Abstract
Background: Residual peri-device leakage (PDL) is frequent after left atrial appendage occlusion (LAAO). Little is known about management strategies, procedural aspects and outcomes of interventional PDL closure.
Aims: The aim of this study was to assess the safety and feasibility of PDL closure after LAAO.
Methods: Fifteen centres contributed data on baseline characteristics, in-hospital and follow-up outcomes of patients who underwent PDL closure after LAAO. Outcomes of interest included acute success and complication rates and long-term efficacy of the procedure.
Results: A total of 95 patients were included and a cumulative number of 104 leaks were closed. The majority of PDLs were detected within 90 days (range 41-231). Detachable coils were the most frequent approach (42.3%), followed by the use of the AMPLATZER Vascular Plug II (29.8%) and the AMPLATZER Duct Occluder II (17.3%). Technical success was 100% with 94.2% of devices placed successfully within the first attempt. There were no major complications requiring surgical or transcatheter interventions. During follow-up (96 days [range 49-526]), persistent leaks were found in 18 patients (18.9%), yielding a functional success rate of 82.7%, although PDLs were significantly reduced in size (pre-leak sizemax: 6.1±3.6 mm vs post-leak sizemax: 2.5±1.3 mm, p<0.001). None of the patients had a leak >5 mm. Major adverse events during follow-up occurred in 5 patients (2 ischaemic strokes, 2 intracranial haemorrhages, and 1 major gastrointestinal bleeding).
Conclusions: Several interventional techniques have become available to achieve PDL closure. They are associated with high technical and functional success and low complication rates.
Introduction
Left atrial appendage occlusion (LAAO) has emerged as a feasible stroke prevention strategy in selected patients with non-valvular atrial fibrillation1,2. A wide variability in LAA shape and size has been described, which may contribute to incomplete LAA closure or new leak formation in up to 30% of cases, as a result of a mismatch in size and shape between the LAA and the occlusion device or cardiac remodelling after the procedure3. Those leaks can cause turbulent flow and the incomplete closure may lead to stasis. Both conditions may potentially increase the risk of thrombus formation and subsequent thromboembolic events4. The clinical significance of peri-device leakage (PDL) has yet to be determined5,6, although, if significant leaks are present, prolongation or reintroduction of oral anticoagulation is current clinical practice. This may cause important safety issues to arise in some patients. As a consequence, alternative strategies such as interventional PDL closure may be desirable. As well as the latest strategy of working with detachable coils7, peri-device leaks are also closed with various structural devices. Until now, no structured investigation has been carried out to assess leak morphology and size subject to closure and evaluate different closure strategies with associated clinical outcomes.
Methods
This multicentre, retrospective registry utilises data collected via standardised case report forms from centres worldwide performing interventional PDL closure after LAAO. We included patients treated with PDL closure irrespective of the initial percutaneous LAAO device choice and generation.
DATA AND OUTCOMES
Baseline characteristics, information on the initial LAA closure, temporal occurrence and size of PDL, detected either by transoesophageal echocardiography (TEE) or by computed tomography (CT), were collected. Procedural aspects, in-hospital, and the latest known clinical follow-up data available were gathered. An anonymised patient-level data set was created and analysed.
The decision to close leak(s) as well as the interventional strategy adopted were left to the discretion of the implanting physician and based on previous experience, material availability and size and location of leak(s). Technical success was defined as a correctly deployed device without major complications (i.e., clinically relevant pericardial tamponade, periprocedural stroke or procedure-related mortality). Functional success was defined as a complete PDL closure (acutely or during follow-up). Persistent leaks after the procedure were documented and classified into three groups according to the maximum leak size (either mild [0-3 mm], moderate [>3-5 mm], or severe [>5 mm]). Other outcomes included major adverse cardiac events (MACE; a composite endpoint of non-fatal ischaemic stroke, systemic embolism, major bleeding complications [Bleeding Academic Research Consortium, BARC ≥type 2] or device embolisation). Outcomes were defined based on the Munich consensus document on definitions, endpoints and data collection requirements for LAAO clinical studies8. All necessary ethical oversight was secured. The study is in line with the Declaration of Helsinki, and the local ethics committee (Landesärztekammer Hessen, No. 2020-1873) approved the investigation. The study is registered at ClinicalTrials.gov (NCT04590898).
STATISTICAL ANALYSIS
Descriptive statistics summarise baseline characteristics, procedural and follow-up data. Categorical data are expressed as numbers and percentage of total. Continuous variables are presented as median with interquartile range (first-third quartile, IQR) or mean with standard deviation, if appropriate. The data set was evaluated for normal distribution with the Kolmogorov-Smirnov-Lilliefors test. Differences between groups were determined by chi-square testing or Fisher’s exact test. With regard to variable distribution, continuous variables were compared using either the Student’s t-test or the Mann-Whitney U test. All tests were two-sided and a p-value <0.05 was considered statistically significant. SPSS, Version 20 (IBM Corp., Armonk, NY, USA) was used for analysis and Prism 8 (GraphPad Software Inc., San Diego, CA, USA) for graphing.
