
Abstract
Left atrial appendage (LAA) closure for stroke prevention in the setting of non-valvular atrial fibrillation is an alternative to oral anticoagulation in patients with increased bleeding risk. It allows similar reduction in thromboembolic events, in particular stroke, compared to warfarin. A common clinical dilemma is the management of patients with peri-device leak after LAA occlusion. This has been documented in both percutaneous as well as surgical approaches. The specific definitions of leak severity, and the longer-term clinical implications are poorly understood. Here we review the mechanisms of incomplete occlusion for the different percutaneous closure devices, the data regarding thromboembolic risk in patients with incomplete appendage closure for both percutaneous and surgical strategies, and provide recommendations for management in these patients.
Introduction
Left atrial appendage (LAA) occlusion has been shown to reduce the risk of thromboembolic stroke in patients with atrial fibrillation (AF)1-3. Although oral anticoagulation agents are typically the first-line management for stroke prevention4, 30-40% of AF patients who meet criteria for warfarin are not anticoagulated due to the relative or absolute risks of bleeding1,5,6,. The majority of left atrial thrombus is thought to form in the LAA in patients with non-valvular AF7,8. LAA occlusion was first performed surgically with the intention of reducing stroke risk9. More recently, percutaneous devices have been evaluated and several are now commercially available10. Following LAA occlusion with the WATCHMAN® device (Boston Scientific, Marlborough, MA, USA), stroke rate was significantly reduced11, and individual trial data have demonstrated non-inferiority to warfarin3.
The LAA shape and size are very variable, and incomplete appendage closure or persistent leaks around the device are common following device placement. Methods of LAA closure vary from occlusion devices such as the WATCHMAN, the AMPLATZER™ Cardiac Plug (ACP) and the AMPLATZER™ Amulet® (St. Jude Medical, St. Paul, MN, USA), which occlude the ostium of the appendage, to external ligation devices such as the LARIAT® (SentreHEART, Redwood City, CA, USA), which exclude the LAA via a suture delivered epicardially. The Amulet is the second generation of the ACP device and has a larger volume in the device lobe, a longer waist, and more stabilising wires. Implantation is usually slightly deeper than with the ACP, also improving device stability. More recently, the WaveCrest device (Biosense Webster, Inc., Irvine, CA, USA) has been developed, which is suitable for short LAAs and can be deployed with minimal catheter manipulation in the LAA12.
Incomplete LAA occlusion may be identified at the time of the procedure or develop later due to remodelling of the LAA tissue around the device13. Whether these peri-device leaks, “perileaks”, are clinically significant is uncertain and there is no consensus at present as to how to manage these patients best. We review the evidence to date regarding the clinical significance and management of incomplete LAA closure.
POTENTIAL RISKS OF INCOMPLETE APPENDAGE CLOSURE
A fibrillating atrium has minimal contraction, leading to local stasis and predisposition to thrombus formation. The blind-ended LAA is particularly prone to thrombus, with low blood flow velocities14,15, and so incomplete LAA closure may increase the risk of thrombus formation and resultant systemic embolisation. For surgical LAA closure, rates of residual communication between the LAA and left atrium have been documented in 20-40% of cases at follow-up imaging16,17. Thrombus was commonly present in patients with a partially closed LAA16. This was associated with higher rates of stroke or systemic embolisation compared to patients with a completely occluded LAA17,18. Surgical ligation may also be followed by late reconnection of the LAA and the left atrium.
Experience to date suggests that incomplete LAA closure using percutaneous closure devices is not associated with the same elevated stroke risk as incomplete surgical closure. Although there were higher rates of ischaemic stroke post LAA occlusion with the WATCHMAN device compared to the warfarin group2, these were predominantly periprocedural events. Theoretically, the presence of perileak may result in turbulent blood flow adjacent to the device, enhancing platelet adhesion and thrombus formation on the device and/or within the LAA. However, small residual leaks are unlikely to have clinical significance as, even if thrombus were to form behind the LAA closure device, it would be difficult for it to embolise through a small perileak into the left atrium19. On the other hand, a large perileak with a larger diameter aperture may allow such thrombus to escape the LAA into the systemic circulation.
DEFINITIONS OF SUCCESSFUL LAA EXCLUSION
Incomplete closure following surgical LAA occlusion is common18,20, with only 40% complete LAA occlusion at follow-up transoesophageal echocardiography (TEE) in a subset of a surgical cohort following suture/staple exclusion or appendage excision18. LAA thrombus was present in almost half of the patients with unsuccessful LAA closure18. Patients with thrombus and incomplete LAA closure had higher rates of stroke or systemic embolisation, particularly if they were not anticoagulated17. Surgical LAA closure is not routinely recommended at the time of cardiothoracic surgery due to these concerns21 as well as the potential to lacerate the LAA during attempts at exclusion.
