
Abstract
Aims: We aimed to assess the feasibility, efficacy and safety of short-term dual antiplatelet therapy (DAPT) for six weeks after left atrial appendage closure (LAAC).
Methods and results: Data of the Cardioangiologisches Centrum Bethanien-LAAC registry were analysed. DAPT (aspirin 100 mg plus clopidogrel 75 mg) was administered until transoesophageal echocardiography (TEE) evaluation six weeks after LAAC. In the absence of significant peri-device flow or device-related thrombus (DRT), the medication was decreased to single antiplatelet therapy (SAPT). Outpatient visits were timed at six-month intervals. The incidences of major bleeding (BARC ≥3) and of thromboembolic events were investigated. A total of 298 patients (76±8 years; 62% male; CHA2DS2-VASc 4.3±1.5; HAS-BLED 3.5±1.0; 61% with history of bleeding) with successful LAAC were included. TEE revealed DRT in 7/298 (2.3%) patients (five at six-week follow-up [FU] 45±10 days after implant, two during a median long-term FU of 731 days). Non-procedure-related bleeding events occurred in 25/298 (8.4%) patients and non-procedure-related thromboembolic events in 11/298 (3.7%) patients. This translated into 3.9 bleeding events/100 patient-years and 1.7 thromboembolic events/100 patient-years, respectively. Procedure-related events consisted of major bleeding in 7/298 (2.3%) patients and stroke in 2/298 (0.7%) patients. Age ≥75 years (OR 3.2; CI: 1.2-8.0; p=0.015) and renal impairment (OR 2.5; CI: 1.1-5.7; p=0.027) were identified as independent predictors for major bleeding after LAAC.
Conclusions: Short-term DAPT for six weeks appears to be a viable alternative for patients after LAAC. Age ≥75 years and renal impairment increase major bleeding events threefold.
Abbreviations
AF: atrial fibrillation
BARC: Bleeding Academic Research Consortium
CCB: Cardioangiologisches Centrum Bethanien
DAPT: dual antiplatelet therapy
DRT: device-related thrombus
FU: follow-up
GIB: gastrointestinal bleeding
LAA: left atrial appendage
LAAC: left atrial appendage closure
NOAC: new oral anticoagulants
OAC: oral anticoagulation
SAPT: single antiplatelet therapy
SE: systemic embolism
TEE: transoesophageal echocardiography
TIA: transient ischaemic attack
VKA: vitamin K antagonists
Introduction
Interventional left atrial appendage closure (LAAC) is an established therapeutic option for atrial fibrillation (AF) patients at risk for thromboembolic complications and a contraindication to long-term oral anticoagulation (OAC)1,2. After successful device placement, antithrombotic therapy has to be applied transiently in order to avoid device-related thrombus (DRT) formation and subsequent embolic events. Uncertainty exists on the optimal antithrombotic regimen as well as the optimal treatment duration. In large randomised clinical trials using the WATCHMAN® device (Boston Scientific, Marlborough, MA, USA), OAC plus aspirin for six weeks followed by six months of dual antiplatelet therapy (DAPT) and lifelong aspirin therapy has been proposed3,4. For the same device, six months of DAPT followed by lifelong aspirin has been proven to be efficacious in patients contraindicated to OAC5. Scarce data from controlled studies are available for alternative devices (AMPLATZER™ Cardiac Plug [ACP], Amulet™ [both St. Jude Medical, St. Paul, MN, USA]), but it is expert consensus to establish a six-month dual antiplatelet therapy6.
All regimens bear the potential risk for bleeding complications, particularly for patients with increased HAS-BLED scores7. Since patient characteristics have changed considerably with a shift towards more frailty and a very high bleeding risk, a foreshortened treatment period would be desirable.
In this single-centre observational study we investigated the feasibility of a short-term (six-week) DAPT course followed by lifelong aspirin therapy in an all-comers cohort receiving different LAAC devices. The incidence of DRT as well as the incidence of bleeding and thromboembolic events was assessed.
