Abstract
BACKGROUND: The optimal antithrombotic therapy following left atrial appendage occlusion (LAAO) remains debated. Ideally, this therapy should effectively prevent device-related thrombosis (DRT) while minimising the associated bleeding risk.
AIMS: We aimed to evaluate the long-term safety and efficacy of a postprocedural single antiplatelet therapy (SAPT) strategy following Amplatzer LAAO in a large consecutive cohort.
METHODS: This retrospective, single-centre, observational study included all patients discharged on SAPT after LAAO with the Amplatzer Cardiac Plug (ACP) or Amplatzer Amulet between March 2010 and December 2021 at Aarhus University Hospital, Denmark. Baseline, procedural, and imaging data were obtained locally, while clinical outcomes and medication data were extracted from the Danish national health registries.
RESULTS: A total of 553 patients underwent Amplatzer LAAO during the specified time frame. Of these, 431 (77.9%) high bleeding risk patients were discharged on SAPT with either acetylsalicylic acid (n=403, 72.9%) or clopidogrel (n=28, 5.1%). At 6 months, 173 (41.7%) patients were not on any antithrombotic therapy. The mean CHA2DS2-VASc and HAS-BLED scores were 3.9±1.5 and 3.4±1.1, respectively. DRT was detected in 6 (1.5%) patients on 8-week follow-up imaging using cardiac computed tomography (n=386, 89.6%) or transoesophageal echocardiography (n=27, 6.3%). The 1-year ischaemic stroke rate was 2.2% (95% confidence interval [CI]: 1.1-4.2). One-year rates for major bleeding and cardiovascular death were 5.9% (95% CI: 4.0-8.9) and 2.9% (95% CI: 1.6-5.1), respectively.
CONCLUSIONS: SAPT following Amplatzer LAAO displayed rates of DRT and stroke comparable to those reported with more intensive antithrombotic regimens. Meanwhile, we observed low rates of major bleeding.
In accordance with current guidelines, percutaneous left atrial appendage occlusion (LAAO) should be considered in patients with atrial fibrillation (AF) and a simultaneous contraindication to long-term oral anticoagulation12.
Although LAAO is an effective alternative for thromboprophylaxis in AF, it remains challenged by the inherent risk of device-related thrombosis (DRT), necessitating the use of postprocedural antithrombotic therapy345. However, the optimal antithrombotic treatment remains debated, and different therapeutic regimens have been employed across various trials and registries678910.
Although the initial landmark trials, PROTECT AF and PREVAIL, utilised a period of postprocedural warfarin, the contraindication to oral anticoagulation among real-world LAAO patients has resulted in many patients being discharged on dual antiplatelet therapy (DAPT) following implantation of either the WATCHMAN series (Boston Scientific), Amplatzer Cardiac Plug (ACP; Abbott) or Amplatzer Amulet (Abbott) devices. Recently, this approach was also approved for use in the USA.
Still, as a consequence of current treatment indications, most patients have a history of intracranial haemorrhage or other major bleeding incidents, prompting physicians to further reduce postprocedural antithrombotic treatment. Moreover, major bleeding remains the most frequently reported adverse event after LAAO and is associated with an increase in all-cause and cardiovascular mortality, further highlighting the need for additional investigation to find the right balance in postprocedural antithrombotic therapy1112.
Previously, low 1-year rates of DRT, ischaemic stroke and bleeding incidents have been reported in limited populations discharged on single antiplatelet therapy (SAPT) alone1314.
In the present study, we aimed to further evaluate the long-term safety and efficacy of a postprocedural SAPT strategy using either acetylsalicylic acid (ASA) or clopidogrel following Amplatzer LAAO.
Methods
This single-centre, retrospective cohort study included all LAAO patients discharged on SAPT following the implantation of an Amplatzer device at Aarhus University Hospital, Denmark, from March 2010 until December 2021. Baseline, procedural and device data were obtained from an institutional prospectively collected registry of all LAAO procedures at Aarhus University Hospital (n=942). From this registry, all patients in whom an Amplatzer device had been implanted were identified and cross-referenced with Danish national health registries using a unique 10-digit identifier that is assigned to all Danish citizens. Patient history, medication data and clinical outcomes were obtained from the Danish National Prescription Registry and the Danish National Patient Registry (DNPR), which contain prospectively collected patient-level data on all reimbursed prescriptions and hospitalisations across Denmark15. Events prior to LAAO were defined as any relevant International Classification of Diseases, tenth revision (ICD-10) codes registered more than 24 hours before the procedure. Furthermore, information on vital status was obtained from the Danish Civil Registration system, and cause of death was extracted from the Danish Cause of Death Registry16. Patients were censored at death, emigration or on 1st January 2023, allowing a minimum of 12 months of follow-up for the last included patient. The study was approved by the Danish Data Protection Agency (1-16-02-419-16) and the Danish Patient Safety Authorities (1-45-70-60-20).
PROCEDURAL DETAILS
All patients referred for LAAO underwent preprocedural planning by cardiac computed tomography (CT). In patients with a glomerular filtration rate <30 ml/min/1.73 m2, transoesophageal echocardiography (TOE) was performed instead of CT. Patients who were not already on ASA treatment received an immediate preprocedural 300 mg loading dose. Procedures were carried out under local or general anaesthesia, guided by intracardiac echocardiography or TOE, and under full heparinisation (100 IU/kg), aiming at an active clotting time of >250 s (Supplementary Table 1). Femoral vein access was sealed using a figure-of-8 suture, and all patients underwent transthoracic echocardiography prior to discharge to confirm device position and exclude pericardial effusion.
ANTITHROMBOTIC THERAPY
Discharge medication was defined as any relevant prescription claimed from the date of the procedure to 4 months after the procedure. Specifically, this included prescriptions for ASA, clopidogrel, vitamin K antagonists, factor II inhibitors, factor Xa inhibitors and heparins (Supplementary Table 2).
Patients claiming a prescription for either low-dose ASA (75 mg) or clopidogrel (75 mg) within this period were categorised as receiving single antiplatelet therapy (SAPT), while those claiming prescriptions for both were categorised as receiving dual antiplatelet therapy (DAPT). Patients discharged on a vitamin K antagonist, or a factor II or factor Xa inhibitor were categorised as receiving oral anticoagulation (OAC) with or without the addition of ASA. Alternative combinations were labelled as “other”, while patients with no relevant prescriptions within the period were considered not to be receiving antithrombotic or anticoagulant therapy (Supplementary Table 2). Antithrombotic therapy was further evaluated at 6 and 12 months after the procedure.
