Abstract
Aims: The European PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) study was performed to determine the safety and efficacy of left atrial appendage occlusion by catheter technique. Embolic stroke due to atrial fibrillation is a common observation, especially in the elderly. Most thrombi in atrial fibrillation form in the left atrial appendage (LAA), its occlusion may therefore reduce the incidence of stroke in these patients.
Methods and results: One hundred and eighty patients with non-rheumatic atrial fibrillation and contraindication to warfarin therapy were enrolled in the PLAATO study. Patients were eligible if they had a history of transient ischaemic attack (TIA) or stroke or at least two independent risk factors for stroke such as age ≥75 years, hypertension, congestive heart failure or diabetes. The primary endpoint was LAA closure as determined by transesophageal echocardiography (TEE) two months after the procedure and stroke rate at 150 patient years. Left atrial appendage occlusion was successful in 162/180 patients (90%, 95% CI 83.1% to 92.9%). Two patients died within 24 hours of the procedure (1.1%, 95% CI 0.3% to 4%). Six cardiac tamponades were observed (3.3%, 95% CI 1.5% to 7.1%). In two cases, surgical drainage of the tamponade was necessary (1.1%, 95% CI 0.3% to 4%). In one patient, the device that was chosen was too small and embolised into the aorta after its release (0.6%, 95% CI 0.1% to 3.1%). It was snared and replaced without further complications. Successful occlusion of the LAA was achieved in 126/ 140 (90%, 95% CI 83.5% to 94.2%) of patients as noted by TEE at the two months follow-up. In a follow-up time of 129 documented patient years, three strokes occurred (2.3% per year). The expected incidence of stroke according to the CHADS2-Score was 6.6% per year. The trial was halted prematurely during the follow-up phase for financial considerations.
Conclusions: Left atrial appendage closure is relatively safe and effective. However, severe complications can occur. It might become an alternative for atrial fibrillation patients who are ineligible for long-term anticoagulation therapy.
Abbreviations
AF: atrial fibrillation
PLAATO: percutaneous left atrial appendage transcatheter occlusion
LAA: left atrial appendage
AE: adverse events
MAE: major adverse events
TEE: transesophageal echocardiography
TIA: transient ischaemic attack
CI: confidence interval
Introduction
Atrial fibrillation (AF) is a frequent cardiac arrhythmia with an increasing prevalence in the elderly. As an independent risk factor for stroke, it accounts for as much as one fifth of all strokes1. These strokes tend to be more severe than embolic events from non-cardiac sources, probably due to the larger size of thrombi in AF2,3. Although highly effective in preventing stroke, oral anticoagulation continues to be widely underused even in AF patients who have concomitant diseases that put them at higher risk for stroke4,5.
It has been shown that more than 90% of thrombi in patients with AF form in the left atrial appendage (LAA)6. Therefore, LAA removal or occlusion seems a logical approach in patients who do not tolerate oral anticoagulation therapy. Surgical resection of the LAA was first performed in 1948. Nowadays, it is performed during the Maze-operation and routinely during mitral valve surgery, as recommended in the American College of Cardiology and the American Heart Association guidelines7. Percutaneous closure of the LAA has been performed since 2001 with the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) device (ev3 Inc., Plymouth, MN, USA) and has been shown to be feasible and safe in an initial multicentre study with 111 patients8. In 2003, the European PLAATO study was launched with the aim to enrol a larger number of patients and thus to learn more about safety and effectiveness of LAA device closure.
Methods
The European PLAATO study was designed as a prospective, multi-centre, non-randomised study. One hundred and eighty patients were enrolled in 18 European centres from March 2003 until April 2005.
Primary and secondary endpoints
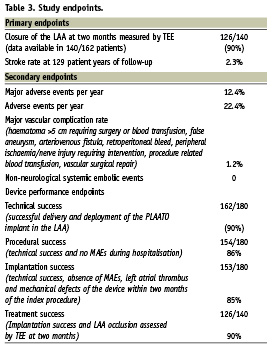
The primary endpoints of the European PLAATO study were successful closure of the LAA as determined by TEE at the two months follow-up and stroke rate at 150 patient years. Left atrial appendage occlusion was defined as successful in case of a trace (< 1 mm in diameter in colour Doppler flow) or mild leak (1-3 mm) and as unsuccessful in case of a moderate (> 3 mm) or severe leak (multiple jets or free flow).