Methods
Data on all LAAC implant procedures at the Cardioangiologisches Centrum Bethanien (CCB) were prospectively collected in the CCB-LAAC registry. From 2010, all patients were treated according to the protocol using DAPT for six weeks after LAAC. The local ethics committee approved the study protocol. Patients gave written informed consent prior to the procedure.
PATIENTS
Two interventional cardiologists evaluated the indication for the LAAC procedure in line with the current expert consensus document6. Data on age, gender, AF type and duration as well as concomitant medical diseases were assessed. Contemporary risk scores for thromboembolism (CHA2DS2-VASc) as well as bleeding (HAS-BLED) were calculated7-9.
PROCEDURE
All procedures were performed in deep sedation, and unfractionated heparin was given at a dose of 70-100 IU/kg body weight. A single transseptal puncture with an 8 Fr sheath (SL1™; St. Jude Medical) using the modified Brockenbrough technique was performed under transoesophageal echocardiography (TEE) (Vivid™ 7; GE Healthcare, Horten, Norway) guidance. Left atrial appendage (LAA) angiographies were obtained in two different angulations. The diameters of the putative landing zone were assessed and compared to echocardiographic measurements.
WATCHMAN AND WATCHMAN FLX™ IMPLANTATION
The delivery sheath (14 Fr) was advanced to the LAA. Device release was realised by unsheathing, i.e., slowly retracting the sheath across the compressed device remaining in its position.
ACP/AMULET IMPLANTATION
After insertion of the delivery sheath (TorqVue™ 12-14 Fr; St. Jude Medical), the device was advanced to the left atrium and partially released for cannulation of the LAA landing zone. Sequential device release was performed by unsheathing and actively pushing the lobe. Thereafter, the device disc was positioned outside the LAA ostium.
COHEREX WAVECREST™ (COHEREX MEDICAL, SALT LAKE CITY, UT, USA) IMPLANTATION
A pre-shaped delivery sheath (17 Fr; 60-90°) was directed to the LAA neck. The pre-loaded device attached to a delivery catheter was advanced, and device release was accomplished by an unsheathing manoeuvre followed by an active deployment of the device anchors.
ECHOCARDIOGRAPHIC EVALUATION
Device compression and residual flow were assessed. Pericardial effusion was ruled out.
ANTITHROMBOTIC REGIMEN
Immediately after the implantation procedure, a loading dose of 250 mg aspirin intravenously in addition to 300 mg clopidogrel per os was administered. Thereafter, aspirin 100 mg in combination with clopidogrel 75 mg once daily was prescribed for six weeks. Oral anticoagulants were stopped.
FOLLOW-UP
A chest X-ray was obtained to exclude device embolisation. Six weeks after LAAC, a TEE was performed to assess DRT and peri-device flow. In case of patent LAAC without DRT, clopidogrel was stopped. Single antiplatelet therapy (SAPT) using aspirin 100 mg/d was continued indefinitely.
In case of peri-device flow >5 mm, DAPT was continued for another six weeks. In case of DRT, patients were treated with low-molecular-weight heparin (1 mg/kg body weight enoxaparin subcutaneously twice daily) for four weeks in addition to DAPT.
Patients were scheduled for outpatient visits at six-month intervals to assess any adverse events (stroke, bleeding, hospitalisation), current medication, 12-lead electrocardiogram and transthoracic echo. For patients not attending, telephonic follow-ups were performed instead. In case of thromboembolic complications, a meticulous medical chart review was performed to determine the type of event, current medication and outcome. Two physicians independently adjudicated the events.
Bleeding events were categorised according to the Bleeding Academic Research Consortium (BARC) classification10.
ENDPOINTS
The primary safety endpoint was the occurrence of major bleeding defined as BARC >2. The primary efficacy endpoint was the incidence of any stroke or systemic embolism. The secondary efficacy endpoint was the incidence of DRT.
According to the aetiology, events were classified into i) procedure-related events (bleeding due to vascular access and pericardial effusion; stroke/systemic embolism [SE] or death ≤7 days within implant), ii) non-procedure-related events occurring in the DAPT phase, and iii) non-procedure-related events after stopping DAPT until last follow-up (FU).
