Abstract
Background: The AngioJet rheolytic thrombectomy (ART) system can quickly fragment and aspirate thrombi according to Bernoulli’s principle.
Aims: This retrospective study aimed to evaluate the therapeutic effects of the ART system in treating severe acute pulmonary embolism (APE), including high-risk pulmonary embolism (HR-PE) and intermediate-high-risk pulmonary embolism (IHR-PE).
Methods: Forty-four APE patients (21 HR-PE and 23 IHR-PE) were enrolled and underwent pulmonary ART using the 6 Fr Solent Omni AngioJet device. Nineteen patients were diagnosed with APE and lower extremity deep venous thrombosis (LEDVT), and underwent thrombectomy of APE and LEDVT simultaneously using ART. All patients also received local thrombolysis with urokinase.
Results: The results showed that the mean length of stay in intensive care units was 2.4±1.9 days. Significant improvements in clinical, haemodynamic and angiographic parameters were observed in both groups; the improvements in shock index, PaO2, and angiographic parameters were more obvious in the IHR-PE group. Six of the 44 patients died in hospital. During the follow-up, 35 of 38 patients were functioning well and no recurrence of APE was observed.
Conclusions: Pulmonary ART plus local thrombolysis of the pulmonary artery for HR-PE or IHR-PE is feasible and appears to be safe. Further studies are warranted to investigate comparative efficacy compared to existing treatments.
Introduction
Venous thromboembolism (VTE) mainly includes deep venous thrombosis (DVT) and pulmonary embolism, resulting in more than 300,000 deaths worldwide annually1,2. Severe acute pulmonary embolism (APE) can be stratified into two main categories based on the clinical presentation and haemodynamic criteria - intermediate-high-risk PE (IHR-PE) and high-risk PE (HR-PE)3. The in-hospital mortality of both categories is as high as 60%4. Therefore, exploring ideal strategies for treating APE has become urgent in recent years.
The aims of treating APE include rapid reduction of the thrombus load, immediate improvement of the patient’s haemodynamic stabilisation, and reduction of the long-term risk of pulmonary hypertension5. Anticoagulant and thrombolytic therapies are the standard medical treatments for APE patients. Systemic thrombolysis, surgical thrombectomy, or percutaneous mechanical thrombectomy (PMT) is generally performed in highly selected APE patients3. However, up to 40% of APE patients present with contraindications for both fibrinolytic therapy and surgical embolectomy6. Furthermore, the delayed treatment of lower extremity DVT (LEDVT), which is an important embolic source of APE, will result in an increase of post-thrombotic syndrome (PTS) for patients with both APE and LEDVT1.
Recently, PMT has offered advantages in treating APE and LEDVT simultaneously, providing a one-step endovascular strategy1. The AngioJet™ rheolytic thrombectomy (ART) system (Boston Scientific, Marlborough, MA, USA) can quickly fragment and aspirate thrombi according to Bernoulli’s principle2,7. However, the safety and indications of ART in treating APE remain unclear8,9. In this study, we conducted a retrospective analysis to evaluate the safety, effectiveness, and long-term outcome of ART for treating APE (HR-PE or IHR-PE) patients with or without LEDVT.
Material and methods
STUDY POPULATION
A retrospective review of consecutive patients (n=44) who were diagnosed with APE (HR-PE or IHR-PE) in Henan Provincial People’s Hospital between January 2016 and June 2018 was performed. HR-PE was defined as systolic blood pressure <90 mmHg for at least 15 minutes, pressure drop of more than 40 mmHg or requiring inotropic support, and imaging results showing embolisms in the main pulmonary artery, “saddle embolism” in the pulmonary trunk, or embolisms in two lobar arteries10. IHR-PE was defined as no systemic hypotension, but presenting severe blood gas disturbances, significant right ventricle strain or myocardial necrosis with positive cardiac biomarkers. ART was performed in APE (HR-PE or IHR-PE) patients with contraindications to thrombolysis or thrombolysis failure, along with time from symptom onset <72 hours, transferred from emergency medicine and other medical divisions to our catheterisation laboratory, and initially diagnosed by echocardiography and computed tomography pulmonary angiography (CTPA). Baseline clinical, laboratory, and instrumental anonymous data were collected and the pulmonary embolism severity index (PESI) was calculated11. Written informed consent was not obtained in all patients because of the observational retrospective nature of the study. Ethics committee approval was required from Henan Provincial People’s Hospital.
