of extremities or non-CNS organs with clinical, imaging or surgical evidence of occlusion without other likely mechanism such as trauma or atherosclerosis. Bleeding was defined according to the VARC-2 definitions26, as described in the latest consensus paper on LAAO25.
Statistical analysis
Continuous variables were reported as means with standard deviations, or as medians with interquartile ranges (IQR) as appropriate. Categorical variables were reported as absolute frequencies and percentages. A two-sided p-value <0.05 was considered statistically significant. Stata software (Stata/IC for Mac, Version 14.1; StataCorp LLC, College Station, TX, USA) was used for statistical analyses.
The Danish Health and Medicines Authorities and the Danish Data Protection Agency (ID 1-16-02-419-16) approved the study.
Results
A total of 110 patients underwent successful LAAO during the study period. One patient had two procedures. During the first, the device embolised to the abdominal aorta. It was snared without complications and a new LAAO was performed successfully a few months later. Three patients with mechanical heart valves underwent LAAO due to stroke despite well-regulated INR. They continued on life-long warfarin, and were excluded from data analysis. Thus, 107 patients formed the basis for the analyses. An ACP device was implanted in 72 cases (67%), while 35 received an Amulet device (33%). Baseline characteristics, predicted stroke risk (CHA2DS2-VASc) and HAS-BLED scores for the total cohort are shown in Table 1. Permanent AF was present in 51 patients (48%), and 54 patients (51%) had a history of ischaemic stroke or TIA. The most common risk factor for stroke was hypertension in 81% of patients, and 96% of patients had a CHA2DS2-VASc score ≥2. Mean HAS-BLED score was 4.1±1.1 and 92% had a HAS-BLED score ≥3. Previous serious bleeding during OAC was the primary indication for LAAO in 86 patients (80%), with prior intracranial haemorrhage (ICH) being the most common (Figure 1).
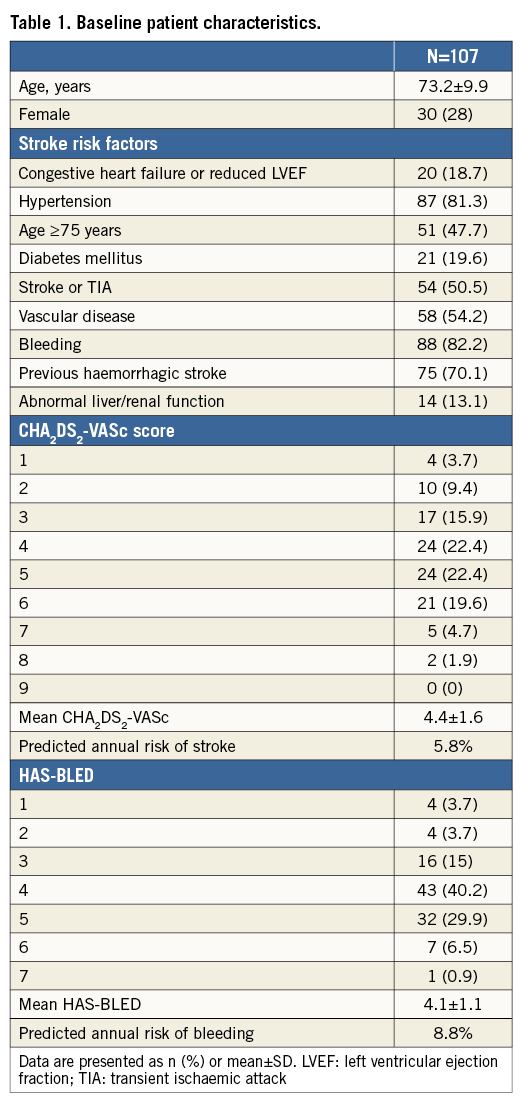
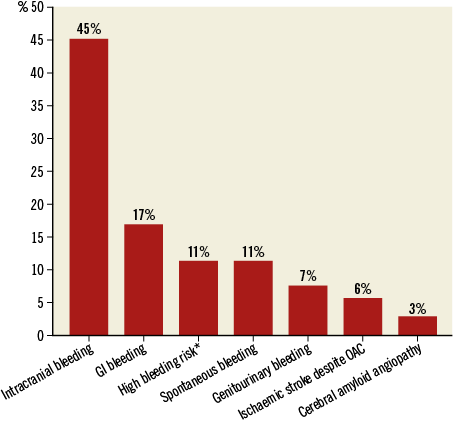
Figure 1. Primary indication for LAAO. *High bleeding risk and, at the same time, comorbidities such as intracerebral aneurysm, oesophageal varices or recurrent falls.