STATISTICAL ANALYSIS
Mean±standard deviation was used to describe continuous variables with normal distribution. Median and interquartile range were used when appropriate. The Student’s t-test was performed to calculate differences between groups of variables with normal distribution. The chi-squared test or the Fisher’s exact test was used to perform between-group comparisons. For time-to-event data, Kaplan-Meier curves and log-rank test were computed using custom software, SPSS for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA).
Univariate and multivariate analyses were computed to identify predictors of major bleeding. Renal impairment was defined as serum-creatinine of ≥1.5 mg/dl11.
Results
PATIENTS
Between July 2010 and December 2015 a total of 298 patients successfully received an LAAC. The demographic data are given in Table 1. Patients had a mean age of 76±8 years and 62% were male. In 61% of patients prior bleeding had occurred, and the risk both for stroke as well as for bleeding was high as expressed by the calculated risk scores: CHA2DS2-VASc 4.3±1.5; HAS-BLED 3.5±1.0. During the course of the study, five different device types were implanted. The distribution was as follows: WATCHMAN and WATCHMAN FLX in 140/298 (47%) patients; AMPLATZER Cardiac Plug and Amulet in 126/298 (42%) patients; WaveCrest in 32/298 (11%) patients.
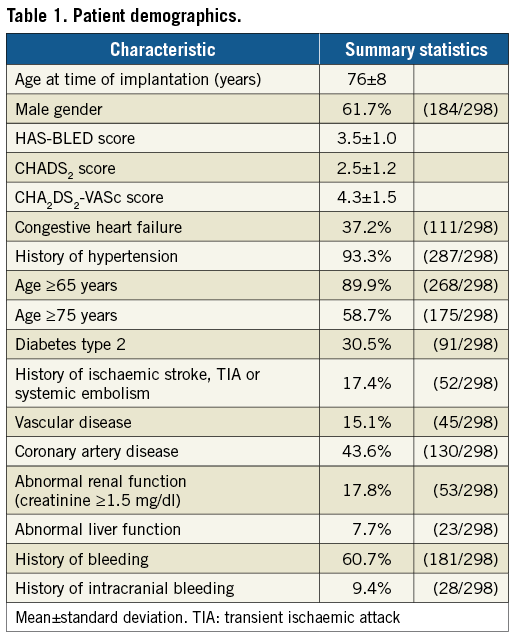
FOLLOW-UP
The first FU visit was attended by 267/298 (89.6%) patients 45±10 days after the implant. Long-term FU was completed by 288/298 (96.6%) patients. The mean FU was 820±547 days after LAAC (644.7 patient-years; median 731 days) (Table 2).
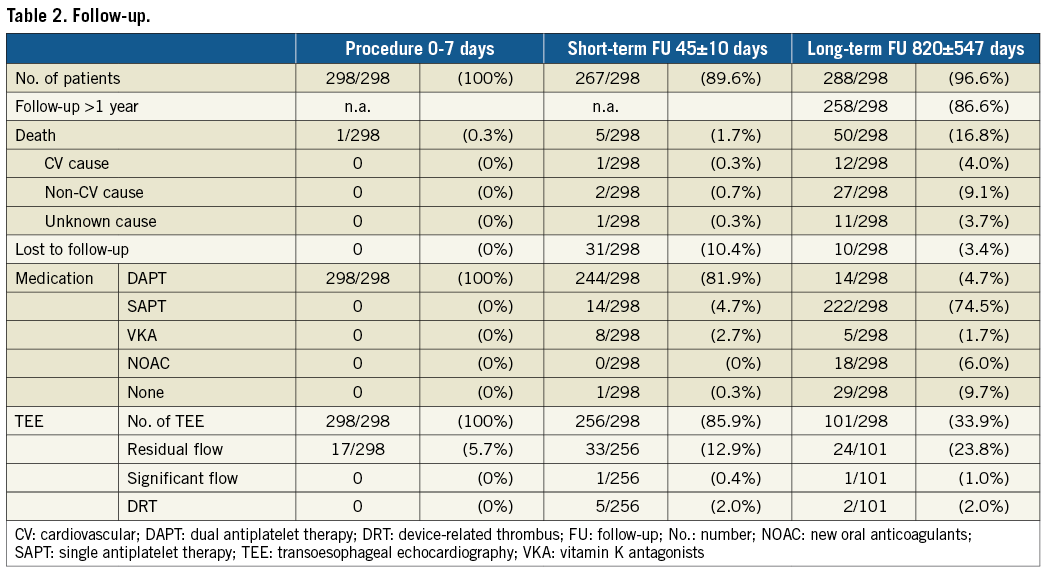
PROCEDURE-RELATED EVENTS
In all patients LAAC was successfully performed and patients were discharged on DAPT. Residual peri-device flow ≤5 mm was detected in 17 (5.7%) patients, whereas significant peri-device flow >5 mm was not seen.