DEVICE-RELATED THROMBOSIS
The primary outcome of this study was the presence of DRT on a cardiac CT or TOE performed 6-12 weeks after device implantation.
As per the institutional protocol, all patients with a glomerular filtration rate >30 ml/min/1.73 m2 were scheduled for early follow-up cardiac CT, while patients unsuitable for CT imaging were evaluated by TOE.
The cardiac CT acquisition protocol has been previously described in detail1718. In short, scans were executed using a prospective electrocardiogram-gated high-pitch spiral protocol and iodine contrast. Images were analysed using the syngo.via imaging software package (Siemens Healthineers).
Cardiac CT scans were evaluated for hypoattenuated thickening (HAT) across the atrial surface of the device. Based on its morphology and extent, any HAT present was categorised as either low- or high-grade HAT, the latter interpreted as definite DRT18. All cardiac CT scans were evaluated by reviewers blinded to the clinical history of the patient. TOE images were evaluated applying the existing consensus on DRT detection and adjudication3519. Accordingly, TOE-adjudicated DRT was defined based on the mobility, morphology, and perceived texture of a suspected echo-reflective mass. All images suspected of showing HAT/DRT were evaluated by the primary investigator and subsequently confirmed or dismissed by a second researcher.
ADDITIONAL CT ANALYSIS
For all patients with a follow-up cardiac CT scan, the presence and degree of peridevice leak (PDL) were analysed based on a previously published grading system (Supplementary Table 3)20. In short, all scans were evaluated for contrast intensity in the distal left atrial appendage (LAA) as well as for any visible leaks at the device disc and at the device lobe.
The implant depth was measured as the distance from the left upper pulmonary vein ridge to the most proximal surface of the device disc. Proximal and distal implantion were defined when this distance was either <5 mm or ≥5 mm, respectively21.
CLINICAL OUTCOMES
Clinical outcomes were defined based on ICD-10 discharge diagnoses prospectively recorded in the DNPR (Supplementary Table 4). Outcomes of interest included ischaemic stroke, intracranial haemorrhage, extracranial major bleeding, cardiovascular and all-cause mortality. In accordance with the Munich consensus on reporting in LAAO research and as all cases of acute stroke in Denmark are handled within a neurological unit, the codes for ischaemic stroke and intracranial haemorrhage were restricted to diagnoses coded in a neurological department22. Major bleeding was defined as any bleeding requiring medical intervention, hospitalisation or prompting evaluation, corresponding to Bleeding Academic Research Consortium (BARC) type 2 or above or major bleeding according to the Munich consensus2223. Cardiovascular mortality was defined as any cause of death registered within the cardiovascular section (DI*) of the ICD-10 index, as well as death without any registered cause22.
STATISTICAL ANALYSIS
All statistical analysis was performed using Stata, version 17.0 (StataCorp). Data distribution was evaluated using histograms and Q-Q plots. Continuous data are presented as mean values with standard deviation (SD) or medians with interquartile range (IQR) and were compared using the Wilcoxon rank-sum test, two-sample t-test or analysis of variance, as appropriate. Meanwhile, categorical values are presented as frequencies with percentages and were compared using Pearson’s χ2 or Fisher’s exact test. Clinical outcomes were evaluated using time-to-event analysis and are reported as absolute frequencies, crude incidence rates (IR) and cumulative incidence proportions (CIP), considering death as a competing risk. Hazard ratios were derived using the Cox proportional hazards model and adjusted for differing baseline risks of stroke and bleeding (renal insufficiency, prior stroke, prior major bleeding, CHA2DS2-VASc and HAS-BLED score).
Results
During the study period, a total of 553 patients underwent LAAO with either an ACP (n=75, 13.6%) or Amulet device (n=478, 86.4%) at Aarhus University Hospital. Among these, 431 (77.9%) patients were discharged on SAPT, using either ASA (n=403, 72.9%) or clopidogrel (n=28, 5.1%) monotherapy (Figure 1). The remaining 122 (22.1%) patients were discharged on either DAPT (n=55, 10.0%), OAC with or without concomitant antiplatelet therapy (APT; n=26, 4.7%), or without antithrombotic therapy (n=41, 7.4%) (Figure 1).
The SAPT cohort had a median follow-up time of 4.1 years (IQR: 2.2-5.9). Among the SAPT cohort, prior intra- and extracranial bleeding was common, while there were fewer patients with a history of ischaemic stroke, compared to the non-SAPT cohort. Consequently, at the time of their procedure, the mean CHA2DS2-VASc and HAS-BLED scores of the SAPT cohort were 3.9 (±1.5) and 3.4 (±1.1), respectively. Patients in the non-SAPT cohort had a higher mean CHA2DS2-VASc score of 4.4 (±1.7) and a lower mean HAS-BLED score of 3.0 (±1.1) (Table 1).