ANTITHROMBOTIC TREATMENT AT FOLLOW-UP
Antiplatelet treatment after LAAO is shown in Table 2. In 91 cases (85%), patients were discharged with ASA monotherapy after LAAO. Two patients discontinued all antithrombotic treatment during the first week due to bleeding episodes. The first patient was treated with ASA monotherapy and experienced an ICH the day after LAAO. This patient had cerebral amyloid vasculopathy and five prior ICHs. The second patient had a serious gastrointestinal bleeding six days after LAAO while on dual antiplatelet therapy due to coronary stenting. Thirteen patients were on clopidogrel or dual antiplatelet therapy upon admission, and three and 13 patients were discharged with clopidogrel and dual antiplatelet therapy, respectively. The reasons for dual antiplatelet therapy were recent coronary stent implantation (n=5), known intracerebral atherosclerosis or stenosis (n=5), recent intracerebral thrombectomy with stent implantation (n=1), thrombectomy of the carotid arteries (n=1) or stent implantation in the lower extremities (n=1). Three patients were prescribed clopidogrel monotherapy due to known ASA intolerance. At 12-month follow-up, 26% of the LAAO patients were without ASA or any other antithrombotic treatment. Only one patient resumed OAC in the post-implant period, which was due to device thrombosis. No patients had treatment by NOAC after LAAO implantation.
DEVICE-RELATED THROMBOSIS DURING FOLLOW-UP
Median follow-up time was 2.3 years (IQR: 1.6-3.2), resulting in a total of 265.5 patient-years. Twenty patients died during follow-up; none of the deaths was procedure- or device-related. Clinical follow-up after six weeks was complete for 103/107 (96.3%) patients. At six weeks, 92.5% had TEE, 87.9% had cardiac CT and 84.1% had both TEE and cardiac CT. Three of the four patients who were not followed up at six weeks had follow-up at 12 months. After 12 months, 84/107 (78.5%) patients had follow-up. TEE was performed in 67.3%, cardiac CT in 61.7% and both TEE and cardiac CT in 50.5% of the initial cohort. The reasons for incomplete 12-month attendance were death prior to the scheduled visit (n=10) and patient unwillingness to undergo repeat imaging (n=13).
Two cases (1.9%) of DRT occurred during follow-up. Only one of these resulted in a symptomatic event. In this case, the patient had been treated with clopidogrel monotherapy (75 mg daily) after LAAO, due to ASA intolerance. The DRT developed during the first month after implantation, and was complicated by ischaemia in the lower extremities. The patient had previously undergone bifemoral bypass due to severe atherosclerosis. The patient underwent successful re-operation. Dose-adjusted warfarin was initiated in addition to clopidogrel and the thrombus had disappeared after seven months. In the other case, the DRT was discovered during routine six-week follow-up TEE, as very thin fibrin strands on the LAAO disc that could not be visualised on cardiac CT. It was conservatively treated with low molecular weight heparin for four weeks and resolved completely without complications. The patient resumed monotherapy with ASA without further events.
PERIPROCEDURAL AND FOLLOW-UP ADVERSE EVENTS
Table 3 shows event rates related to the procedure and during follow-up, while Figure 2 shows the effectiveness of LAAO in reducing the risk of stroke and bleeding according to the predicted risk. After a total of 265 patient-years of follow-up, ischaemic stroke occurred in six patients. One patient died during admission, before TEE could be performed. One patient had a stroke the day after the procedure. None of the events was associated with DRT or proven cardioembolism. Four of the six patients suffering a stroke were known to have cerebral atherosclerosis or stenosis of the carotid arteries. One patient underwent successful cerebral bypass surgery.