Acute (n=3) or delayed (n=7) pericardial tamponade requiring subxyphoidal drainage occurred in 10/298 (3.4%) patients. Per device distribution was as follows: WATCHMAN and WATCHMAN FLX 3/140 (2.1%) patients, AMPLATZER Cardiac Plug and Amulet 4/126 (3.2%) patients and WaveCrest 3/32 (9.4%) patients (Supplementary Table 1). All patients were managed conservatively without surgery and recovered without sequelae.
Procedure-related major bleeding events occurred in 7/298 (2.3%) patients and were all related to vascular access (Figure 1). Two of 298 (0.7%) patients experienced an embolic stroke one day after the implant (Figure 2).
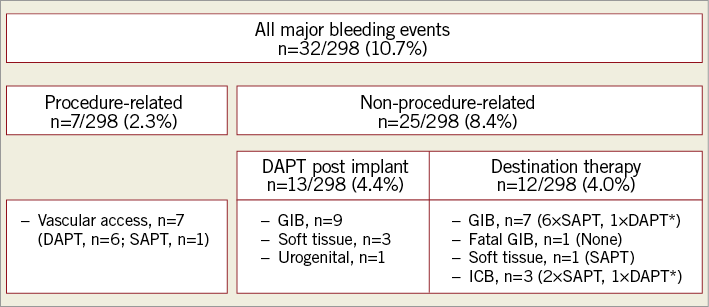
Figure 1. Summary of major bleeding events. *Transient DAPT due to medical intervention and individual decision, respectively. DAPT: dual antiplatelet therapy; GIB: gastrointestinal bleeding; ICB: intracranial bleeding; SAPT: single antiplatelet therapy
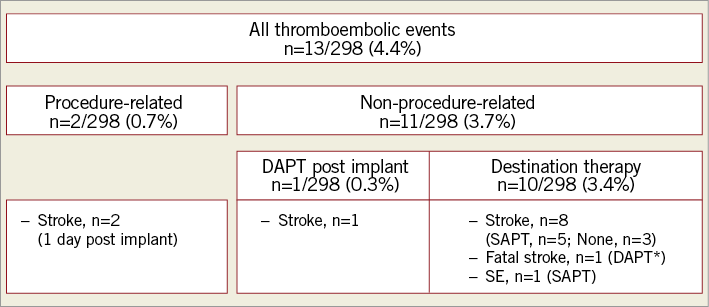
Figure 2. Summary of thromboembolic events. *Transient DAPT due to medical intervention. DAPT: dual antiplatelet therapy; SAPT: single antiplatelet therapy; SE: systemic embolism
At day 6 following the implant of a WATCHMAN device one patient died at home after an uneventful hospital course. The cause of death could not be elucidated.
NON-PROCEDURE-RELATED MAJOR BLEEDING EVENTS
Non-procedure-related major bleeding events occurred in 13/298 (4.4%) patients taking DAPT 11±16 days after LAAC. This included gastrointestinal bleeding (GIB, n=9), soft tissue bleeding (n=3) and urogenital bleeding (n=1).
After cessation of DAPT, 12/298 (4.0%) patients experienced bleeding events including GIB (n=8), soft tissue bleeding (n=1) and intracranial bleeding (n=3). While nine of these patients were on SAPT, one of the GIB was fatal and occurred in a patient without any antithrombotic medication. Two patients resumed DAPT due to medical intervention and individual decision, respectively. The chronology is illustrated in Figure 3.