PROCEDURE
All patients received IV heparin immediately after APE was suspected and routinely underwent pulmonary angiography before and after ART. The characteristics of the 6 Fr Solent™ Omni, AngioJet and the thrombectomy procedure have been described previously12 (Figure 1). Details of the procedure are shown in Supplementary Appendix 1. When the procedure was finished, the patients were transferred to the intensive care unit (ICU) for continuous monitoring. Patients were then administered therapeutic doses of low-molecular-weight heparin for 3-5 days, and subsequently received oral anticoagulation therapy.
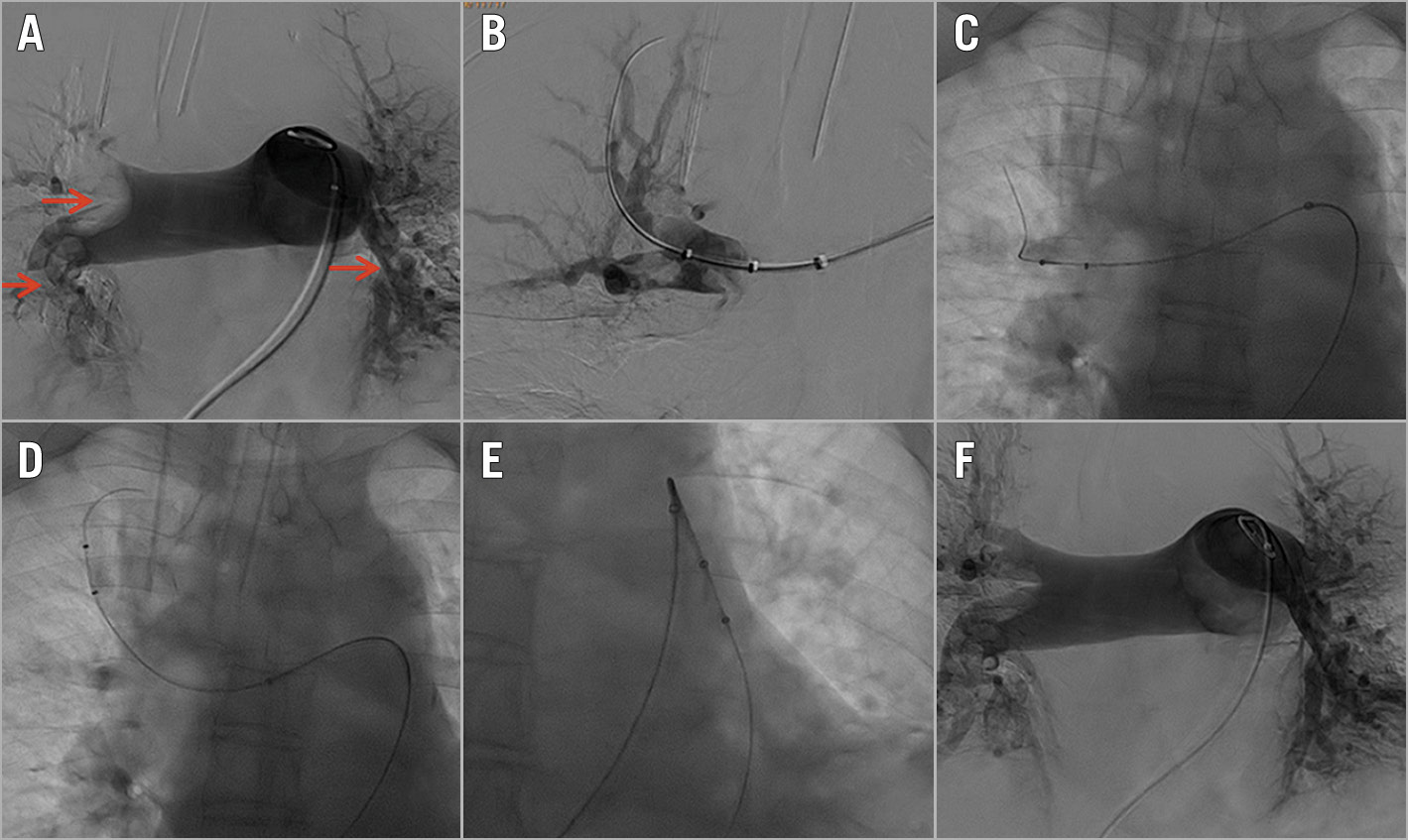
Figure 1. Angiography of a 66-year-old APE female with bilateral pulmonary embolism who underwent ART. A) “Saddle embolism” with occlusion of the superior and middle lobar arteries and partial occlusion of the bilateral inferior lobar artery. Thrombus with filling defects indicated by red arrows. B) The distal vessels. C) - E) AngioJet system was introduced to the pulmonary artery and ART was performed. F) Post-procedural angiography.