In contrast, rates of successful appendage closure with percutaneous devices appear higher, although the rates of thromboembolism or stroke appear similar in patients with small residual perileak compared to complete occlusion (Figure 1). However, data are again based on a limited number of events and limited follow-up time, and may have been confounded by management of some perileaks with oral anticoagulation or additional closure devices.
LAA occlusion studies have used varying definitions of procedural success. Most have used TEE to assess incomplete appendage closure. Criteria for successful closure have ranged from <1 mm residual flow into the LAA on Doppler colour flow22 to <5 mm23 (Table 1). The only two randomised trials of LAA occlusion using the WATCHMAN device both used <5 mm residual flow into the LAA as the criterion for successful closure3,24, while trials using the ACP and Amulet devices have reported leaks >5 mm as significant and 1-3 mm as moderate25,26. Recent consensus documents have suggested that the <5 mm definition should be used as part of the technical definition for procedural success27.
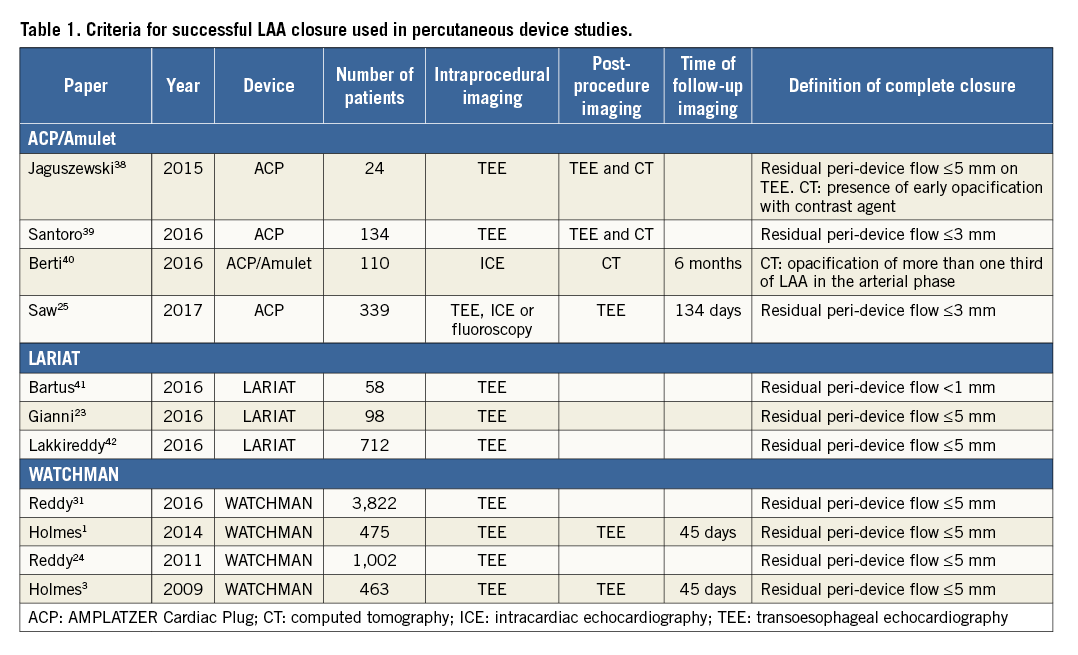
Small leaks may close spontaneously over time: patients with leaks <2-3 mm after LARIAT LAA exclusion showed complete closure on TEE at one year22. However, more recent registry data for the LARIAT investigators have also demonstrated the potential for new leaks during longer-term follow-up in patients who had appeared to have complete LAA closure at the time of index device therapy23. Non-randomised data have suggested that the LARIAT may be associated with lower rates of thrombus at six months after appendage occlusion compared to the WATCHMAN device28, although whether this relates to anatomic factors or patient selection is unclear.
OUTCOMES FOLLOWING INCOMPLETE LAA OCCLUSION
Five studies (three percutaneous closure devices, two surgical) have reported outcomes in patients with incomplete LAA closure (Figure 1). Perileak was present in 41% of patients in the PROTECT AF trial using the WATCHMAN device at 45 days, 34% at six months and 32% at 12 months19. Approximately one third of patients had a perileak >3 mm in diameter. There was no difference in clinical outcome in patients with a perileak compared to those with complete closure; however, clinicians were aware of the TEE results and many of the patients with a significant perileak remained on warfarin for an extended period of time.