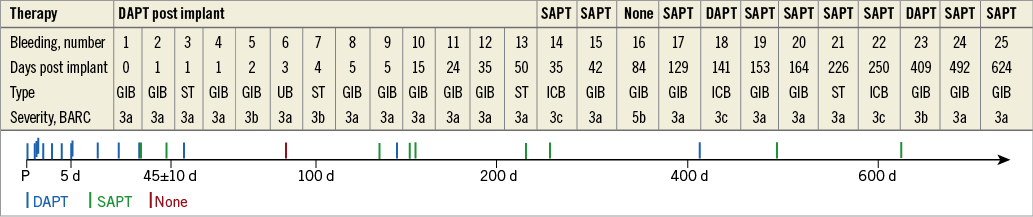
Figure 3. Timing of non-procedure-related major bleeding events. BARC: Bleeding Academic Research Consortium; d: days post procedure; DAPT: dual antiplatelet therapy; GIB: gastrointestinal bleeding; ICB: intracranial bleeding; P: procedure; SAPT: single antiplatelet therapy; ST: soft tissue; UB: urogenital bleeding
Indeed, 21/25 (84.0%) patients with non-procedure-related major bleeding events had a history of bleeding. In 17/21 (81.0%) patients with bleeding history, the same type of bleeding occurred. Of these, 14/17 (82.4%) patients with GIB had a prior history of GIB.
The annual major bleeding rate was 3.9%. Given the expected bleeding rate of 8.7%, this reflects a reduction of 55.2%7.
NON-PROCEDURE-RELATED THROMBOEMBOLIC EVENTS
In total, 11/298 (3.7%) patients experienced a non-procedure-related thromboembolic event (stroke, n=10; SE, n=1) during the course of the study (Figure 4). Given the total FU time of 644.7 patient-years, this resulted in an annual stroke/SE rate of 1.7%. Compared to the expected stroke/SE rate of 7.8%, this represented a risk reduction of 78.2%9.
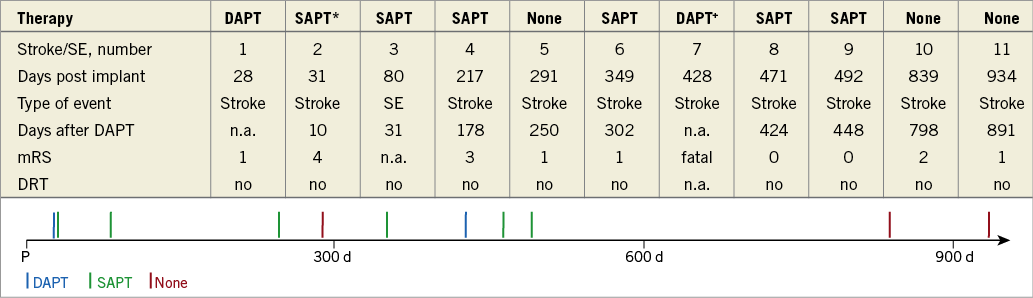
Figure 4. Timing of non-procedure-related thromboembolic events. *DAPT stopped 21 days after implant due to gastrointestinal bleeding. +Transient DAPT due to medical intervention. d: days post procedure; DAPT: dual antiplatelet therapy; DRT: device-related thrombus; mRS: modified Rankin Scale; n.a.: not applicable; P: procedure; SAPT: single antiplatelet therapy; SE: systemic embolism
During the first six weeks after LAAC, two patients experienced embolic stroke: one patient was on DAPT and one stroke occurred 31 days post implant under SAPT in a patient with shortened time of DAPT (21 days) due to recurrence of GIB. After the post-interventional DAPT period, nine patients experienced a stroke/SE under SAPT (n=5) or no therapy (n=3) or DAPT (n=1). One stroke was fatal and occurred despite DAPT due to previous medical intervention (peripheral artery disease).
The first stroke after the designated time of DAPT was noted 178 days after its cessation.
In 10/11 (91%) patients with thromboembolic events, DRT as well as significant peri-device flow were excluded.