Secondary endpoints were the occurrence of major adverse events (MAE) and adverse events (AE) during hospitalisation and as noted through one, two, six, twelve, 18 and 24 months after the index procedure. Major adverse events were defined as cardiac or neurological death, stroke, myocardial infarction, cardiac tamponade or cardiovascular surgery related to the PLAATO procedure. Other secondary endpoints were major vascular complication rate and incidence of non-neurological systemic embolic events.
Device performance endpoints comprehended technical success, defined as successful delivery and deployment of the PLAATO device into the LAA; procedural success, defined as technical success and no MAEs during hospitalisation; implantation success, defined as technical success, absence of MAEs, thrombi and defects of the implant within two months of the index procedure; and treatment success, defined as implantation success and LAA occlusion confirmed by TEE after two months.
Patient population
To be eligible for the PLAATO study, patients had to have a diagnosis of continuous or paroxysmal, non-rheumatic AF of at least three months duration and have a contraindication to warfarin therapy. Contraindications were defined as patient refusal to take warfarin, contraindication to warfarin (according to product label), condition in which the hazard of haemorrhage might be greater than the potential benefits of warfarin, inability to maintain a stable INR and embolism despite warfarin therapy.
Additionally, only patients with at least two points according to the CHADS2-Score9 were enrolled, defined as a history of stroke or transient ischaemic attack (TIA) or at least two of the following: congestive heart failure, arterial hypertension, age ≥ 75 years or diabetes mellitus. The distribution of the CHADS2-score is shown in Figure 1.
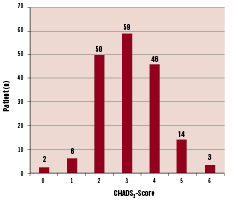
Figure 1. CHADS2-Score distribution in 180 patients. Mean CHADS2 score was 3.1±0.8, corresponding to a mean expected incidence of stroke of 6.6±0.8% per year.
Key criteria for patient exclusion were thrombus formation in the left atrium or LAA as well as complex aortic plaque, mitral or aortic stenosis or regurgitation, left atrial diameter > 6.5 cm, acute myocardial infarction or unstable angina, and recent stroke (within two months prior to the index procedure) as well as symptomatic carotid disease. The PLAATO device needs to be oversized by 20-50% to achieve a stable position within the LAA. Because 32 mm is the largest device available, the LAA orifice diameter must not exceed 26.6 mm. Baseline patient demographics are summarised in Table 1.
Procedure and follow-up
Prior to their first PLAATO device implantation, investigators had to participate in a training session to familiarise themselves with the device and the implantation technique.
Prior to the procedure, patients had to undergo multiplane TEE to exclude thrombi in the left atrium (LA) and LAA and written informed consent was obtained in all subjects. Forty-eight hours prior to the intervention, patients were placed on 300-325 mg aspirin daily and received a loading dose of 300 mg clopidogrel. Preprocedural administration of endocarditis prophylaxis was recommended.
The implantation technique of the PLAATO device has been described before10. The key steps are shown in Figure 2.
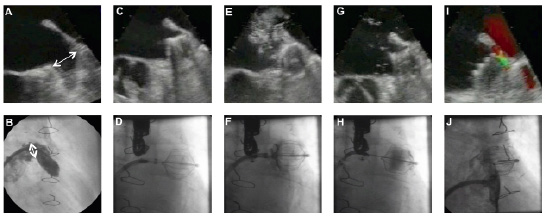
Figure 2. Device implantation and measurements. A,B: assessment of LAA morphology. Measuring LAA orifice diameter in TEE (A) and angiography (B) to chose appropriate device size. Excluding possible thrombi in the LAA. C,D: positioning and expansion of the device. Performing proximal (E,F) and distal (G,H) dye injections to assess the seal quality of the occluder. After a successful device stability test, the PLAATO occluder is released from its delivery catheter. To assess the final leak status, colour Doppler (I) is used and a final dye injection (J) is performed.
The postprocedural medical regime included aspirin 300-325 mg indefinitely, whereas administration of clopidogrel 75 mg daily for six months and endocarditis prophylaxis were at the discretion of the investigators.
Follow-up tests were planned after one, two, six, twelve, 18 and 24 months of the index procedure including NIH stroke scale to detect neurological changes that might be attributable to embolic events. A TEE was scheduled in all patients at the two months follow-up. Pre- and postprocedural echocardiographic examinations were taped and then reviewed by an independent echo core laboratory.