DEFINITIONS AND STUDY ENDPOINTS
Preprocedural and post-procedural pulmonary angiography as well as CTPA were analysed by an experienced blinded observer to calculate the thrombus burden for all patients according to criteria described by Miller et al13. The Miller index (MI) is a sum of two components including obstruction and perfusion indices, ranging from 0 = best to 34 = worst. A value of MI >17 indicates more than 50% of pulmonary vascular bed involvement and mainly corresponds to an HR-PE. Saddle emboli was defined as a thrombus located in the right and/or left main pulmonary artery occluding more than 50% of each lumen, or at the bifurcation of the pulmonary trunk3. Shock index (SI) was calculated for each patient as heart rate divided by systolic blood pressure; SI >1 is a relative indication for urgent intervention. Right ventricle strain on echocardiography was defined as paradoxical right ventricular septal motion during systole, right ventricular dilatation and increased pulmonary artery pressures. Serum cardiac troponin I (cTnI) was recorded before intervention. After treating LEDVT with ART, thrombus burden in the extremity was assessed by grading the portion of thrombolysis according to the reporting standards of a national multicentre registry: complete (grade III, >90% clearance), near complete (grade II, 50%-90% clearance), or partial (grade I, <50% clearance)14.
EVENTS AND FOLLOW-UP
Details of the following events which occurred during hospital stay were collected when present: all-cause death, recurrence of APE, major bleeding mainly comprising any bleeding associated with a haemoglobin decrease ≥5 g/dL or requiring surgical repair, cerebral haemorrhage, transient renal dysfunction with increased serum creatinine but not requiring haemodialysis, renal failure requiring haemodialysis, and severe thrombocytopaenia (i.e., platelets <100,000×109/L). Procedure-related arrhythmias were also recorded. Echocardiography was repeated 72 hours after the procedure. The venous duplex ultrasound (DUS) scan of the lower limb was repeated. After discharge, oral anticoagulation with warfarin or rivaroxaban was continued for six months at least. Finally, patients were followed up by clinic visit and/or telephone interview with a primary endpoint of all-cause death. Clinic visit including physical examination, DUS and CTPA to evaluate symptomatic improvement, pulmonary artery pressure and the cardiopulmonary and lower limb venous angiographic results was imperative at six months after discharge.
STATISTICAL ANALYSIS
Categorical variables were expressed as n (%) and continuous variables were expressed as mean±standard deviation. The chi-square test was performed for categorical variables and between-group comparisons were performed with the Student’s t-test. Within-group comparisons were performed with the paired-samples t-test. For all tests, statistical significance was set at p<0.05. Survival curves were determined by the Kaplan-Meier method. Statistical analysis was performed using SPSS, Version 22 (IBM Corp., Armonk, NY, USA).