Figure 5 shows the Kaplan-Meier plots of all major bleeding and thromboembolic events.
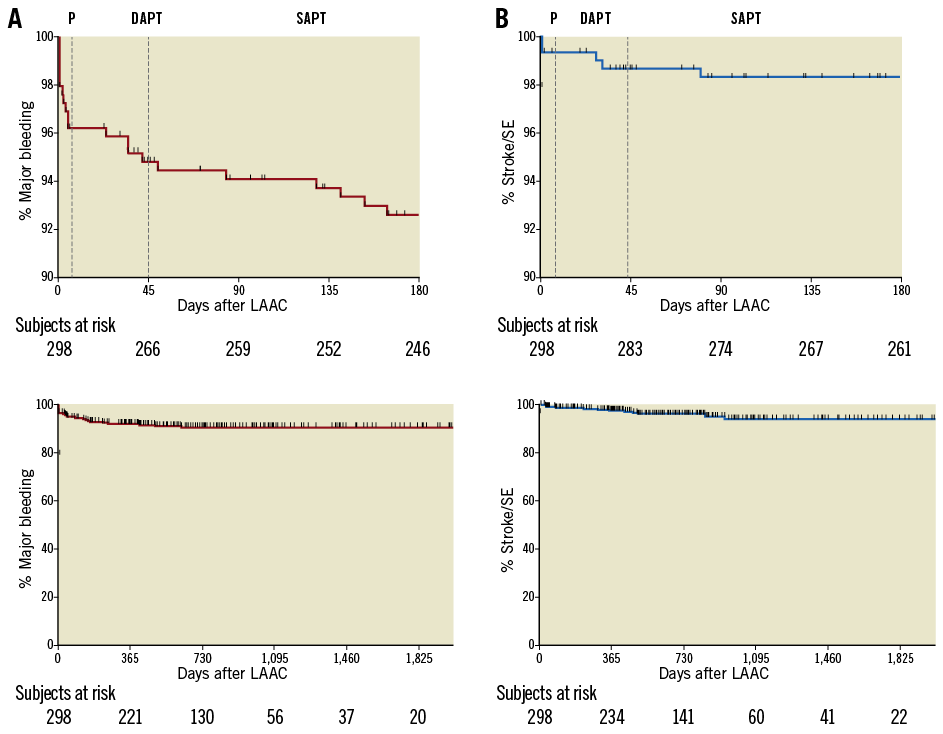
Figure 5. Kaplan-Meier plots of major bleeding and thromboembolic events. A) Major bleeding events. B) Thromboembolic events (stroke/SE). The upper graphs depict the transition from DAPT to destination therapy after TEE at six weeks (dotted lines), while the lower graphs illustrate the entire follow-up. DAPT: dual antiplatelet therapy; LAAC: left atrial appendage closure; P: procedure; SAPT: single antiplatelet therapy; SE: systemic embolism
DEVICE-RELATED THROMBUS
At first FU 45±10 days after LAAC, TEE was performed in 256/298 (85.9%) patients. In 5/256 (2.0%) patients on DAPT, DRT was detected (WATCHMAN and WATCHMAN FLX, n=3; AMPLATZER Cardiac Plug and Amulet, n=2). Significant peri-device flow was seen in one patient (WATCHMAN).
During the course of the study, TEE was performed in 101/298 (33.9%) patients for various clinical indications at 341±271 days after LAAC. DRT was detected in 2/101 (2.0%) patients on SAPT. The significant peri-device flow in the above-mentioned patient was still present.
Throughout long-term FU, therapy regimens consisted of no antithrombotic medication (n=29/298; 9%), SAPT (n=222/298; 75%) and DAPT (n=14/298; 5%). Only 5/298 (2%) and 18/298 (6%) patients had resumed vitamin K antagonist (VKA) therapy and new oral anticoagulants (NOAC), respectively. The reasons for resuming long-term OAC were stroke (n=7), pulmonary embolism/deep vein thrombosis (n=5) and individual decision of the general practitioner (n=7). In two cases the reason could not be elucidated. Transient OAC resumption (n=2) was performed after left atrial ablation.