The estimated risk reduction achieved by LAA occlusion was calculated according to the CHADS2-Score published by Gage and colleagues9.
Statistics
Analyses were conducted on an intention to treat basis. Continuous variables were summarised by mean, standard deviation, and minimum and maximum values. The frequencies of occurrence of events are stated as percentages with 95% confidence intervals (CIs).
Ethics
Written informed consent was obtained from all patients. The procedures were performed in accordance with the ethical standards of the responsible ethical committees.
Results
Of the 180 candidates enrolled in the European study, device implantation was successful in 162 patients (90%). The reasons why device implantation was not successful were the challenging anatomy of the LAA (eight patients), too large a LAA orifice diameter (six patients), access to the LAA not possible (two patients), complications during transseptal puncture (one patient) and failure of device stability test prior to its release from the delivery catheter (one patient).
As per fluoroscopy, LAA orifice diameters ranged from 12-36 mm with a mean diameter of 21±4 mm. The PLAATO occluders used ranged from 20 to 36 mm in diameter; the median size was 29 mm. In detail, four devices of 20 mm, nine of 23 mm, 35 of 26 mm, 46 of 29 mm, and 67 of 32 mm size were used. Additionally, one patient received a novel 36 mm device. In 35 patients (19.4%), the initially chosen device had to be exchanged during the procedure: in 10 patients, an occluder of the same size was implanted, in 17 patients a larger occluder and in eight patients a smaller occluder, were implanted. Mean procedure time was 65± 31 minutes.
Patients of the European PLAATO study were followed for 129 documented patient years and mean follow-up time was 9.6±6.9 months. All but one patient finished their two month follow-up, 124 finished their six month follow-up and 70 patients finished their one year follow-up.
Primary endpoints
As per the 140 TEE examinations available two months after the procedure, successful LAA occlusion with only a trace or mild leak was achieved in 126 patients (90%, 95% CI 83.5% to 94.2%).
Within 129 patient implant years, three strokes occurred (2.3%, 95% CI 0.6% to 7.2%). A 75-year-old patient with congestive heart failure and severely reduced left ventricular ejection fraction suffered from a major ischaemic stroke 15 months after implantation of the PLAATO device and died due to secondary complications. Transesophageal echocardiography performed during the index procedure and at the two month follow-up had shown that the device was well seated with only a trace leak. A 57-year-old female patient sustained a minor stroke one month after implantation of the PLAATO device. An MRI and CT-scan were performed, detecting an infarction in the right middle cerebral artery area. A third stroke occurred three months after the PLAATO procedure: a 72-year-old patient with a history of three embolic strokes prior to device embolisation was admitted to the hospital for recurrent cerebral ischaemia with coordination, motor and speech deficits. Two days after hospitalisation, he was found unconscious and pulseless. Cardiopulmonary resuscitation was unsuccessful, and sudden death due to arrhythmia was presumed.
No systemic embolic events were noted during follow-up.
Secondary endpoints
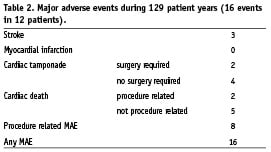
In a follow-up time of 129 patient years, there were 16 major adverse events (12.4%, 95% CI 7.5% to 19.6%) in twelve patients (Table 2). Eight major adverse events (6.2%) were procedure related. Two patients died within 24 hours after the implantation procedure. An 82-year-old male patient with severe coronary artery disease received a PLAATO device without complications under general anaesthesia. Thirty minutes after implantation, the patient showed hypotension and decreasing oxygen saturations, requiring cardiopulmonary resuscitation and administration of catecholamines. Cardiac catheterisation showed a severe three vessel coronary artery disease without evidence for an acute myocardial infarction. TEE demonstrated a normal PLAATO device position and no cardiac tamponade. The patient died on the following day. The cause of death was thought to be secondary to exacerbation of congestive heart failure aggravated by the administration of anaesthesia. The request for autopsy was rejected by the patient’s family.
In a 74-year-old male patient, LAA perforation with subsequent tamponade occurred during the procedure and the patient was transferred for emergent cardiac surgery. The tamponade was successfully drained and the LAA was ligated. However, the PLAATO device had embolised during resuscitation and was not removed for unknown reasons. It was found in the aortic arch in a x-ray control after surgery. The device was snared, however, during removal, perforation of the iliac artery occurred and the patient died due to haemorrhagic shock.