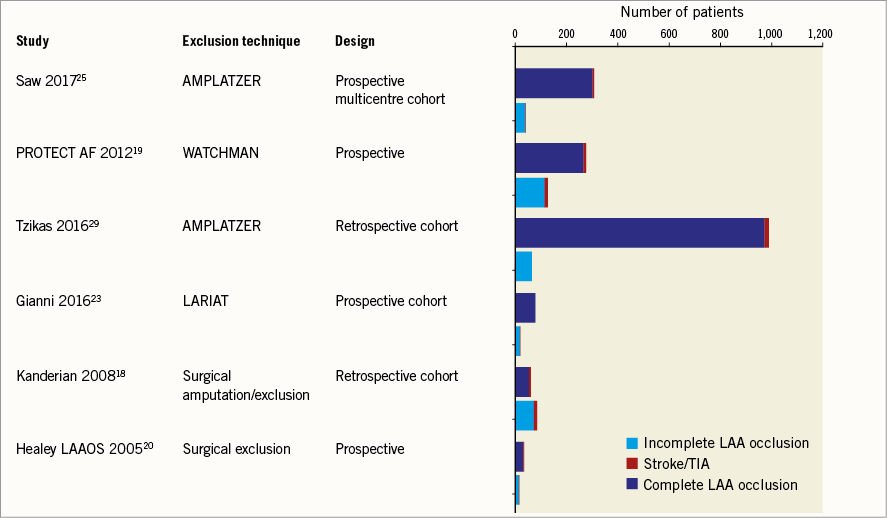
Figure 1. Studies of LAA occlusion reporting clinical outcomes in patients with and without complete occlusion.
European registry data following ACP insertion in 1,047 consecutive patients across 22 centres reported 73 (7%) patients with a perileak on follow-up (median time to TEE seven months)29. Leak was significant (>3 mm) in 2% and mild (1-3 mm) in 5%. Patients with a major leak were not restarted on warfarin, with the exception of one patient. There were no strokes/transient ischaemic attacks in the patients with incomplete closure at follow-up. In the core lab-adjudicated cohort of this multicentre series (n=339), the overall incidence of peri-device leak was 12.5% (5.5% minimal leak <1 mm, 5.8% mild leak 1-3 mm, 0.6% moderate leak 3-5 mm, and 0.6% severe leak >5 mm). Presence of any leak was not associated with higher clinical events compared to no leak. In fact there were no stroke, TIA or death events in patients with mild to severe leaks25. However, it is important to emphasise that total patient-years of follow-up and absolute number of events were small in all of these studies and further data are needed to determine whether perileaks are benign or associated with an increased risk of stroke.
MECHANISMS AND DETECTION OF PERILEAK FOLLOWING LAA OCCLUSION
The mechanism of leaks following LAA occlusion appears to vary between devices. For the WATCHMAN device, leaks are typically between the edge of the device and the wall of the LAA. With the LARIAT device, the leaks are described as “gunny sack”-like, with incomplete tightening of the suture resulting in a central leak where the residual LAA tissue begins to unfurl28. The ACP device consists of two lobes. When the lobes are well aligned, the left atrial lobe typically is placed to occlude the mouth of the appendage fully. However, when this lobe is off axis, a leak can result around one side of the lobe and disc into the LAA27 (Figure 2).
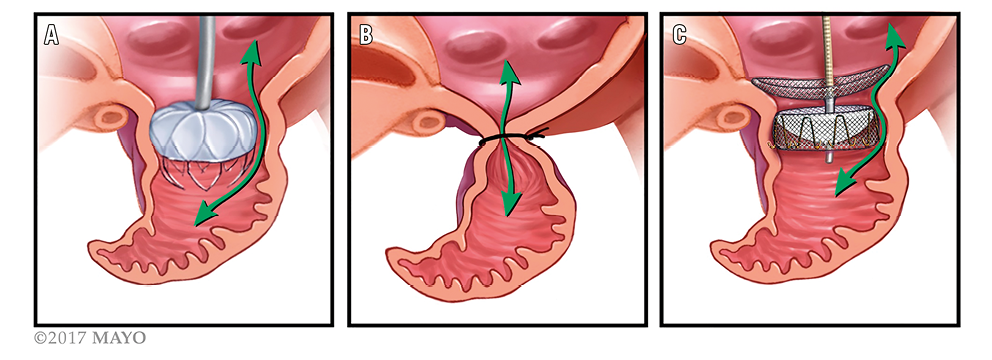
Figure 2. Likely mechanisms of perileak for different devices. The WATCHMAN (A) is a nitinol frame coated with polyethylene terephthalate which sits in the mouth of the LAA and is anchored inside the LAA by multiple barbs. Perileaks are typically between the side of the LAA and the device. The LARIAT suture device (B) snares and then ligates the LAA. Leaks are at the site of snare due to incomplete closure and unfurling of the residual LAA tissue. The AMPLATZER Amulet and AMPLATZER Cardiac Plug (C) are composed of self-expanding nitinol mesh; they sit at the mouth of the LAA and attach to an anchoring lobe with stabilising wires which fix into the body of the LAA. Leaks can occur when the lobe is off axis and occur around this edge of the LAA lobe.