Results
PREPROCEDURAL RESULTS
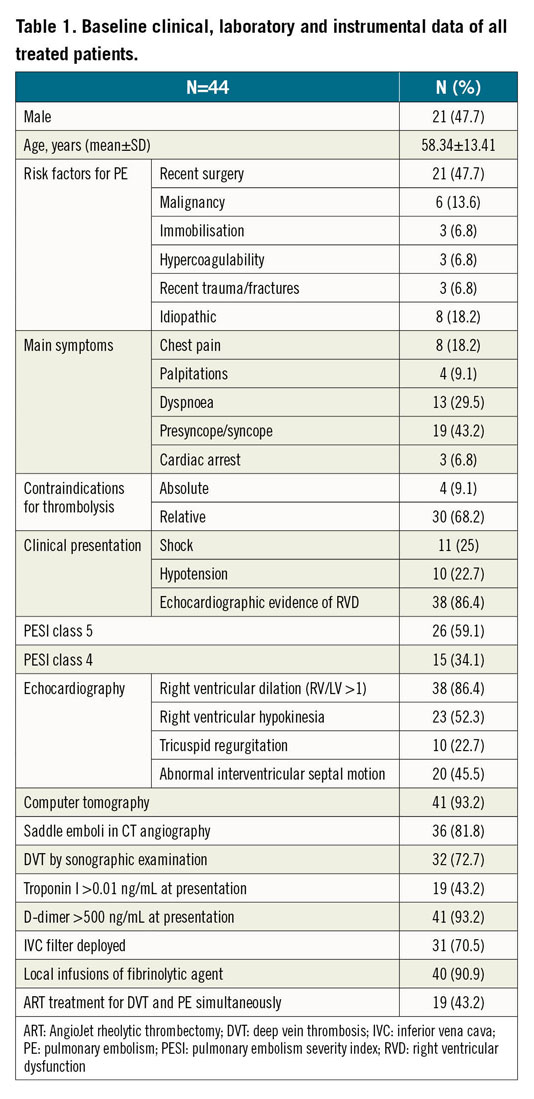
A total of 44 APE patients with haemodynamic compromise were treated with ART. The average age of the patients involved was 58.34±13.41 years (ranging from 21 to 74 years), and 23 (52.3%) were female. Preprocedural CTPA was performed in 41 of 44 patients. Three patients did not have CTPA performed due to severe haemodynamic instability; they underwent pulmonary angiography. Shock was present in 11 of 21 HR-PE patients (47.7%). There were 32 patients (72.7%) who were diagnosed with both APE and LEDVT. There were 10 patients (22.7%) with thrombolysis failure; the remainder were unsuitable for systemic fibrinolytic administration due to contraindications to thrombolysis. Thrombophilia was routinely checked in patients with a first unprovoked PE or recurrent VTE without common aetiology, especially in young patients. Three patients were considered as having thrombophilia, two with heterozygous protein S deficiency and one with antiphospholipid syndrome. Baseline clinical, instrumental, and laboratory data of the 44 patients are listed in Table 1. The baseline clinical data of the IHR-PE and the HR-PE groups are shown in Supplementary Table 1.