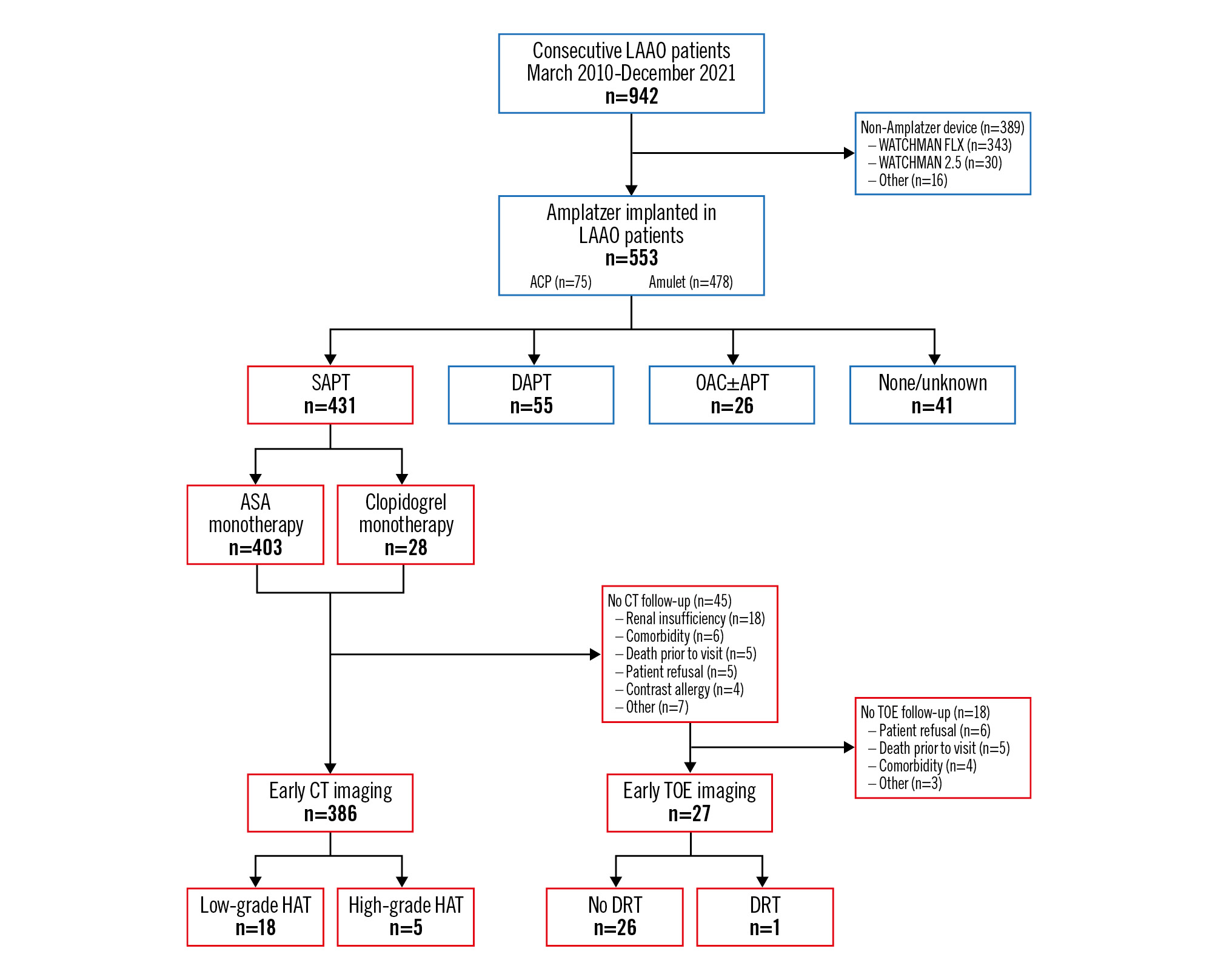
Figure 1. Flowchart of study patients, antithrombotic therapy at discharge, and follow-up imaging. ACP: Amplatzer Cardiac Plug; APT: antiplatelet therapy; ASA: acetylsalicylic acid; CT: computed tomography; DAPT: dual antiplatelet therapy; DRT: device-related thrombosis; HAT: hypoattenuated thickening; LAAO: left atrial appendage occlusion; OAC: oral anticoagulation; SAPT: single antiplatelet therapy; TOE: transoesophageal echocardiography
Table 1. Baseline characteristics and comparison to non-SAPT cohort.
| Baseline characteristics | SAPT at discharge n=431 | Other at discharge n=122 | p-value |
|---|---|---|---|
| Age at LAAO, years | 74.4 (69.0-79.6) | 73.9 (68.3-79.2) | 0.35 |
| Follow-up, years | 4.1 (2.2-5.9) | 4.6 (1.9-6.0) | 0.51 |
| Sex, female | 144 (33.4) | 47 (38.5) | 0.29 |
| Atrial fibrillation category | 0.82 | ||
| Unknown | 143 (33.2) | 37 (30.3) | |
| Paroxysmal | 196 (45.5) | 59 (45.5) | |
| Permanent | 92 (21.3) | 26 (21.3) | |
| Renal insufficiency | 97 (23.2) | 15 (12.9) | 0.02 |
| Chronic ischaemic heart disease | 62 (14.4) | 23 (18.9) | 0.23 |
| Prior events | |||
| Prior stroke | 119 (23.2) | 50 (41.0) | 0.01 |
| Prior TIA | 26 (6.0) | 16 (13.1) | 0.01 |
| Prior systemic embolism | 3 (0.7) | 2 (1.6) | 0.33 |
| Prior ICH | 103 (23.9) | 15 (12.3) | 0.01 |
| Prior major bleeding (incl. ICH) | 338 (78.4) | 67 (54.9) | <0.001 |
| Prior AMI | 43 (10.0) | 16 (13.1) | 0.32 |
| Prior PCI | 58 (13.5) | 21 (17.2) | 0.30 |
| Prior CABG | 25 (5.8) | 8 (6.6) | 0.76 |
| CHA2DS2-VASc score | 3.9±1.5 | 4.4±1.7 | 0.01 |
| HAS-BLED score | 3.4±1.1 | 3.0±1.1 | <0.001 |
| Data are presented as median (IQR), n (%) or mean±SD. AMI: acute myocardial infarction; CABG: coronary artery bypass graft; ICH: intracranial haemorrhage; IQR: interquartile range; LAAO: left atrial appendage occlusion; PCI: percutaneous coronary intervention; SAPT: single antiplatelet therapy; SD: standard deviation; TIA: transient ischaemic attack | |||
ANTITHROMBOTIC THERAPY
At 6-month follow-up, 173 (41.7%) patients in the SAPT cohort were not on antithrombotic therapy, while just 25 (6%) had intensified their APT to include DAPT (1.9%) or OAC (4.1%). In comparison, for the non-SAPT cohort, 34 (29.8%) patients were without any registered therapy at 6 months.
At 12 months post-procedure, 192 (48.0%) and 42 (37.5%) patients were off pharmacological thromboprophylaxis in the SAPT and non-SAPT cohorts, respectively (Figure 2, Supplementary Table 5).