TEE is considered the gold standard for imaging of the LAA and guiding LAA procedures10,12 (Figure 3). Although the majority of data on perileaks have been derived from TEE, cardiac CT may have improved sensitivity in detection of perileak post LAA closure26. No data are available for direct comparison; however, a series of 45 patients who had intraprocedural TEE with a perileak rate of 14% at the end of the procedure demonstrated residual leak on CT in over 60% of cases. Twenty-three of these patients had both follow-up CT and TEE; five had no evidence of perileak on TEE but a residual communication demonstrated on CT26. CT also allows evaluation of the mechanism of residual leak and may provide valuable data for patient-specific device selection as further devices are developed and evaluated. The majority of patients with ACP/Amulet with a perileak had off-axis lobes and a lower mean maximum lobe compression. Two patients had a fabric leak on CT, which subsequently occluded on repeat CT three months later. For the WATCHMAN device, leaks appeared to result from ostial gaps around the device26.
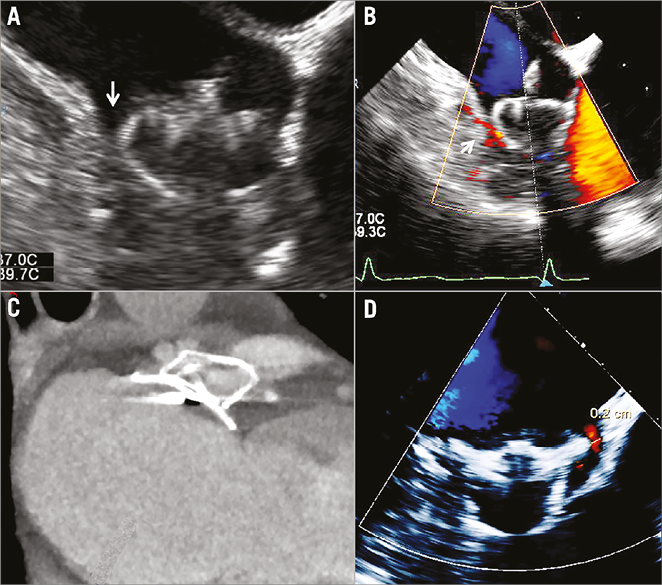
Figure 3. Periprocedural imaging of a 27 mm WATCHMAN using TEE (A) with Doppler colour flow (B). Although the device was adequately compressed, a perileak was evident on 2D TEE (arrowed). C) CT of an Amulet device in situ. D) An Amulet with a 0.2 mm residual perileak evident on TEE.
OPTIMISATION OF DEVICE SELECTION AND PROCEDURAL PLANNING TO MINIMISE RISK OF PERILEAK
Recent registry data have suggested high rates of procedural success, with lower rates of acute complications following the early learning curve compared to earlier studies29,30. A strategy of deliberate oversizing of the WATCHMAN device by 20% or more31, rather than the initially recommended 10-20%, may have contributed to the lower rates of perileak seen in recent registry studies30. The EWOLUTION prospective multicentre registry reported acute procedural success in 98.5% of patients treated with WATCHMAN. On follow-up TEE, only 1% of patients had a residual perileak of greater than 5 mm30. Similarly, for the Amulet and ACP devices, CT and TEE sizing pre-procedure are useful for sizing, with careful measurement of the maximum and mean LAA neck diameter27. Consecutive case series with the Amulet demonstrated high procedural success (98%) with rates of perileak reported between 032 and 7%33, and significantly lower prevalence of perileak compared to ACP33. For small LAA treated with the Amulet, expert opinion suggests slightly greater oversizing than the manufacturer recommendations to ensure a good seal27.