INTRAPROCEDURAL RESULTS
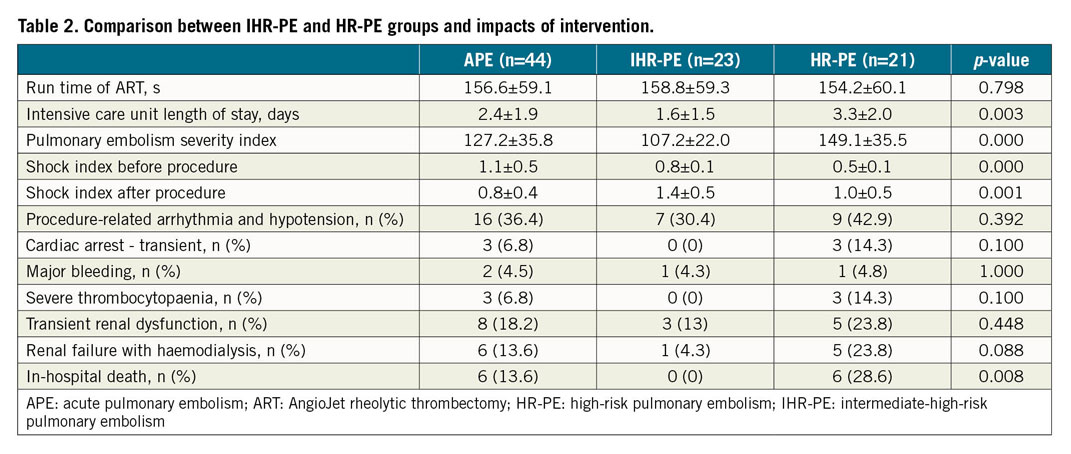
ART was performed in all patients within a mean time of 28.6±31.2 hours (range 6.5-72 hours) from symptom onset, and LEDVT thrombectomy was performed simultaneously with ART in 19 of 44 patients (43.2%). Adjunctive therapy with local infusions of a fibrinolytic agent was performed in all patients except for four patients with absolute contraindications to thrombolysis. The mean run time of the AngioJet was 156.6±59.1 sec (ranging from 78 to 280 sec) (Figure 2). Procedure-related severe arrhythmias, including bradycardia and hypotension, were observed in 16 of 44 patients (36.4%). Three of them presented with intraprocedural transient cardiac arrests and five patients presented with refractory bradycardia and hypotension, most of whom had shock or cardiopulmonary resuscitation before the procedure (Table 2). A detailed description of the three transient cardiac arrest patients is shown in Supplementary Appendix 2. In these patients, temporary transvenous pacing was needed in 4 of 44 patients (9.1%); intra-aortic balloon pumping was used in 2 of 44 patients (4.6%), and extracorporeal membrane oxygenation (ECMO) was used in 2 of 44 patients (4.6%) due to refractory shock, hypotension, and severe respiratory insufficiency after the procedure. The mean ICU length of stay was 2.4±1.9 days (range 0.3-7 days); the clinical events occurring during hospitalisation are summarised in Table 2. Major bleedings were observed in 2 of 44 patients (4.5%) - one pelvic cavity retroperitoneal haematoma requiring transfusion and one cerebral haemorrhage for which the patient received local fibrinolytics.
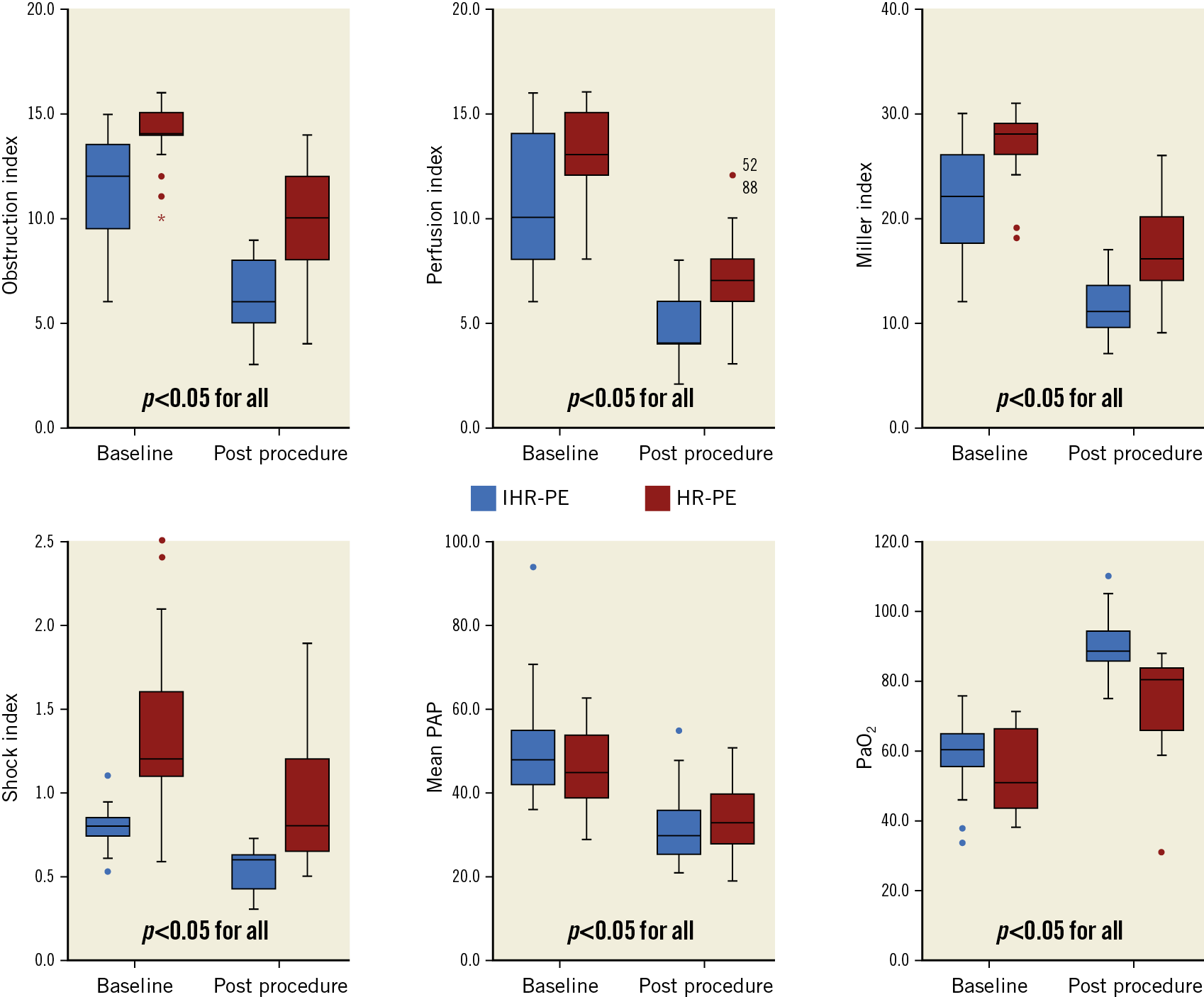