Results
Fifteen international centres contributed 95 patients who underwent interventional PDL closure of 104 leaks after LAAO. Procedures were performed in the period between 06/2013 and 08/2020.
BASELINE CHARACTERISTICS
Patients were in their early seventies (72±9 years) and predominantly male (71.6%). Median CHA2DS2-VASc score was 4 points (range 3-5) and median HAS-BLED score was 3 points (range 2-4). The most common reason for initial device implantation was gastrointestinal bleeding (26.3%). Intracranial haemorrhage as the reason for device implantation was noted in 6 patients (6.3%). Further baseline characteristics are displayed in Supplementary Table 1. The WATCHMAN™ (Boston Scientific, Marlborough, MA, USA) device was chosen in over half of the patients (66.3%), whereas the LARIAT® (SentreHEART, Redwood City, CA, USA) suture system was used in 17.9% of cases. The WATCHMAN FLX™ (Boston Scientific), AMPLATZER™ Amulet™ (Abbott Vascular, Santa Clara, CA, USA) and AMPLATZER™ Cardiac Plug (Abbott Vascular) for initial LAAO were used in 5.3%, 2.1%, and 2.1% of cases, respectively. Other devices were chosen in 6.3% of cases. Figure 1A provides a visual overview.
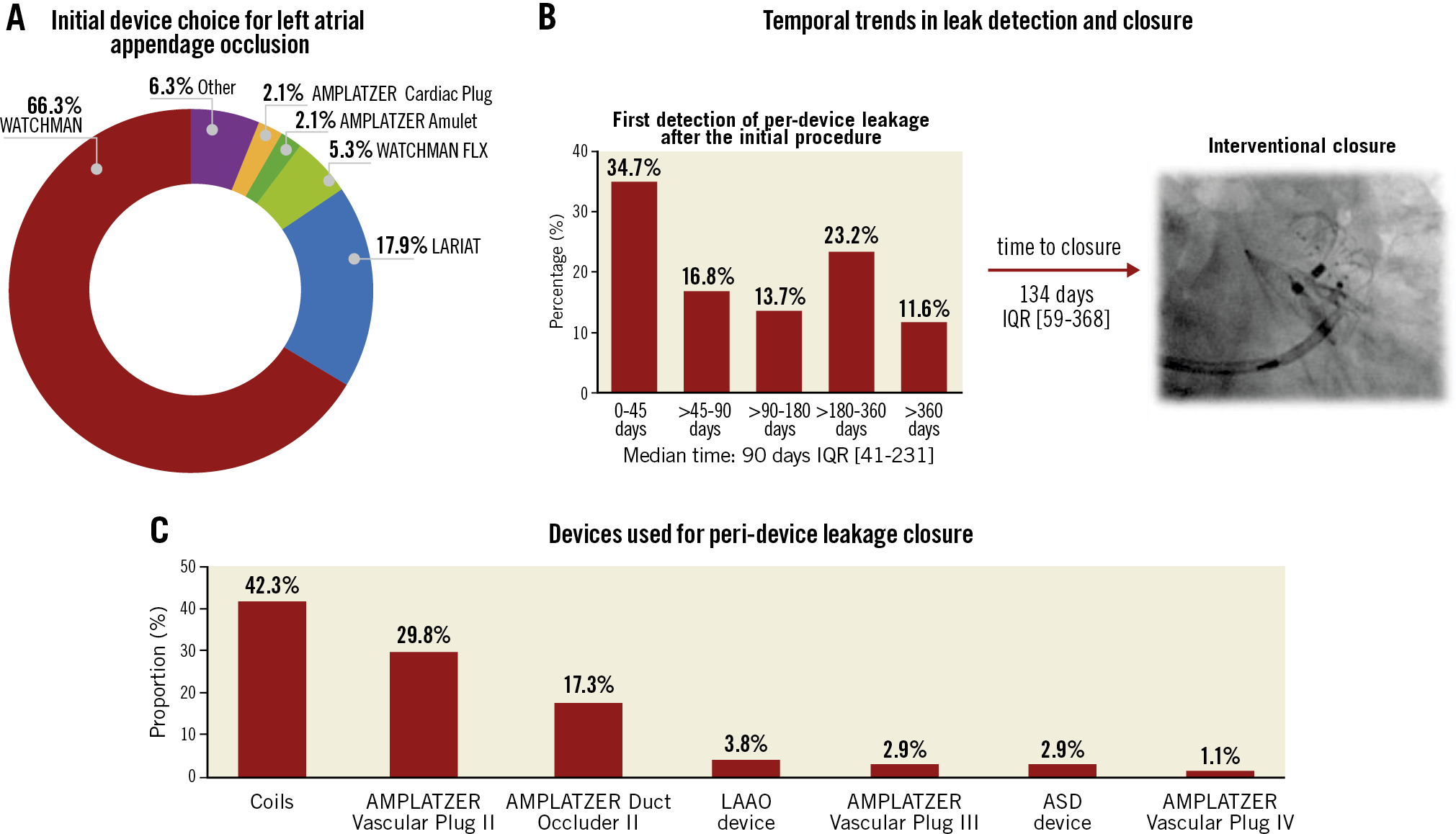
Figure 1. Device choice and temporal trends in patients undergoing peri-device leakage closure after left atrial appendage occlusion (LAAO). A) Initial device choice of patients undergoing peri-device leakage closure after insufficient percutaneous LAAO. B) Temporal trends in detection of peri-device and subsequent interventional closure. C) Device choice for peri-device leakage closure. ASD: atrial septal defect: IQR: interquartile range
First detection of PDL was within 90 days (range 41-231) after the index procedure. Most of the cases were found within the first 45 days (34.7%), although 11.6% of peri-device leaks were reported >365 days after the procedure. Median time from PDL detection to PDL closure was 134 days (range 59.5-368). Temporal trends of PDL occurrence and closure can be found in Figure 1B. The preferred method for PDL detection was a combination of 2D/3D TEE assessment (88.4%), followed by CT (10.5%). In one case (1.1%), intracardiac imaging (ICE) was used. One leak was present in 86 patients (91.6%), two leaks in 9 patients (9.4%). A total of 104 leaks were subject to interventional closure. The maximum leak diameter was 6.1±3.6 mm. Leaks to be closed were most commonly classified as round (32.7%), crescent-shaped (30.8%) or oval (26%) and located at the posterior portion of the device (36.5%). Further information on leaks subject to closure can be found in Table 1.
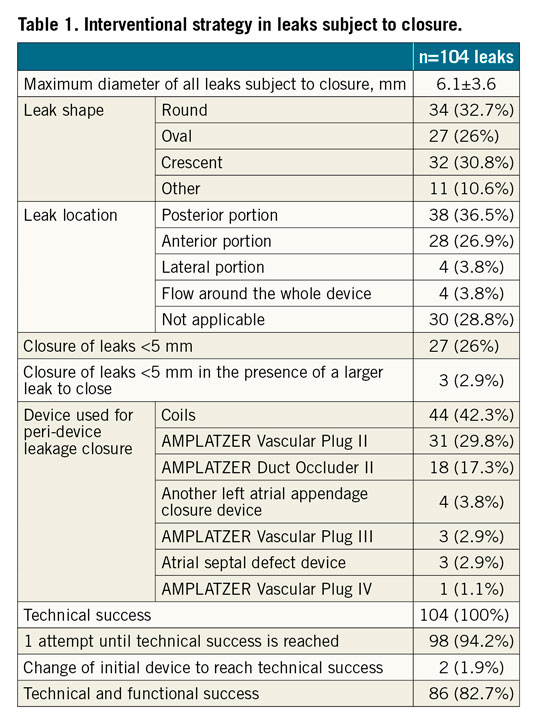
PROCEDURAL AND IN-HOSPITAL OUTCOMES
Informed patient preference (46.3%) to close PDL was the driving reason for closure. In 11.6% of patients, the leaks were closed following an embolic stroke or transient ischaemic attack (TIA). Detachable coils were the most frequent approach (42.3%), followed by the use of AMPLATZER™ Vascular Plug II (AVP; Abbott Vascular, 29.8%), AMPLATZER™ Duct Occluder II (ADO; Abbott Vascular, 17.3%) and other LAAO devices (3.6%) (Figure 1C). Procedure duration was 50 minutes (range 40.2-88.2), fluoroscopy time was 16.5 minutes (range 9-24.7) and 60 mL (range 40-110) contrast medium volume was used (Figure 2A). A two-leak closure strategy within the same procedure was performed in 9 (9.5%) patients. Technical success was reached in 100%; 94.2% of devices were placed successfully within the first attempt. Change of the initial device intended for closure was noted in 1.9% of cases. Clinically non-relevant pericardial effusion and/or tamponade (i.e., not requiring therapeutic pericardiocentesis, surgical intervention, blood transfusions, or resulting in death or shock) were seen in 5.3% of patients. One patient (1.1%) experienced a TIA during the in-hospital stay. Severe complications such as device embolisation/fracture/erosion/laceration, clinically relevant pericardial tamponade, cardiopulmonary resuscitation, conversion to open heart surgery, myocardial infarction, stroke or death did not occur. In-hospital stay was 2 days (range 1-2). In-hospital data are presented in Figure 2B. Further information is displayed in Table 2.