Preprocedural work-up with CT for device sizing rather than TEE-only measurements may be associated with larger device selection and lower rates of perileak27,34 and should be considered prior to LAA occlusion procedures. During the procedure, careful imaging immediately post device insertion should be performed to assess for presence of any perileak before final deployment. Use of multiple views, with a complete sweep of the LAA from 0 to 135 degrees on TEE is recommended for thorough assessment for the presence of any perileak27.
It has yet to be determined whether choice of device will alter rates of perileak. Future trials will perform direct comparison of commercially available devices, including randomisation of patients to either the Amulet or the WATCHMAN device, with a primary endpoint of procedure-related complications and stroke at 18 months, and a secondary endpoint of residual perileak at 45-day TEE35. However, with contemporary studies reporting low rates of significant perileak with many commercially available devices30 and improved sealing with second-generation devices such as the Amulet, it may be difficult to prove superiority of one device over another. Finally, second-/third-generation and new devices will take into account the mechanisms of perileak that have occurred with the current commercially available devices, and modifications in design, as with the TAVR experience, may further reduce rates of perileak.
UNANSWERED QUESTIONS
Further data will become available in the next few years to allow a better assessment of thromboembolic event rates in patients with persistent perileaks post LAA occlusion. The prevalence of perileaks in the early trials may have been representative of operator learning curves for both device sizing and implantation12. This appears to have reduced with increased operator experience and further refinement to device design. Going forward, it will be important to accrue greater patient numbers and follow-up duration to determine definitely whether perileaks are associated with an increased risk of stroke or thromboembolism, as this will guide management in these patients. It will be important to collect these data for each of the commercially available devices, since the mechanism of leak varies between device designs, and the clinical implications of a persistent leak may therefore be device-specific. Such data will guide management of patients with persistent perileaks. The current data lack sufficient numbers and follow-up to determine whether small perileaks may confer an increased risk of stroke or should be intervened upon.
Large leaks (e.g., those >5 mm or uncovered lobes) can potentially be closed with vascular plugs, coils or septal occluder devices. Case series have demonstrated successful closure36,37; alternatively, patients may be managed on oral anticoagulants if tolerated. In a large single-centre retrospective series, 2% of 631 patients who underwent LAA closure required a second LAA closure procedure for peri-device leak >3 mm. These 12 cases of second LAA closure procedure were all successful and none of the patients had a stroke on follow-up32. Thus, a second LAA closure procedure can be feasible; however, current experience is limited and the long-term clinical benefit of this approach should be explored further.
The natural history of perileaks following LAA appendage closure also requires further study. Reports on the LARIAT device have demonstrated increased incidence of leaks at one-year follow-up, suggesting that leaks may develop late after LAA occlusion23; however, this has not been a consistent finding across studies. Rates of new leaks may vary with different devices. More information is required as to whether patients may require follow-up TEE or CT to check for new perileaks as time since the LAA closure increases.
With increased data on outcomes and natural history of perileaks, evidence-based management recommendations will emerge. The major unanswered questions at present are whether patients with a persistent perileak require long-term anticoagulation or antiplatelet therapy, and whether additional mechanical perileak closure should be attempted. Imaging of perileaks is also a developing field. When and if repeat imaging should be performed to check for development of new perileaks is still uncertain.
Based on the available evidence, it would seem reasonable to observe perileaks less than 5 mm serially for patients post WATCHMAN or Amulet/ACP device. For leaks of 5 mm and greater, management with continued oral anticoagulation would be reasonable if clinically safe, while perileak closure may be considered on an individualised basis. For patients with incomplete surgical appendage closure, ongoing anticoagulation or consideration of percutaneous closure would be recommended. The data for the LARIAT device are less clear but a similar strategy to surgery may be reasonable.
Summary
Prospective analysis of contemporary percutaneous therapies has not revealed an increased event rate with persistent perileaks, although data are limited. Different devices appear to be associated with different mechanisms of device leak post LAA closure. Further work is required to assess the thromboembolic risks associated with persistent perileak and to guide management recommendations in these patients.
| Impact on daily practice Persistent leaks (perileaks) following left atrial appendage closure occur in up to 30% of patients. Use of preprocedural imaging and improved decision making regarding device size appear to have reduced contemporary perileak rates. Limited data from early trials did not suggest a higher rate of stroke in patients with persistent perileaks; however, the number of events was small and further data are needed. In patients with perileaks of 5 mm or greater, clinicians should consider continued oral anticoagulation if tolerated or percutaneous closure of the perileak. |
Conflict of interest statement
D. Holmes (along with the Mayo Clinic) would like to declare a financial interest in the WATCHMAN device. The other authors have no conflicts of interest to declare.