Including the event mentioned above, there were six cardiac tamponades (3.3%, 95% CI 1.5% to 7.1%). In two cases, surgical drain of the tamponade was necessary. Four patients were treated with pericardiocentesis in the catheterisation laboratory without sequelae. Pericardial effusion was noted in five patients (2.8%, 95% CI 1% to 6.7%) during the procedure, no treatment was necessary. During follow-up, there were no further pericardial effusions. Seventeen (9.4%, 95% CI 5.8% to 14.9%) minor bleeding complications occurred that were not device or procedure related patients.
Thrombus formation on the delivery sheath occurred in four patients (2.2%, 95% CI 0.7% to 6%) and was resolved without sequelae in all cases. In one patient (0.6%), a thrombus was found on the occluder one month after the procedure. It resolved without sequelae after administration of low-molecular weight heparin for two months.
In one patient (0.6%), the device was too small and embolised into the aorta after its release. It was snared and retrieved by catheter technique. A larger device was successfully implanted in the same procedure.
Device implantation was successful in 162/180 patients (90%, 95% CI 83.1% to 92.9%). Results of primary and secondary endpoints, including device performance endpoints, are summarised in Table 3.
Discussion
Atrial fibrillation is the most common cardiac arrhythmia. It occurs in 2-4% of the population over 60 years and its prevalence continues to increase with age1. Atrial fibrillation is responsible for 20% of all strokes1, in those patients older than 80 years even up to one third11. Stroke is a leading cause for disability and has been shown to generate long-term costs of as high as $200,000 per patient12.
Nowadays, catheter ablation of foci triggering AF can be performed safely in an outpatient procedure. However, many patients still require oral anticoagulation thereafter because of high recurrence rates of up to one-third within the first month13 and 16-22% within the first three years14.
Aspirin alone was found to reduce the incidence of stroke by one-fifth, with no significant increase in the risk of major haemorrhages15. Compared to aspirin, oral anticoagulation is known to decrease the risk for all cause stroke and cardiovascular events significantly, whereas there is an increase in the incidence of major bleeding events of up to 2.2%16,17. Despite the knowledge of the effectiveness of warfarin, it continues to be widely underused for different concerns. Besides possible drug interactions and the need for frequent dose adjustments, the risk for major haemorrhage is considered more important than the potential benefits of oral anticoagulation therapy in most patients. Therefore, only 55% of high-risk AF patients receive warfarin4,5.
When attempting to occlude the LAA for stroke prevention, a profound knowledge of its anatomy is necessary. It has paper thin walls which can be perforated when accessing the LAA, as observed in one of the first patients to receive a PLAATO device18. In 80% of the population, it consists of two or more lobes19. Visualising the LAA in multiple planes is therefore important to avoid occlusion of one lobe only when multiple lobes are present or if they have no common orifice. It can be found in close proximity to the left upper pulmonary vein, the mitral valve, the left anterior descending artery and the circumflex artery20. To our knowledge, no impairment of these surrounding structures has been observed due to the implantation of a PLAATO device so far.
Transcatheter occlusion of the LAA was successfully performed in 90% of patients. Device implantation was not performed when one lobe of a multi-lobulated LAA with a common ostium could have been occluded only and when the LAA orifice diameter was too large. The wide anatomical variability of the LAA makes device selection crucial. When the device was selected too large, blood flow into the LAA became possible because the expanded polytetraflouroethylene (ePTFE) layer between the device struts was not stretched sufficiently to seal off the LAA. Yet, a moderate or severe residual leak around the device was only found in 10% of the patients who completed the two months follow-up TEE. Although the device is equipped with hooks to avoid its embolisation, the device compression had to be at least 10% to guarantee a stable position within the LAA. However, the observed number of necessary device exchanges for another size (13.8%) reflects certain device limitations: PLAATO is a rather rigid, self-expanding occluder that needs to be oversized by 20-50% compared to the orifice diameter of the LAA to guarantee a stable position after implantation. Another LAA occlusion system, the WATCHMAN® device (Atritech, Inc., Plymouth, MN, USA), however, requires over sizing of 8-20% only and may fit oval shaped LAAs better due to its softer configuration. Also, the flatter shape of the WATCHMAN device may allow the occlusion of a common orifice of a multi-lobulated LAA, whereas the longer PLAATO device requires deeper implantation for safe anchoring.