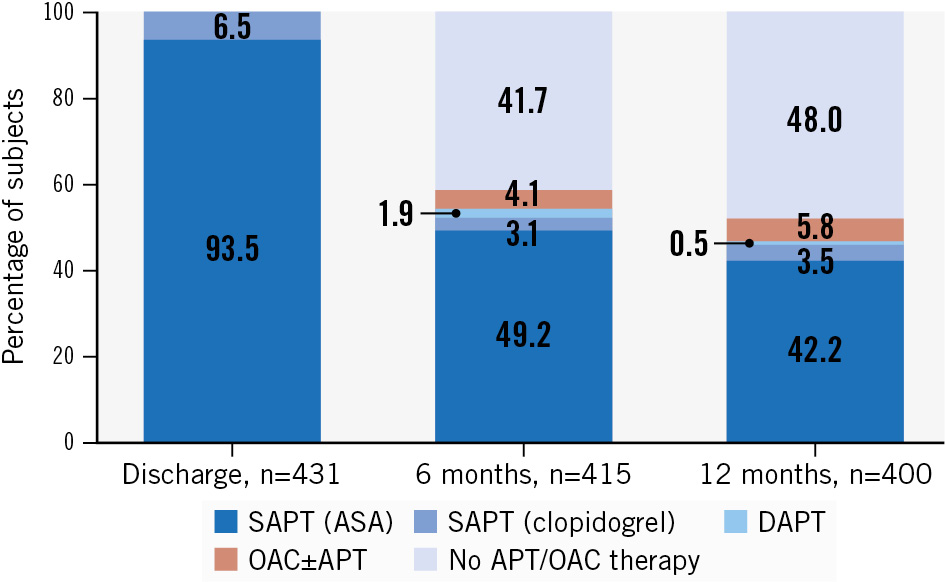
Figure 2. Bar plot showing the continued antithrombotic therapy at 6 and 12 months among Amplatzer LAAO patients discharged on SAPT. APT: antiplatelet therapy; ASA: acetylsalicylic acid; DAPT: dual antiplatelet therapy; OAC: oral anticoagulation; SAPT: single antiplatelet therapy
DEVICE-RELATED THROMBOSIS
Of the 431 patients within the SAPT cohort, 386 (89.6%) underwent early follow-up imaging using cardiac CT. Among the 45 (10.4%) patients without CT follow-up, early follow-up TOE imaging was performed in 27 patients (Figure 1). The mean time from procedure to CT follow-up was 59.0 (±21.7) days. The primary reasons for incomplete follow-up imaging among the SAPT cohort were renal impairment (n=18, 40.0%), patient comorbidities (n=6, 15.6%), death prior to imaging (n=5, 11.1%), and a known allergy to iodine contrast (n=4, 8.9%).
Five patients (1.3%) were found to have high-grade HAT at their early follow-up CT, while 18 (4.7%) presented with low-grade HAT, corresponding to an effective DRT rate on CT of 1.3%. The mean time to TOE was 56.6 (±16.9) days. One case of DRT was detected within this limited cohort (Supplementary Table 6).
Collectively, 6 patients (1.5%) within the SAPT cohort were found to have DRT on early follow-up imaging (Central illustration). Four patients received intensified antithrombotic treatment in response to the follow-up imaging. One patient had already been changed from SAPT to DAPT following a myocardial infarction, while intensified therapy was withheld in 1 patient because of a high risk of bleeding. All DRT patients were still on antithrombotic therapy at 6 months (Supplementary Table 6). In the highly heterogeneous non-SAPT cohort, a total of 3 patients (2.7%) were found to have DRT on either follow-up TOE (n=1) or cardiac CT (n=2) (Supplementary Table 7).
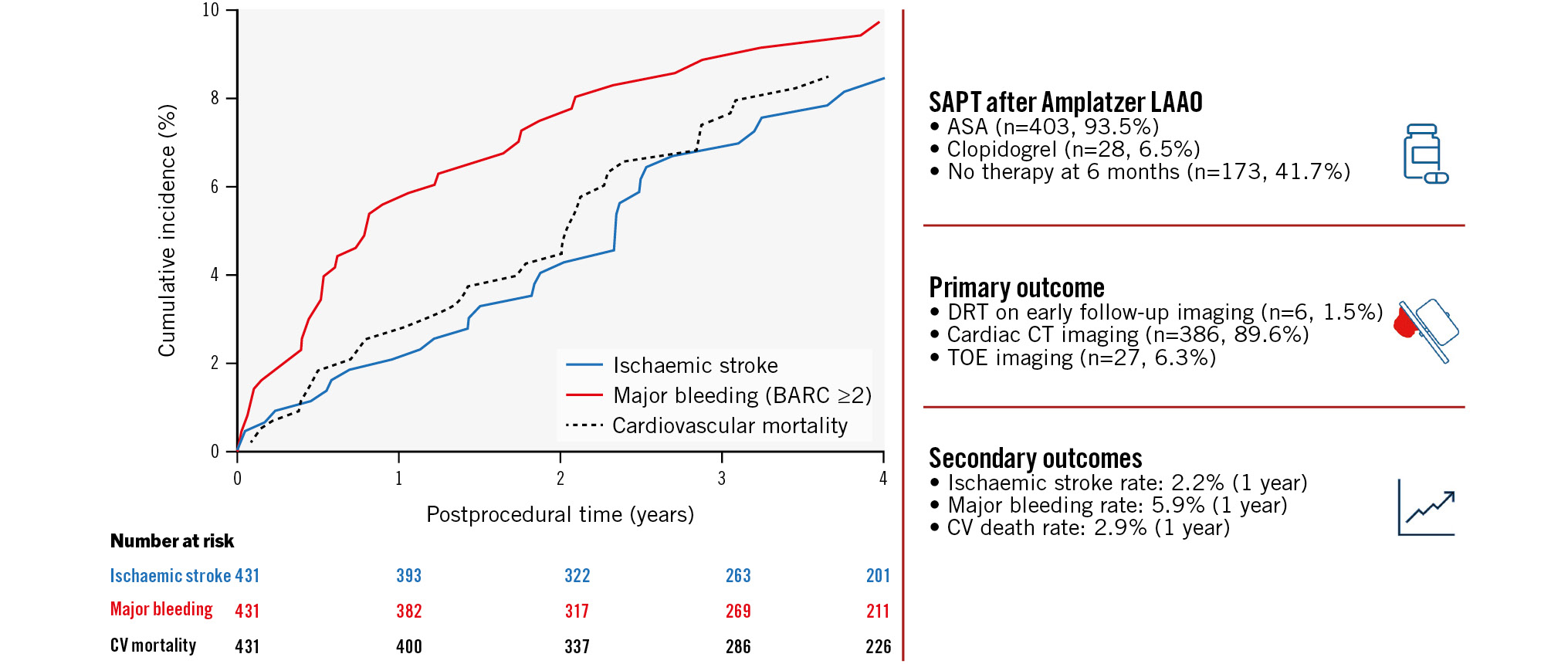
Central illustration. Primary and secondary outcomes of patients treated with single antiplatelet therapy (SAPT) following Amplatzer LAAO. Mortality-adjusted cumulative incidences of ischaemic stroke (blue, solid), major bleeding (red, solid) and cardiovascular death (black, dotted) throughout 4 years of follow-up. The number at risk is represented in relation to the relevant outcome. Clinical event rates are presented as annual incidence rates. ASA: acetylsalicylic acid; BARC: Bleeding Academic Research Consortium; CT: computed tomography; CV: cardiovascular; DRT: device-related thrombosis; LAAO: left atrial appendage occlusion; TOE: transoesophageal echocardiography
ADDITIONAL CT FINDINGS
Among patients in the SAPT cohort with an available follow-up CT, LAA patency was observed in 64% of patients. A grade 3 leak was seen in 79 patients (20.5%), while the LAA was found to be completely occluded in 103 patients (26.7%). The frequencies of LAA patency and PDL grade did not differ between patients with continued or discontinued antithrombotic therapy at 6 months. A deeper implantation depth was observed among patients on continued therapy at 6 months (Table 2). Moreover, 3 out of 5 patients (60.0%) with high-grade HAT on their 8-week follow-up CT had an implantation depth ≥5 mm. In comparison, 104 patients (24.7%) without high-grade HAT had an implantation depth ≥5 mm (p=0.07). While there was no significant difference between groups, this tendency is in line with several previous publications which have identified a deep device implantation as a risk factor for DRT21.