Altogether 50/298 (16.8%) patients had died from cardiovascular (n=12), non-cardiovascular (n=27) and unknown (n=11) causes. One patient died during the DAPT phase 31 days after implant for an unknown reason.
UNIVARIATE AND MULTIVARIATE ANALYSIS
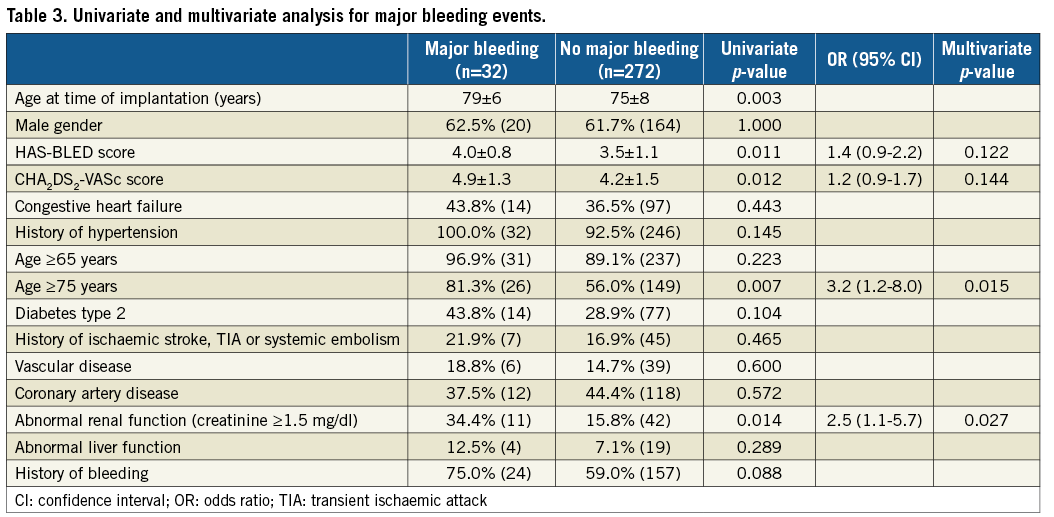
In order to determine risk factors for bleeding, univariate and multivariate analyses were computed (Table 3). Age >75 years (OR 3.2; CI: 1.2-8.0; p=0.015) and renal impairment (OR 2.5; CI: 1.1-5.7; p=0.027) were identified as independent predictors of recurrent bleeding after LAAC.
Discussion
LAAC has evolved to an established therapeutic option for stroke prophylaxis in patients with contraindications to OAC. Recently published real-life registries underscore the efficacy in stroke risk reduction as well as the procedural safety of this intervention12,13. However, uncertainty exists on the optimal post-implant antithrombotic strategy in this patient population with a high bleeding risk. An expert consensus statement provided recommendations for the two most commonly used devices (WATCHMAN and AMPLATZER Cardiac Plug)14. Depending on device type and individual bleeding risk, different antithrombotic regimens including VKA or DAPT were recommended. However, the consensus document remains relatively vague with respect to DAPT treatment duration, ranging from one to six months. The absence of a clear-cut standard is reflected by the results of the aforementioned real-life registries (EWOLUTION and AMULET) that showed a wide variety of antithrombotic regimens being used in everyday clinical practice with a clear trend towards DAPT. Alternatively, individualised strategies were applied using SAPT, VKA, NOAC or even no therapy at all without any significant differences between groups with regard to DRT or clinical events15. This has ultimately led to a change in labelling for the WATCHMAN device.
The optimal duration of DAPT, however, remains unclear. Intermediate-term DAPT for six months after LAAC proved ample efficacy in preventing DRT and thromboembolic events in the ASAP study but was also found to be associated with a considerable number of bleeding events5. Whilst others have addressed this dilemma by de-escalating DAPT to SAPT using aspirin, the present concept shortened DAPT duration to six weeks16. As expected, the incidence of bleeding was strongly correlated to the intensity of antithrombotic therapy. While 4.4% of patients using DAPT experienced a major bleeding, fewer events were noted after DAPT cessation during extended FU.