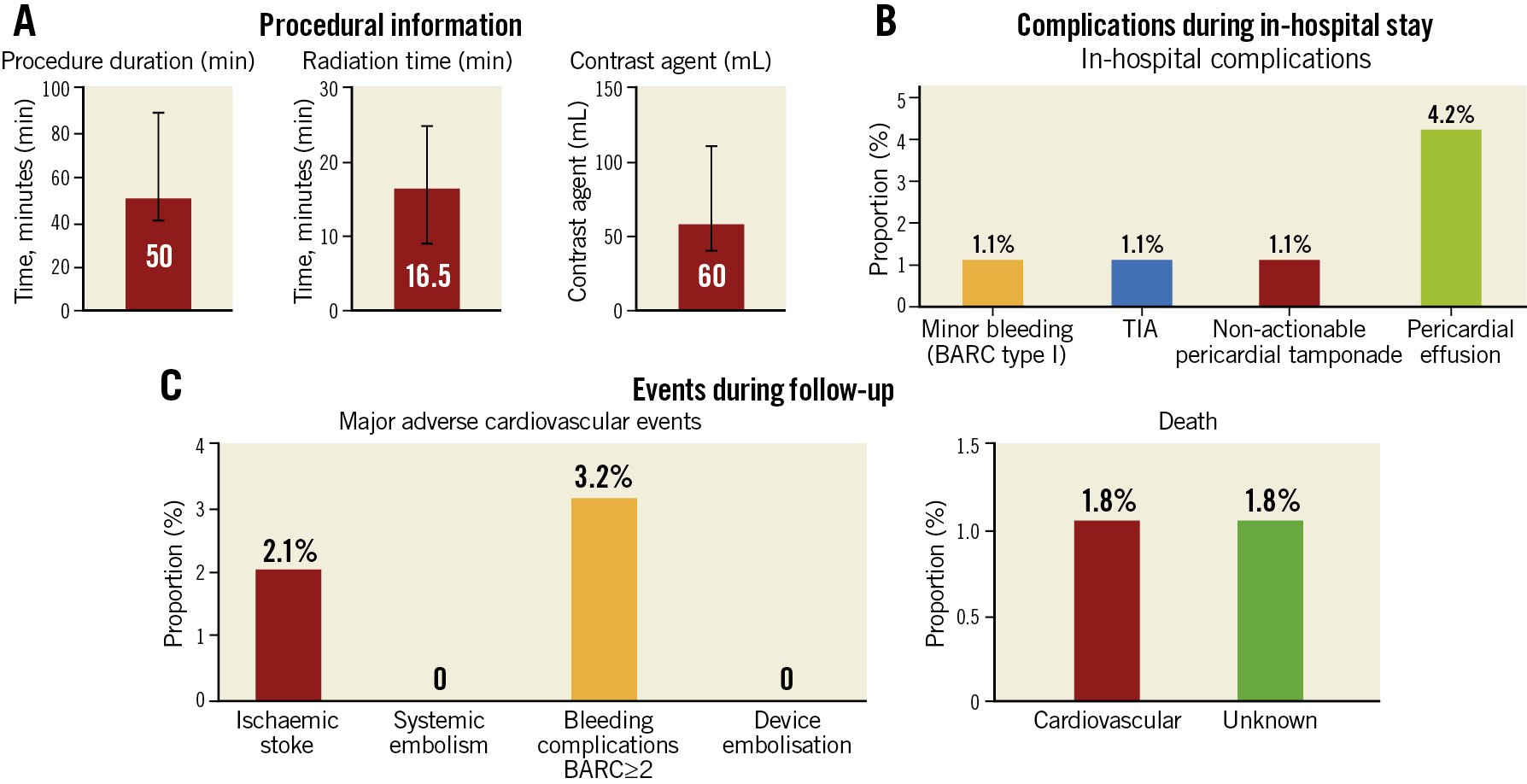
Figure 2. In-hospital information and follow-up of patients undergoing peri-device leakage closure. A) Procedure characteristics. B) Complications during in-hospital stay. C) MACE during follow-up. BARC: Bleeding Academic Research Consortium;TIA: transient ischaemic attack
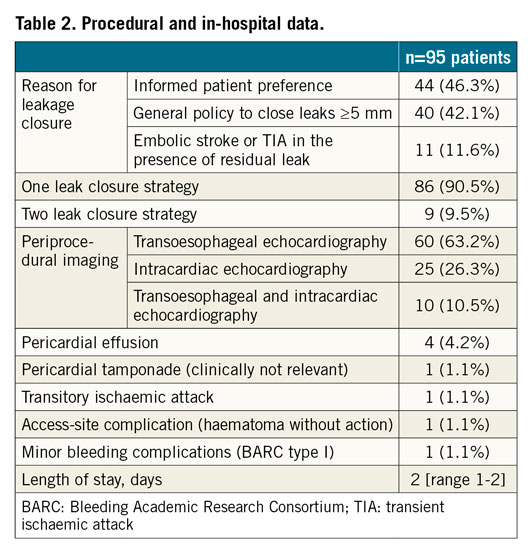
FOLLOW-UP DATA
Median follow-up time after PDL closure was 96 days (range 49-526). MACE occurred in 5 patients (5.3%), and were mainly bleeding complications (3.2%): one patient experienced two gastrointestinal bleedings 345 days and 348 days after the procedure with the need for re-hospitalisation. The first one was on dual antiplatelet therapy (DAPT; aspirin+clopidogrel) and the second one on aspirin alone. One patient still on DAPT suffered from a massive intracranial haemorrhage 40 days after PDL closure and died the same day. One other patient on aspirin and warfarin had a fall one day after discharge and experienced a subdural haematoma. She recovered without sequelae.