Table 2. Additional CT findings at early follow-up imaging.
| CT characteristics | Total SAPT cohort with CT follow-up n=386 | Continued AT at 6 months n=221 |
Discontinued AT at 6 months n=158 |
p-value |
|---|---|---|---|---|
| HAT category | 0.16 | |||
| No HAT | 363 (94.0) | 206 (93.2) | 150 (94.9) | |
| Low-grade HAT | 18 (4.7) | 10 (4.5) | 8 (5.1) | |
| High-grade HAT | 5 (1.3) | 5 (2.3) | 0 (0) | |
| PDL category | 0.89 | |||
| Complete occlusion | 103 (26.7) | 62 (28.1) | 40 (25.3) | |
| Grade 1 leak | 58 (15.0) | 31 (14.0) | 26 (16.5) | |
| Grade 2 leak | 146 (37.8) | 83 (37.6) | 60 (38.0) | |
| Grade 3 leak | 79 (20.5) | 45 (20.4) | 32 (20.3) | |
| LAA patency | 247 (64.0) | 140 (63.3) | 102 (64.6) | 0.81 |
| Distance from LUPV | 0.03 | |||
| <5 mm | 289 (74.9) | 156 (70.6) | 128 (81.0) | |
| 5 to <10 mm | 38 (9.8) | 23 (10.4) | 14 (8.9) | |
| 10 to <15 mm | 44 (11.4) | 34 (15.4) | 9 (5.7) | |
| ≥15 mm | 15 (3.9) | 8 (3.6) | 7 (4.4) | |
| Distal implant | 97 (25.1) | 65 (29.4) | 30 (19.0) | 0.02 |
| Data are presented as absolute frequencies (%) among patients with an early follow-up CT scan available. Findings are presented both for the collective SAPT-discharged cohort as well as stratified by continuation of antithrombotic therapy at 6-month follow-up. Seven patients were censored earlier than 6 months and have not been included in the comparison. AT: antithrombotic therapy; CT: computed tomography; HAT: hypoattenuated thickening; LAA: left atrial appendage; LUPV: left upper pulmonary vein; PDL: peridevice leak; SAPT: single antiplatelet therapy | ||||
CLINICAL OUTCOMES
Clinical events were evaluated at 1 and 4 years after LAAO, the latter approximating the median follow-up time (Table 3). At 1 year, the crude annual ischaemic stroke rate was 2.19% (95% confidence interval [CI]: 1.14-4.21). Equivalent rates for major bleeding and cardiovascular death were 5.94% (95% CI: 3.98-8.87) and 2.89% (95% CI: 1.64-5.09), respectively.
From a total of 1,296.49 patient years at 4 years of follow-up, 33 patients (7.66%) experienced an ischaemic stroke. Meanwhile, 40 patients (9.28%) experienced major bleeding (BARC ≥2). The CIPs of ischaemic stroke and major bleeding at 4-year follow-up were 8.44% (95% CI: 5.94-11.48) and 9.74% (95% CI: 7.10-12.86), respectively (Central illustration). The crude annual major bleeding rate declined from 1- to 4-year follow-up without a significant increase in ischaemic stroke rates (Central illustration, Table 3). Moreover, no increased risk of ischaemic stroke was observed among SAPT-discharged patients who discontinued APT after 6 months (Table 4, Figure 3A). At 4 years, the cumulative incidence proportion of all-cause mortality was 28.30% (95% CI: 23.87-32.87), suggesting an overall high mortality rate in this LAAO population.
No statistically significant difference in clinical event rates or hazard ratios were seen between the SAPT and non-SAPT cohorts (Supplementary Table 8). Likewise, the chosen discharge regimens were not predictive of the risk of postprocedural pericardial effusion (Supplementary Table 9).