One aim of LAA occlusion in suboptimal anticoagulation candidates is prevention of severe bleeding complications. Whereas oral anticoagulation accounts for approximately 2.2% of severe bleedings per year, implantation of the PLAATO device caused cardiac tamponade in 3.3% of the study patients during the procedure, but no more bleeding complications in the later follow-up. The occurrence of procedural bleedings might be part of a learning curve, as none of these complications occurred in the three centres that performed LAA occlusion in prior studies. This trend was also observed in the multicentre PLAATO feasibility study published in 20058: three of four bleeding complications (2.7%) occurred in the early experience of the participating centres. The same rate of interventional bleeding events requiring treatment occurred in the WATCHMAN feasibility study21. However, in all studies, most procedural bleeding complications did not require intervention and might be considered less important once long term follow-up data will be available.
The revised CHADS2 Score by Gage and colleagues is usually calculated to evaluate a patient’s indication for oral anticoagulation. Whereas the need for oral anticoagulation may be discussed controversially in patients with CHADS2-Scores less than two, those patients with several additional risk factors for stroke besides AF should receive a warfarin/ coumadine therapy if there are no absolute contraindications to these agents.
We found that the PLAATO study patients matched the CHADS2 population in mean age (70 vs. 72 years), number of subjects aged ≥ 75 years (39% in both), and number of female patients (34% vs. 37%). In both studies, aspirin was prescribed on a continuing basis in all patients.
Given a stroke risk reduction of 20% by aspirin alone, we estimated the additional stroke risk reduction achieved by LAA occlusion according to the CHADS2-Score. PLAATO patients were at a high risk for stroke with a mean CHADS2 score of 3.1±0.8, equating to a mean expected incidence of stroke of 6.6±0.8% per year in the study population. The actual risk for embolic events can be estimated even higher, as echocardiographic risk factors such as dense spontaneous echocardiographic contrast or blood flow velocity ≤ 20 cm/sec within the LAA were observed in some patients. The risk for stroke in these patients has been described as high as 13-18% per year22. However, these echocardiographic features were not assessed in all patients systematically.
Given three strokes in a mean follow-up time of 129 documented patient years (2.3% annual stroke rate), the stroke risk reduction achieved by LAA occlusion and aspirin would be 65% in the current European PLAATO study. Compared to the multicentre PLAATO feasibility study8, the estimated stroke risk reduction was the same (mean CHADS2 score 2.5); in the initial experience with the WATCHMAN LAA system, no strokes at all were observed (mean CHADS2 score 1.8)21.
These estimations based on the CHADS2 score were confirmed in the recently published PROTECT AF study (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation)23. Seven hundred and seven patients were randomly assigned to either left atrial appendage occlusion followed by warfarin therapy for 45 days or continuous anticoagulation treatment in a 2:1 ratio. Procedural bleeding rates (4.8%) were similar to those in the present PLAATO study (3.3%) and bleeding events declined with investigator experience. In a combined endpoint of all stroke, death and peripheral embolism, non-inferiority of LAA occlusion to chronic warfarin therapy could be demonstrated. Based on the CHADS2 score, stroke risk was reduced by 57% in the intervention arm of the PROTECT AF study.
Study limitations
This trial was stopped earlier than planned for financial considerations in December 2006. Therefore, follow-up data is limited to 129 patient years in the present study and results of the two months TEE are available in 140/162 patients only. The mean follow-up period of 9.6 months in the present study is not sufficient to estimate the long-term benefits of LAA occlusion regarding stroke risk reduction. However, Block and colleagues demonstrated promising long-term outcomes of 64 patients from the initial PLAATO feasibility study with a mean follow-up time of 3.75 years. While the estimated annual stroke rate according to the CHADS2 score in these patients was 6.6%, the actual stroke rate was only 3.8% (estimated risk reduction 42%)24.
Conclusion
This study has shown that LAA occlusion with the PLAATO device can be performed at an acceptable risk. However, complications may occur and they can be serious. The number of procedural bleedings shows that this procedure requires experience with transseptal puncture and knowledge of possible pitfalls of LAA anatomy. Almost all complications occurred during or early after the procedure. The results regarding stroke risk reduction are promising and were recently confirmed in the PROTECT AF study. Transcatheter occlusion of the left atrial appendage occlusion might become an alternative for atrial fibrillation patients who are ineligible for long-term anticoagulation therapy.