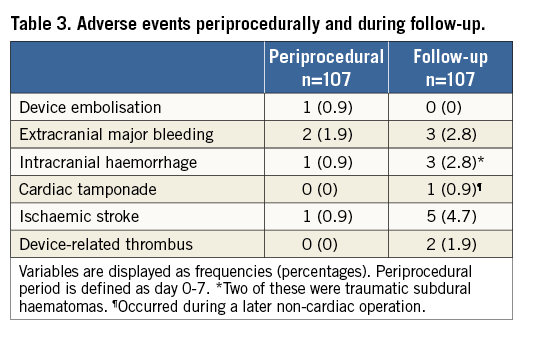
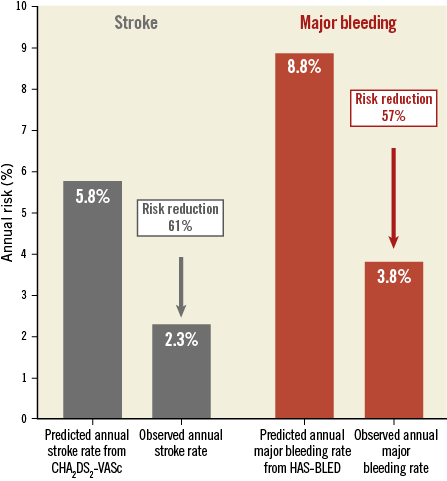
Figure 2. Predicted and observed rates of stroke and major bleeding. Effectiveness of LAAO in reducing the risk of stroke and bleeding among 107 high-risk AF patients. ASA was used as monotherapy for post-implant antithrombotic therapy. Both periprocedural and follow-up events were included.
Major bleeding events occurred in 10 patients. None of these was fatal. One patient experienced a late cardiac tamponade with need of surgical drainage. The event occurred three months after LAAO during non-cardiac surgery under general anaesthesia. The follow-up transthoracic echocardiography and TEE after six weeks were reviewed, revealing no signs of pericardial effusion. Of the three patients who experienced intracranial haemorrhage during follow-up, two had a traumatic subdural haematoma treated conservatively. Gastrointestinal bleeding was the most common extracranial bleeding event.
Discussion
This is the first prospective study to evaluate monotherapy with ASA after LAAO with the ACP and Amulet devices. The data presented in this study suggest ASA monotherapy to be feasible and safe to prevent DRT without attenuating the efficacy of LAAO to reduce the risk of stroke.
DEVICE-RELATED THROMBOSIS
Dual antiplatelet therapy is the recommended and most widely used antithrombotic regimen after LAAO with AMPLATZER devices. However, the results from our study show that the use of ASA monotherapy does not cause an increase in device-related thrombosis. In fact, our study has shown a 50% lower incidence of device-related thrombosis compared to other registry data of the AMPLATZER and WATCHMAN devices2,6,8-13. The follow-up TEE protocol in our study and the meticulous approach with TEE if stroke or TIA was suspected should minimise the risk of under-reporting DRT.
Factors other than antithrombotic therapy probably play a role in the development of DRT. In a small-scale study, it was suggested that notably reduced left ventricular ejection fraction, higher CHA2DS2-VASc score and platelet count might be risk factors for DRT12. Another small case study reported high rates of DRT (17%) but, as pointed out by the authors, all occurred in patients where a remnant of the LAA was left between the upper pulmonary vein ridge and the disc14. Thus, a deep implantation of the device in the LAA, leaving an uncovered part of the LAA in front of the disc, may possibly be an important factor contributing to DRT. This emphasises proper device sizing and positioning as potentially important factors to avoid DRT, by making sure that the proximal disc of the AMPLATZER device reaches to the upper pulmonary vein ridge and seals the whole LAA orifice.
The antiplatelet treatment after LAAO must be individualised taking into account the comorbidities of the patient. This was also practised in our cohort with 12% of patients having another indication for dual antiplatelet therapy. Clopidogrel and ASA is the most frequently used dual combination after LAAO. However, it should be kept in mind that up to 25% of patients can be clopidogrel non-responders27,28. This is also a challenge with the use of clopidogrel monotherapy. Interestingly, monotherapy with clopidogrel, due to ASA intolerance, was used in one of the two cases of device thrombosis in this study.