Figure 2. The obstruction index, perfusion index, Miller index, shock index, mean pulmonary artery pressure (PAP), and partial pressure of oxygen (PaO2) before and after ART in patients with high-risk pulmonary embolism (HR-PE) or intermediate-high-risk pulmonary embolism (IHR-PE).
POST-PROCEDURAL RESULTS
There was a statistically significant improvement in all angiographic endpoints including perfusion, obstruction and Miller indices (all p<0.05) in all patients compared to baseline (Figure 2, Figure 3), with a reduction of 49.34±13.33% (ranging from 14.3% to 70%) in the perfusion index, a reduction of 37.76±15.01% (ranging from 6.7% to 75%) in the obstruction index, and a reduction of 43.46±12.18% (ranging from 13.3% to 70.9%) in the MI (p<0.05). Moreover, improvements in the shock index (from 1.08±0.46 at baseline to 0.76±0.42 post procedure, p<0.05) and mean pulmonary artery pressure (PAP) (from 48.41±11.76 mmHg at baseline to 33.48±8.99 mmHg post procedure, p<0.05) were observed. Furthermore, the partial pressure of oxygen (PaO2) in arterial blood was significantly increased (from 56.82±11.24 at baseline to 82.35.1±14.01 mmHg post procedure, p<0.05) (Figure 2). The degree of venous patency was assessed in LEDVT patients using a DUS scan post procedure. The results indicated that 7 of 19 patients (36.8%) were in grade III, 9 of 19 patients (47.4%) were in grade II, and 3 of 19 patients (15.8%) were in grade I (Figure 4). Moreover, a distinct decrease in the circumference of the thigh and calf was also noted in these 19 patients, showing an obvious improvement of VTE symptoms. Six patients (13.6%) died in hospital due to intractable and persistent shock.