Table 3. Clinical outcomes of Amplatzer LAAO patients discharged on SAPT (n=431).
| 1-year follow-up | Events, n (%) | Crude annual IR, % (95% CI) | CIP at 1 year, %* (95% CI) |
|---|---|---|---|
| Ischaemic stroke | 9 (2.09) | 2.19 (1.14-4.21) | 2.09 (1.04-3.79) |
| Any major bleeding | 24 (5.56) | 5.94 (3.98-8.87) | 5.56 (3.67-8.01) |
| Intracranial haemorrhage | 8 (1.86) | 1.94 (0.97-3.89) | 1.86 (0.88-3.49) |
| Extracranial major bleeding | 16 (3.71) | 3.93 (2.41-6.41) | 3.71 (2.21-5.81) |
| All-cause mortality | 31 (7.19) | 7.47 (5.25-10.62) | 7.19 (5.01-9.89) |
| Cardiovascular mortality | 12 (2.78) | 2.89 (1.64-5.09) | 2.78 (1.52-4.67) |
| 4-year follow-up | Events, n (%) | Crude annual IR, % (95% CI) | CIP at 4 years, %* (95% CI) |
| Ischaemic stroke | 33 (7.66) | 2.55 (1.81-3.58) | 8.44 (5.94-11.48) |
| Any major bleeding | 40 (9.28) | 3.11 (2.28-4.23) | 9.74 (7.10-12.86) |
| Intracranial haemorrhage | 10 (2.32) | 0.75 (0.40-1.39) | 2.39 (1.23-4.22) |
| Extracranial major bleeding | 030 (6.96) | 2.30 (1.61-3.28) | 7.33 (5.06-10.14) |
| All-cause mortality | 111 (25.75) | 8.20 (6.80-9.87) | 28.30 (23.87-32.87) |
| Cardiovascular mortality | 34 (7.89) | 2.51 (1.79-3.51) | 8.50 (6.02-11.50) |
| * cumulative incidence with death as a competing risk (for all-cause mortality, emigration was considered a competing risk). Data are presented as absolute frequencies, crude annual incidence rates and cumulative incidence proportions at 1 and 4 years post-procedure. CI: confidence interval; CIP: cumulative incidence proportion; IR: incidence rate; LAAO: left atrial appendage occlusion; SAPT: single antiplatelet therapy | |||
Table 4. Clinical outcomes of patients with or without continued APT 6 months after Amplatzer LAAO (n=415).
| Discontinued APT at 6 months n=173 | ||||
|---|---|---|---|---|
| Events, n (%) | Crude annual IR, % (95% CI) | CIP at 1 year, %*(95% CI) | CIP at 4 years, %*(95% CI) | |
| Ischaemic stroke | 11 (6.36) | 2.02 (1.12-3.66) | 1.16 (0.23-3.78) | 7.04 (3.74-11.78) |
| Any major bleeding | 21 (12.14) | 4.07 (2.65-6.24) | 8.67 (5.08-13.45) | 12.54 (8.05-18.07) |
Intracranial haemorrhage |
7 (4.05) | 1.29 (0.61-2.70) | 3.47 (1.43-6.99) | 4.18 (1.85-8.02) |
Extracranial major bleeding |
14 (8.09) | 2.64 (1.56-4.46) | 5.20 (2.56-9.22) | 8.35 (4.78-13.17) |
| Continued APT beyond 6 monthsn=242 | ||||
| Events, n (%) | Crude annual IR, %(95% CI) | CIP at 1 year, %*(95% CI) | CIP at 4 years, %*(95% CI) | |
| Ischaemic stroke | 26 (10.74) | 2.66 (1.71-4.12) | 2.48 (1.03-5.05) | 9.16 (5.78-13.47) |
| Any major bleeding | 16 (6.61) | 2.08 (1.28-3.40) | 2.48 (1.03-5.05) | 7.14 (4.24-11.02) |
Intracranial haemorrhage |
2 (0.83) | 0.25 (0.06-1.02) | 0.04 (0.00-2.14) | 0.87 (0.0.18-2.89) |
Extracranial major bleeding |
14 (5.79) | 1.81 (1.07-3.06) | 2.07 (0.78-4.49) | 6.26 (3.58-9.98) |
| * cumulative incidence with death as a competing risk (for all-cause mortality, emigration was considered a competing risk). Data are presented as absolute frequencies, cumulative incidence proportions at 1 and 4 years post-procedure, as well as crude annual incidence rates at 4-year follow-up. APT: antiplatelet therapy; CI: confidence interval; CIP: cumulative incidence proportion; IR: incidence rate; LAAO: left atrial appendage occlusion; SAPT: single antiplatelet therapy | ||||
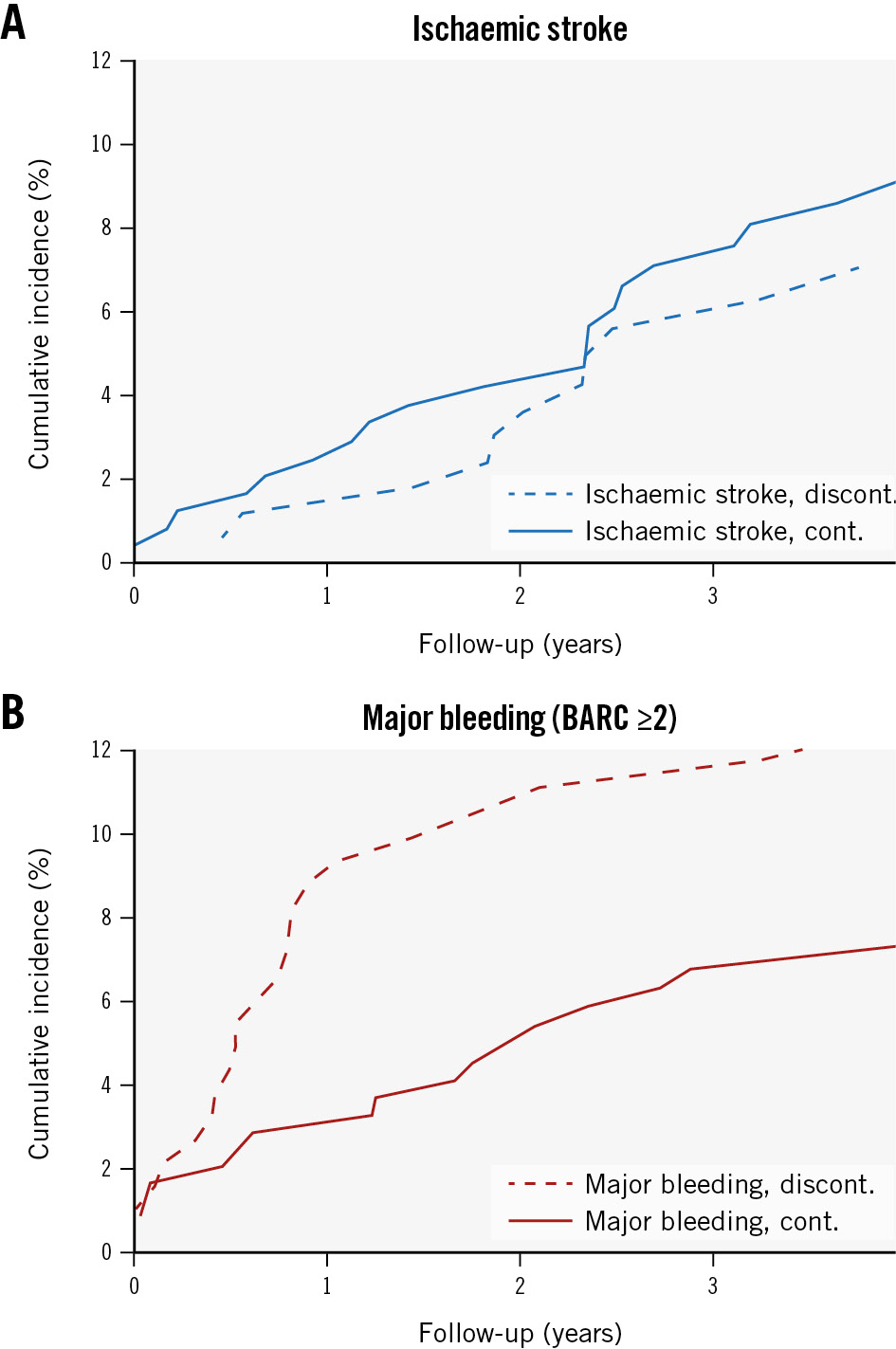
Figure 3. Mortality-adjusted cumulative incidences of secondary outcomes among the SAPT cohort with and without continued APT at 6 months. A) Ischaemic stroke and B) major bleeding. APT: antiplatelet therapy; BARC: Bleeding Academic Research Consortium; cont.: continued; discont.: discontinued; SAPT: single antiplatelet therapy