STROKE PREVENTION
Based on the CHA2DS2-VASc score, the annualised rate of stroke was expected to be 5.8%. However, the observed annual rate of stroke in our cohort was only 2.3%, giving a reduction in stroke risk of 61%. This is very similar to what was reported from the European/Canadian ACP registry, which is remarkable because patients in that registry received more intensive post-implant antithrombotic treatment. In the European/Canadian ACP registry, 55% of the patients were treated with OAC, dual antiplatelet therapy, triple therapy or a combination between antiplatelets and anticoagulation after LAAO, with 23% still on one of these combinations at the latest follow-up2. In our cohort, only 12% were on dual antiplatelet therapy at discharge and 4% after six months. Thus, our data suggest that ASA monotherapy as post-implant antithrombotic therapy is not associated with an increase in the risk of stroke, which is also supported by a subgroup analysis from the European/Canadian registry2.
BLEEDING EVENTS
The main reason to use ASA monotherapy after LAAO is to reduce bleeding events. Eighty percent had a previous serious bleeding episode as indication for LAAO, and 92% had a HAS-BLED score ≥3. Our results showed a 57% reduction in bleeding rate as compared to the predicted rate. The risk reduction for bleeding was 61% in the European/Canadian ACP registry2, with an annualised bleeding rate of 2.1%. The annualised bleeding rates are not fully comparable between the two studies, due to a higher HAS-BLED score in our cohort. The annualised event rate for major bleeding in the PROTECT AF trial was 3.1%1, but the HAS-BLED score was not calculated. As the patients were warfarin eligible, their HAS-BLED scores were probably lower than in our cohort. The present data thus suggest that ASA monotherapy will lead to a reduction in bleeding events compared with the more intensive post-implant antithrombotic treatment currently used.
It should be emphasised that a bleeding risk remains associated with the use of heparin during the procedure (ACT is kept >250 s). One third of bleeding events in our cohort occurred in the immediate periprocedural period. As highlighted by our results, patients with cerebral amyloid angiopathy may potentially be more susceptible for bleeding events and special care should be taken in this patient population29.
In case of DRT during follow-up, temporary anticoagulation is indicated6. The use of OAC, NOAC and low molecular weight heparin seems equally common in other studies2,7,9,10,12. The anticoagulation regimen in case of DRT should be based on an individual assessment, and in high-risk patients (e.g., cerebral amyloid angiopathy) even temporary OAC may be associated with substantial bleeding risk.
Limitations
The non-randomised observational design of our study is limited by the lack of a control group or an alternative treatment group. The use of CHA2DS2-VASc and HAS-BLED scores for comparison of effectiveness is only a proxy measure used in the absence of a control group. A larger randomised controlled trial or at least a comparable control group would be necessary to confirm the superiority of ASA monotherapy after LAAO.
Conclusions
This study suggests that ASA monotherapy is safe and effective to prevent device-related thrombosis after LAAO with AMPLATZER devices and that this approach does not attenuate the efficacy of LAAO to reduce the risk of stroke in AF. The study adds to the evidence that LAAO is effective in reducing the risk of stroke and major bleeding in AF with a low rate of complications.
| Impact on daily practice What is known? The majority of patients undergoing LAAO have a high risk of bleeding. Current guidelines recommend at least dual antiplatelet therapy to prevent device-related thrombosis after implantation. What is new? ASA monotherapy after LAAO seems to be safe and effective with a low rate of device-related thrombosis and without increasing the risk of stroke. What is next? A randomised controlled trial to test the superiority of ASA monotherapy after LAAO compared with dual antiplatelet therapy. |
Funding
The study was supported by the Health Research Fund of Central Denmark.
Conflict of interest statement
J.E. Nielsen-Kudsk is a proctor for St. Jude Medical. J.M. Jensen has received speaker’s honorarium from Bracco Imaging. H.K. Jensen has received speaker’s honorarium from St. Jude Medical. The other authors have no conflicts of interest to declare.