In two (2.1%) patients an ischaemic stroke was observed: one patient, taking aspirin and warfarin, was admitted on day two post-procedure with acute ischaemic stroke, recovered with no residual deficits and was discharged three days later. Another patient, taking aspirin, experienced an acute ischaemic stroke 726 days after PDL closure.
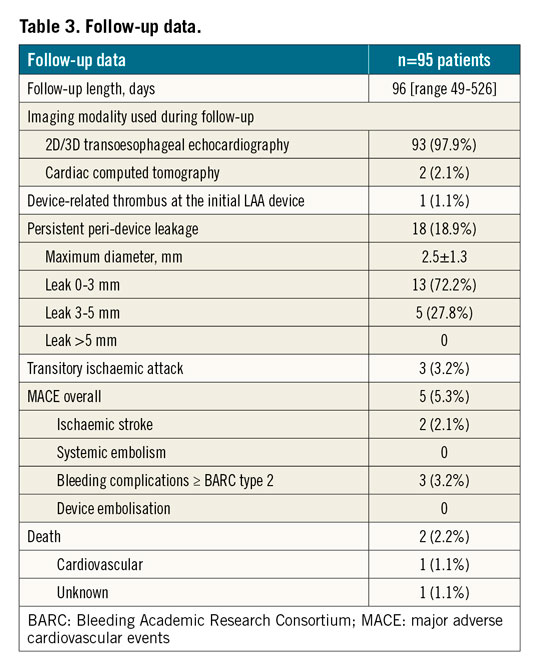
Of note, none of the patients with persistent leaks experienced a stroke or TIA during follow-up. Two (2.1%) patients died: one death was adjudicated as being due to cardiovascular causes (patient still on DAPT after PDL closure, who experienced intracranial haemorrhage and passed away 40 days after the procedure). Clinical outcomes during follow-up are displayed in Figure 2C. Imaging modalities to detect PDL during follow-up were either 2D/3D TEE (97.9%) or CT (2.1%). Persistent leaks were found in 18 patients (18.9%), yielding a functional success rate of 82.7%. Nonetheless, PDLs were significantly reduced in size (pre-leak sizemax 6.1±3.6 mm vs post-leak sizemax 2.5±1.3 mm, p<0.001). Persistent leaks were classified as mild (0-3 mm) in 72.2% of cases, and moderate (3-5 mm) in 27.8% of patients. None of the patients had a severe residual leak with dimensions >5 mm (Figure 3). In patients treated with detachable coils, the initial leak size was smaller (pre-leak sizemax coils 5±1 mm vs pre-leak sizemax other methods 7.1±4.6 mm, p=0.01) as compared to other PDL closure methods. Persistent leaks were evenly distributed between groups (coils n=7/41, 17.1% vs other methods n=11/54, 20.4%, p=0.08). Device-related thrombus was observed in one patient (1.1%). Additional follow-up data can be found in Table 3.