Most importantly, early DAPT cessation did not lead to a higher incidence of thromboembolic events. In fact, the first stroke was noted 178 days after DAPT cessation. The observed annual stroke/SE rate of 1.7% is similar to previous reports such as the PREVAIL study (2.7%)4.
The present data also show that DAPT was effective in preventing DRT on different occluder devices. The 1.7% DRT rate is well in line with contemporary reports in similar patient populations (EWOLUTION 3.7%, AMULET registry 1.5%)12,13.
Although neither the present nor the aforementioned study with a SAPT strategy was designed to prove improved safety and efficacy compared to longer-term DAPT treatment, both may add valuable information in the setting of high-risk patients after LAAC. As an alternative to DAPT, the use of low-dose NOAC appears to be a viable management strategy after LAAC according to recently published data17.
Data from contemporary real-life registries demonstrated that a minority of patients had been treated without any antithrombotic therapy after LAAC without a higher incidence of device thrombosis. Although this may represent a tempting strategy for very high-risk patients, the patient number was too low to justify general recommendations.
Recently, it appeared that bleeding is the most prevalent adverse event after LAAC, occurring at a rate of 4.1%15. Since European guidelines recommend interventional LAAC exclusively for patients with contraindications to long-term OAC, i.e., patients at high risk for bleeding, medical strategies to reduce that risk are warranted1. Among this patient population, this study identified elderly people >75 years and patients with impaired renal function as being at a threefold risk for bleeding events using DAPT after LAAC.
The latter is well in line with previous reports from the retrospective ACP registry, showing that patients with chronic kidney disease had a higher bleeding event rate with more severe renal impairment and even a higher mortality in those with a glomerular filtration rate of <30 ml/min18. On the other hand, the analysis on the association of age with adverse outcome in the ACP registry did not identify patients >75 years as being at a higher risk. However, the post-implant antithrombotic strategy was not standardised and individualised concepts for the elderly might have confounded the data19.
It remains to be determined if this patient population should be treated with less aggressive strategies such as SAPT, as recently suggested for patients at high risk for intracranial haemorrhage, or even no therapy16.
Serious periprocedural complications during LAAC implantations have been substantially reduced to 2.7% during the first seven days after the implant12. In the subacute phase after LAAC, prevention of thrombus on the incompletely endothelialised device and subsequent thromboembolism is of the utmost importance. Treatment strategies include antiplatelet therapy as well as anticoagulation and a combination of these.
Limitations
Several factors need to be taken into consideration when interpreting the present results. First, the study is purely observational and lacks a control group. Future randomised studies may be needed to determine the value of short-term DAPT in comparison to other strategies. Second, the sample size of this single-centre study is rather small and included different device types. The follow-up at 45 days was attended by 89.6% of patients and 85.9% underwent TEE evaluation. Therefore, the total DRT rate may be underestimated, but the protocol compliance rate resembles that of the EWOLUTION registry study (94%)15.
However, the results are well in line with the latest real-life clinical registry studies where DAPT was the predominant strategy after LAAC12,13.
Conclusions
Short-term DAPT for six weeks appears to be a viable alternative for patients after LAAC. Age >75 years and renal impairment increase major bleeding events threefold; therefore, less aggressive antithrombotic strategies in these patient populations deserve investigation.
| Impact on daily practice Bleeding is the most prevalent adverse event after LAAC, and the incidence correlates with the intensity of the antithrombotic regimen. Short-term DAPT for six weeks has the potential to decrease bleeding events while the earlier cessation of DAPT did not lead to a higher rate of thromboembolic events. Age >75 years and renal impairment could be identified as independent risk factors for major bleeding. It remains to be determined if these patient populations should be treated with less aggressive strategies. |
Conflict of interest statement
B. Schmidt reports receiving personal fees from Abbott and Boston Scientific outside the submitted work and is a member of the advisory board of Abbott and Boston Scientific. K.R.J. Chun reports receiving personal fees from Abbott and Boston Scientific outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Per device distribution: pericardial effusion and TEE results.
To read the full content of this article, please download the PDF.