Discussion
This single-centre cohort study evaluated the use of SAPT following Amplatzer LAAO in 431 AF patients. At 4-year follow-up, the presented rates of both DRT and clinical outcomes suggest this strategy to be both safe and efficient as a postprocedural therapy in patients at high risk of bleeding. To our knowledge, this is the largest reported cohort of patients discharged on SAPT following LAAO.
ANTITHROMBOTIC THERAPY
Despite a generally high bleeding risk among LAAO patients, a period of postprocedural antithrombotic treatment is the current consensus to minimise the risk of DRT and subsequent thromboembolic events during endothelialisation of the device. Although limited evidence exists on the natural history of this process in humans, canine studies suggest that complete endothelialisation occurs during the first months following implantation2425. In the present study, approximately 42% and 48% of patients discharged on SAPT were without any antithrombotic therapy at 6 and 12 months, respectively (Figure 2). Accordingly, the incidence rate of major bleeding appears higher within the first 6 months post-procedure, converting to a more stable rate for the rest of the reported follow-up period (Figure 3, Table 3). In accordance with institutional protocol, patients with established coronary heart disease (prior percutaneous coronary intervention [PCI] or coronary artery bypass grafting [CABG]) or with prior ischaemic stroke or carotid artery revascularisation were continued on lifelong antiplatelet therapy following LAAO.
At 6 months, approximately 6% of patients discharged on SAPT received intensified anticoagulation with either an oral anticoagulant or DAPT (Figure 2). While a few of these patients account for the DRT cases identified on early follow-up imaging, this escalation likely also represents patients treated for other indications such as coronary artery disease, venous thromboembolism, or stroke despite LAAO.
DEVICE-RELATED THROMBOSIS
The primary rationale behind postprocedural antithrombotic therapy is the prevention of DRT on the atrial surface of the implanted device – a condition associated with an increased risk of stroke or transient ischaemic attacks3. In accordance with institutional protocol, early follow-up imaging in the presented cohort was predominantly performed using cardiac CT. Previously, cardiac CT has proven to be superior to TOE in detecting DRT, and an algorithm for interpretation has been developed and used in previous studies on Amplatzer devices1826. Despite cardiac CT being the primary imaging modality utilised, the DRT rates reported in this study appear comparable to other recent studies investigating the Amulet device with follow-up TOE (Figure 4)42728.
As differences in imaging modalities and DRT definitions complicate direct comparisons, standardisation is needed for both clinical and scientific purposes. To reduce the effects of any intra- and interobserver variance, all cases with suspected HAT were evaluated twice by the primary investigator and subsequently confirmed or dismissed by a second investigator. Importantly, overdiagnosis and subsequent increased anticoagulation is highly undesirable in the LAAO population who have a generally high risk of bleeding.
While likely underpowered because of a low number of patients in each subgroup, low- or high-grade HAT on cardiac CT was not associated with an increased risk of clinical events (Supplementary Table 10).