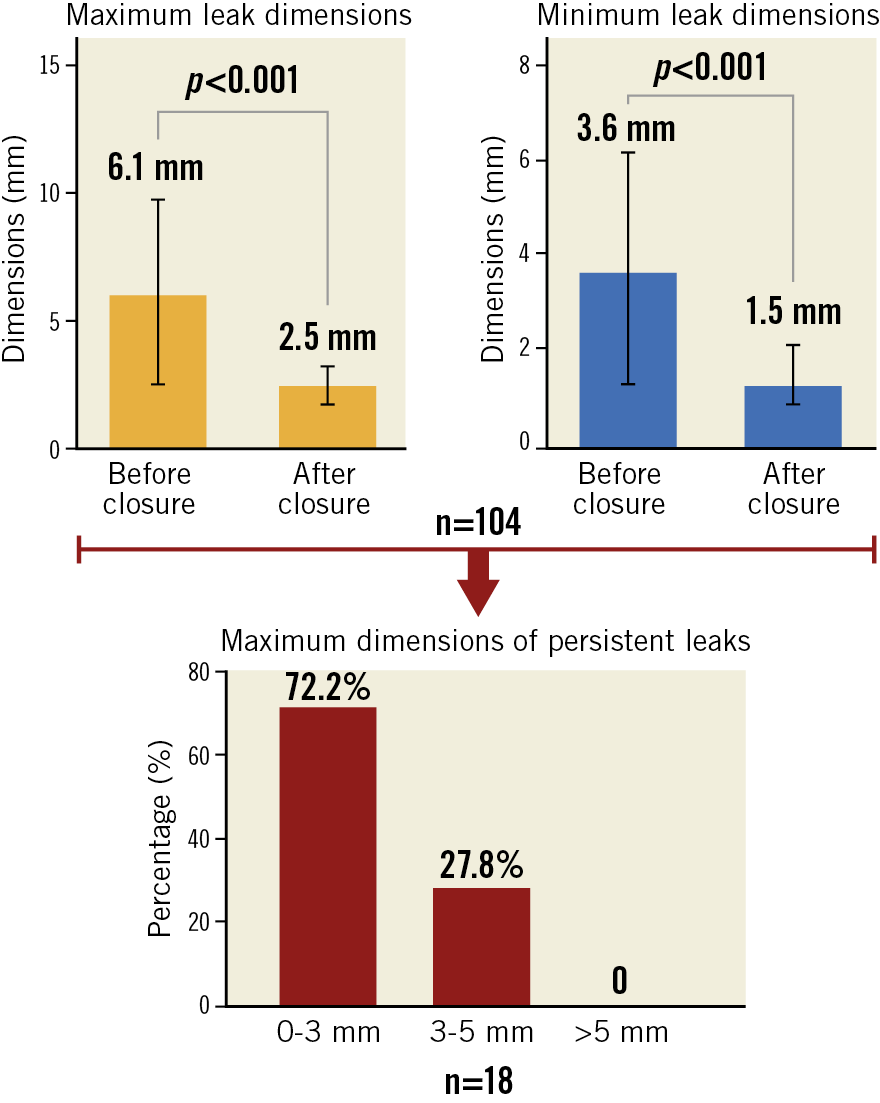
Figure 3. Leak dimensions before and after closure. Peri-device leakage closure led to a significant reduction in the maximum and minimum leak size. In patients with persistent leaks (n=18), leak dimensions between 0-3 mm were noted in 72.2% of cases, and leaks between 3-5 mm in 27.8% of patients. None of the patients had a leak >5 mm.
Discussion
A variety of approaches can be used to achieve PDL closure after LAAO. This is the largest registry to date reporting corresponding interventional strategies and outcomes. Our findings suggest that PDL closure is a feasible and safe strategy with high technical and functional success (Central illustration).
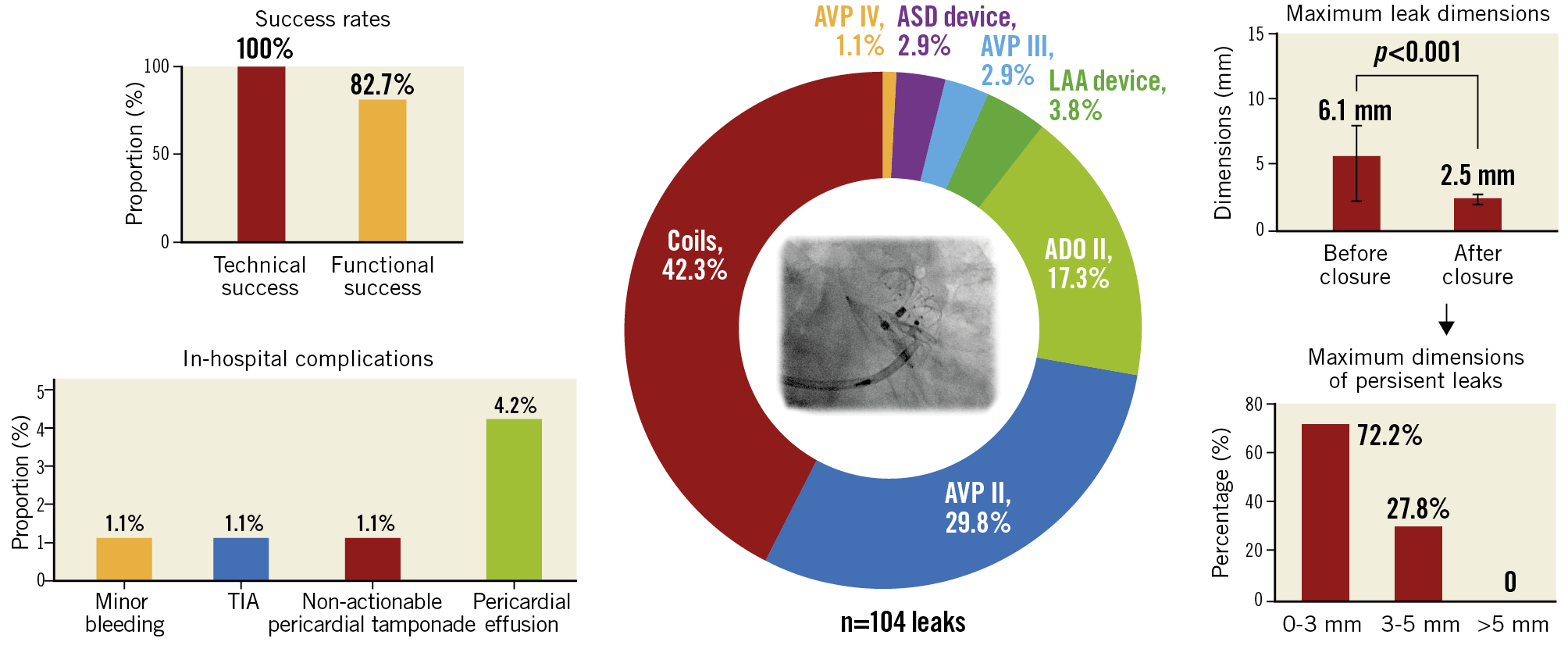
Central illustration. Peri-device leakage (PDL) closure after percutaneous left atrial appendage closure can be performed with a variety of devices. In our registry, detachable coils were the most frequent approach (42.3%), followed by the use of AMPLATZER Vascular Plug II (AVP, 29.8%). The procedure is associated with high technical and functional success. The maximum leak dimensions can be reduced substantially after closure. None of the patients presented with a leak >5 mm after closure. The procedure is associated with low in-hospital complication rates. TIA: transient ischaemic attack
DETECTION OF PDL AND MANAGEMENT STRATEGIES
The efficacy of stroke prophylaxis may be compromised due to incomplete closure of the LAA9. Hence, PDL can be seen as a significant technical limitation of LAAO. The impact of PDL on cardiovascular events remains controversial: whereas patients with incomplete surgical LAAO show an increased risk for device-related thrombus and thromboembolic events irrespective of leak severity10,11,12, patients undergoing percutaneous LAAO with subsequent PDL do not necessarily have an augmented risk for cardiovascular events5,13. However, low event rates, limited sample size and anticoagulation therapy in reported studies may bias results. A revisit of successful LAA closure may also be warranted. PDL can be a manifestation of a residual defect adjacent to the implanted device, a tilted device or a missed lobe. Hence, not only the leak dimensions themselves but also the anatomical conditions may determine the risk for thromboembolic complications. A small (<5 mm) but completely uncovered deep lobe in a multilobulated LAA is more likely to be significant than an uncovered proximal mildly trabeculated side lobe14.
Although PDL after percutaneous LAAO has no demonstrated prognostic value, it is current clinical practice to continue oral anticoagulation after successful surgical LAAO in accordance with European guideline recommendations, and to continue or resume oral anticoagulation if persistent LAA patency (>5 mm) is seen after interventional LAAO. There is no uniform opinion on the management of patients presenting with moderate (3-5 mm) leaks. Oral anticoagulation may be continued or interrupted, depending on patient-related factors such as the history of thromboembolic events, counterbalanced with the risk for major bleeding complications. If oral anticoagulation is not a viable option, patients may benefit from a device-based closure as part of an individualised treatment strategy. Compared to other LAAO series, bleeding complications and ischaemic event rates in our study appear to be quite high during follow-up. This might be attributable to the small sample size and the preselected high-risk LAAO population (baseline CHA2DS2-VASc score: 4 [range 3-5] and HAS-BLED score: 3 [range 2-4]) who underwent PDL closure.
The detection method for PDL after LAAO is also a subject of debate. Historically, 2D-TEE is the most often used technique and forms the basis for leak quantification and management strategies in major LAAO landmark trials15,16,17,18. In recent years, 3D-TEE and especially cardiac CT have gained popularity for procedural planning and follow-up. Preprocedural cardiac CT may improve outcomes since it offers the most accurate detection and characterisation of PDL and the possibility to predict the best C-arm angulation for interventional PDL closure. A large discrepancy between 2D-TEE and cardiac CT is noted regarding the PDL detection rate and leak size quantification. CT has a higher likelihood of showing LAA patency after LAAO than TEE19. However, the prevalence of large leaks (≥5 mm) can be reliably detected with both methods20. Korsholm et al just recently introduced a novel cardiac CT classification for peri-device leaks after AMPLATZER Amulet occluder implantation. However, independent of the detection method, PDL was not associated with impaired clinical outcomes in the aforementioned study21.