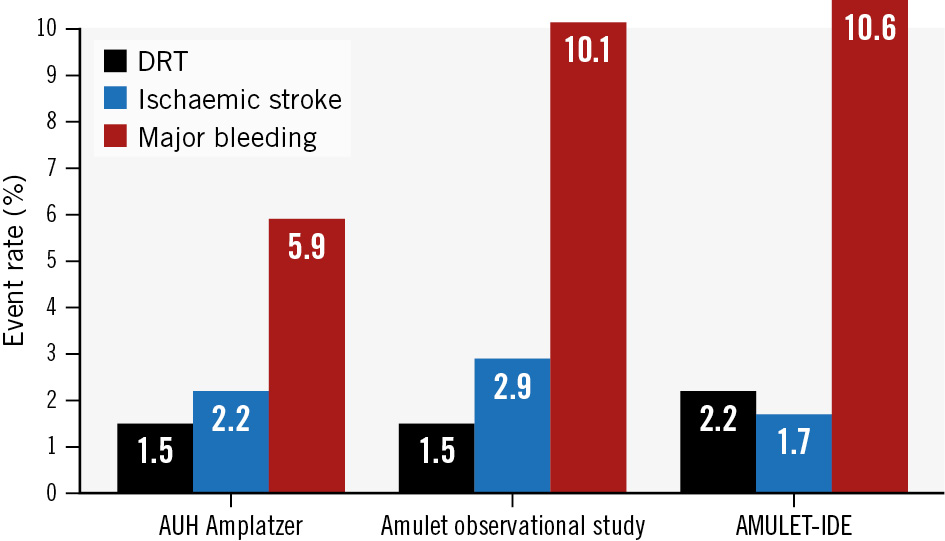
Figure 4. Reported 1-year rates of ischaemic stroke and major bleeding and calculated rates of early DRT among Amulet-implanted patients in contemporary trials and observational studies. DRT rates are calculated from the number of patients with reported DRT or high-grade HAT relative to the number of patients with evaluated early follow-up imaging. AUH Amplatzer cohort (6/413); Amulet observational study (10/673)28; AMULET-IDE Trial (19/856)4. AUH: Aarhus University Hospital; DRT: device-related thrombosis; HAT: hypoattenuated thickening
CLINICAL OUTCOMES
A cohort with very similar characteristics to ours was described in the Amulet observational study10. However, in that study, the majority of patients (57.6%) were discharged on DAPT, while 22.5% were discharged on SAPT. Also, compared to our SAPT cohort, the fraction of patients without any antithrombotic therapy was lower at both 6 (14.5% vs 41.7%) and 12 months (18.9% vs 48.0%). Despite these differences, the present Aarhus University Hospital SAPT cohort displays a comparable annualised rate of ischaemic stroke (2.2%/year vs 2.9%/year), equal rates of DRT at early follow-up (1.5% vs 1.5%), but a lower 1-year rate of major bleeding (5.9%/year vs 10.3%/year) (Figure 4)1028. Similarly, in the AMULET-IDE trial, a vast majority of the Amulet cohort were discharged on DAPT, and they maintained this treatment until 6-month follow-up. Comparable to the Amulet observational study, rates of DRT, ischaemic stroke and major bleeding were 2.2%, 1.7% and 10.6%, respectively (Figure 4)412.
Moreover, within the Amulet observational study, specific event rates are reported for SAPT- and DAPT-discharged patients, and the 1-year rates of DRT were 0.8% versus 1.6%, respectively. Simultaneously, the rate of major bleeding appeared lower in the SAPT-treated cohort (6.6% vs 8.4%). The EWOLUTION study, reporting on the WATCHMAN device, did not find an association between SAPT and an increased risk of DRT or bleeding at 3-month follow-up9.
While this should not be interpreted as a direct comparison with DAPT, the results found in these non-randomised registries may indicate that a more minimalistic approach using SAPT and early discontinuation is viable in patients with a comprehensive bleeding history and no other indications for continued or more intense treatment (e.g., confirmed coronary, cerebral or peripheral arterial disease).
Several ongoing trials are investigating the optimal antithrombotic therapy following LAAO. Of particular interest to the present study, the Italian ARMYDA-AMULET trial (ClinicalTrials.gov: NCT05554822) is randomising patients to SAPT or DAPT following Amulet implantation, evaluating DRT, stroke, and major bleeding at 6 months. Meanwhile, the ANDES (ClinicalTrials.gov: NCT03568890) and FADE-DRT trials (ClinicalTrials.gov: NCT04502017) are investigating the use of full- or half-dose direct oral anticoagulation (DOAC) versus antiplatelet-based strategies.
Limitations
As a single-centre, observational study, a central limitation of this investigation is its translatability. It represents a report on local practice in a high-volume LAAO centre, and, although SAPT was the primary strategy for post-LAAO treatment, this regimen could be modified on a case-by-case basis by the implanting physician. An element of confounding by indication will inherently be present, favouring SAPT treatment in LAAO patients where the a priori risk of bleeding seems to exceed the risk of thromboembolism. Theoretically, this would cause an underestimation of the true stroke risk while overestimating the risk of bleeding in a general LAAO population. Furthermore, due to the registry-based nature of this study, the definition of major bleeding was BARC ≥2 type bleeding. In contrast, the referenced studies all define major bleeding as BARC ≥3. Regardless, we found rates of bleeding comparable, or lower, to those presented in contemporary trials and registries111227.
The interpretation and definition of DRT on both CT and TOE imaging is debatable. In the current study, no cases of low-grade HAT on early cardiac CT were included as DRT. Further validation of the suggested algorithms is needed. However, several studies do point towards low-grade HAT representing benign endothelialisation18262930.
Ultimately, DRT is of interest because of its concomitant risk of thromboembolism. While imaging interpretation lacks validation, the diagnosis of ischaemic stroke holds a very high positive predictive value (97%) in the Danish national registries31.
Conclusions
In a high bleeding risk AF population, SAPT following Amplatzer LAAO displayed rates of DRT and stroke comparable to those reported in large contemporary trials and registries, which utilised more intensive postprocedural antithrombotic regimens. Meanwhile, we observed low rates of major bleeding. The use of SAPT after LAAO appears safe and effective in selected patients but should be further evaluated in clinical trials.
Impact on daily practice
Most patients undergoing left atrial appendage occlusion are at a high risk of bleeding, and major bleeding events remain the most frequent postprocedural adverse event. Current consensus supports the need for postprocedural antithrombotic therapy to minimise the risk of device-related thrombosis (DRT), yet the optimal regimen remains unknown. This large, single-centre, observational study demonstrates low rates of DRT, ischaemic stroke and bleeding in a high bleeding risk population treated only with single antiplatelet therapy upon discharge. Furthermore, a large proportion of patients were no longer receiving any antithrombotic treatment by the 6-month follow-up, suggesting that this minimalistic approach is both safe and efficient in this population.
Funding
This work was funded through a grant from Eva og Henry Frænkels Mindefond (Eva & Henry Frænkels Mindefond c/o Redmark, Dirch Passers Allé 76, 2000 Frederiksberg, Denmark). Moreover, Dr Kramer is currently enrolled as a PhD fellow at Aarhus University (Nordre Ringgade 1, 8000, Aarhus C, Denmark) and has received a full scholarship from the institution. Funders were not involved in any aspect of the study, including its design, collection, analysis and interpretation of the data, or the writing of the manuscript.
Conflict of interest statement
K. Korsholm has received lecture fees from Abbott and Boston Scientific. J.E. Nielsen-Kudsk has received institutional research grants from Abbott and Boston Scientific. A. Kramer has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