PDL CLOSURE WITH REGARD TO THE INITIALLY IMPLANTED DEVICE
In this study, PDL closure was most often performed in patients initially receiving a WATCHMAN occluder, which will now be substituted by the WATCHMAN FLX, and the LARIAT suture system, which achieves LAAO sealing through extraluminal LAA closure. The high proportion of WATCHMAN devices might represent a selection bias since newer-generation devices have a shorter availability period and the market availability of devices differs across participating centres. For example, the AMPLATZER Amulet occluder is approved by the Federal Drug Administration but not yet in widespread clinical use in the USA. On the other hand, it may also reflect the device configuration: the WATCHMAN occluder follows the pacifier design, with only a single barrier between the left atrium and the LAA surface, as compared to a lobe and disc device, which may enhance sealing through a two-layer technique.
Patients treated initially with the LARIAT, a percutaneous suture ligation system to secure LAA exclusion, were the second largest population within this registry. Peri-device gaps are common with the LARIAT suture system and may increase over time22. In those patients, an increased thromboembolic event rate is reported23. Here, interventional PDL closure – if technically feasible – can be favoured as part of an individualised treatment concept to mitigate stroke risk. It has to be noted that the incidence and severity of leaks may not be directly associated with the initial device type chosen for LAAO. The occurrence of PDL varies tremendously throughout available studies5,13,24, and factors such as suboptimal implantation, undersizing, the initial LAA size and anatomy play a crucial role.
DEVICE CHOICE FOR PDL CLOSURE
With new-generation devices and better preprocedural planning, PDL may be reduced through technical improvements. For example, the WATCHMAN FLX has a shorter height, an improved fabric coverage, and a closed distal end and requires less implantation depth as compared to its predecessor the WATCHMAN occluder. It favours a high degree of LAA sealing and good clinical results25. However, the WATCHMAN FLX is still delivered through a non-steerable sheath and remains prone to suboptimal device implantation and subsequent PDL development through non-co-axial alignment during device deployment.
Along with optimising the mechanical properties of the device itself, vast imaging efforts, comparative studies, novel classifications26,27,28,29 and new approaches, are being made to secure optimal sizing and guiding throughout the procedure. However, PDL will still be a subject of debate in a small number of selected patients. The device choice is currently at the implanter’s discretion and based on the leak location, size, previous experience and material availability. No dedicated structural device has yet been developed for PDL closure, reflecting the small number of patients undergoing interventional closure of leaks after LAAO and the large variety of leak size and location. In certain subgroups, such as patients with unsuitable anatomies for conventional percutaneous LAAO or insufficient surgical closure and/or percutaneous ligation, a novel septal occluder device did prove to be a suitable choice30.
Limitations
All information gathered on patients undergoing PDL closure was self-reported by the participating centres and adverse events were not adjudicated by an independent clinical events committee. This registry did not collect information on the PDL mechanism in each individual patient. The device choice for leakage closure does reflect an individual patient-tailored approach. Until now, PDL after percutaneous LAAO has no proven clinical prognostic value, and PDL closure is an off-label procedure with no formal recommendations. The heterogeneity of LAAO devices, the limited number of leak closures per centre, and types of leak and devices used for PDL closure may limit the conclusiveness of this study. Additionally, the large time span of this registry may not necessarily reflect contemporary practice in LAAO.
Conclusions
The concept of peri-device leakage closure encompasses a variety of different techniques to achieve PDL closure after LAAO. They are associated with high technical and functional success and low periprocedural and post-procedural complications.
|
Impact on daily practice Peri-device leakage is a common phenomenon after left atrial appendage occlusion with a controversial impact on adverse events during follow-up. In very few cases, percutaneous peri-device leakage closure is used to enhance sealing after insufficient left atrial appendage occlusion. With this largest-to-date registry we assessed leak morphology and size subject to closure and evaluated different closure strategies with associated clinical outcomes. We showed that peri-device leakage closure has a high technical and functional success rate. Leak size can be significantly reduced and procedural complication rates are low. |
Conflict of interest statement
A. Natale has received speaker honoraria from Abbott, Biosense Webster, Boston Scientific, Biotronik, Baylis, Medtronic and St. Jude Medical, and is a consultant for Biosense Webster, St. Jude Medical, and Janssen. I. Cruz Gonzalez serves as proctor for Abbott and Boston Scientific. L. Raeber has received research grants to his institution from Abbott, Boston Scientific, Biotronik, HeartFlow, Sanofi, and Regeneron and speaker honoraria from Abbott, AstraZeneca, Amgen, Occlutech, Sanofi and Vifor. H. Sievert has received institutional honoraria, travel expenses, and consulting fees from 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Append Medical, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, Cardimed, CeloNova, Comed B.V., Contego, CVRx, Dinova, Edwards Lifesciences, Endologix, Hemoteq, Hangzhou Nuomao Medtech, Holistick Medical, Lifetech, Maquet Getinge Group, Medtronic, Mokita, Occlutech, Recor, RenalGuard, Terumo, Vascular Dynamics, Vectorious Medtech, Venus, Venock, and Vivasure Medical. The other authors have no conflicts of interest to declare with regard to this project.
Supplementary data
To read the full content of this article, please download the PDF.