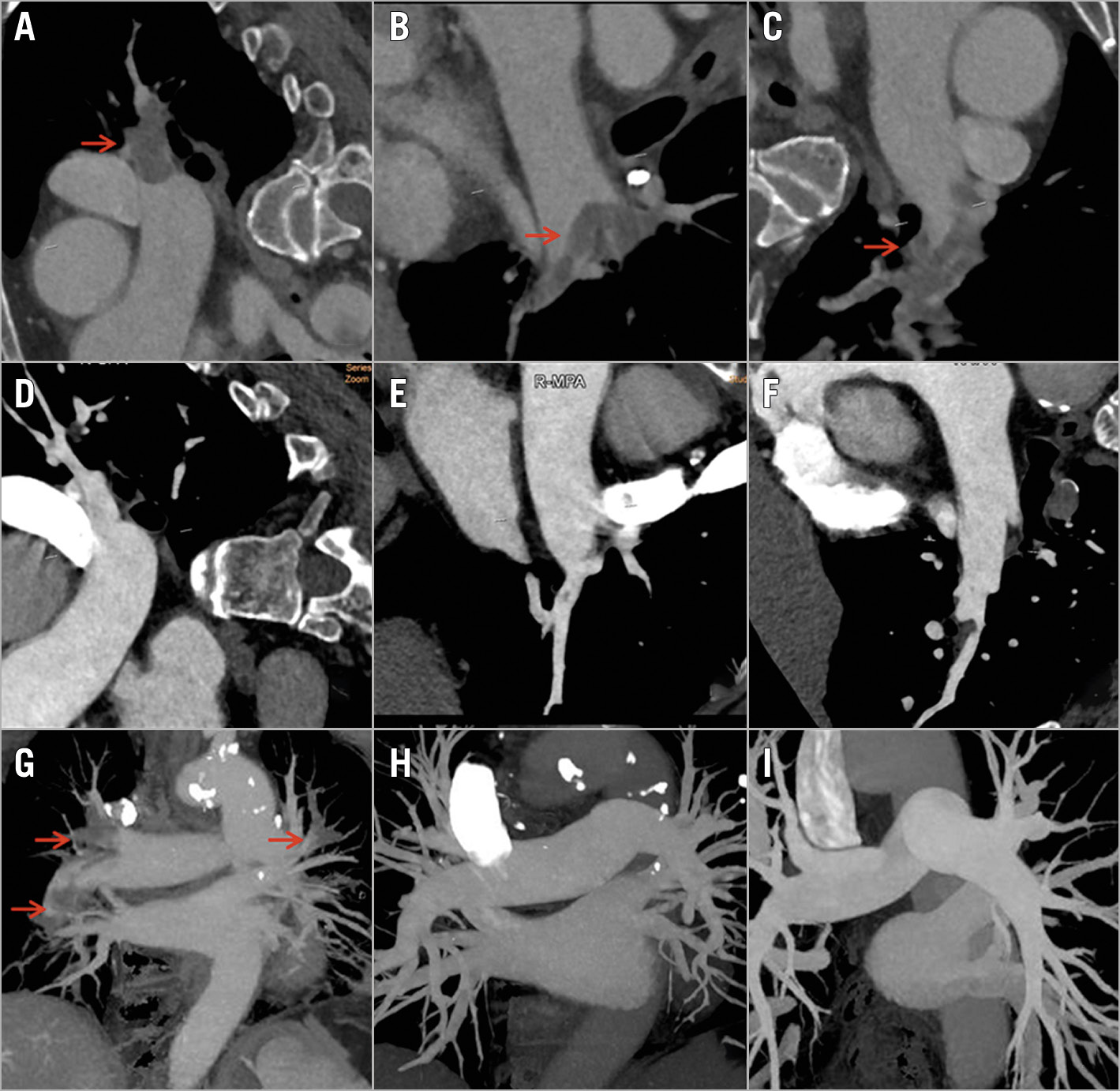
Figure 3. Computed tomography pulmonary angiography (CTPA) before the procedure (A, B, C) and one week after the procedure (D, E, F) in the same patient as in Figure 1. The image shows the post-procedural angiographic results in the same locations of superior (A, D), middle (B, E) and inferior (C, F) lobar arteries of the right lung. CT reconstruction images are shown before the procedure (G), one week post procedure (H), and six months post procedure (I). Thrombus with filling defects is indicated by red arrows.
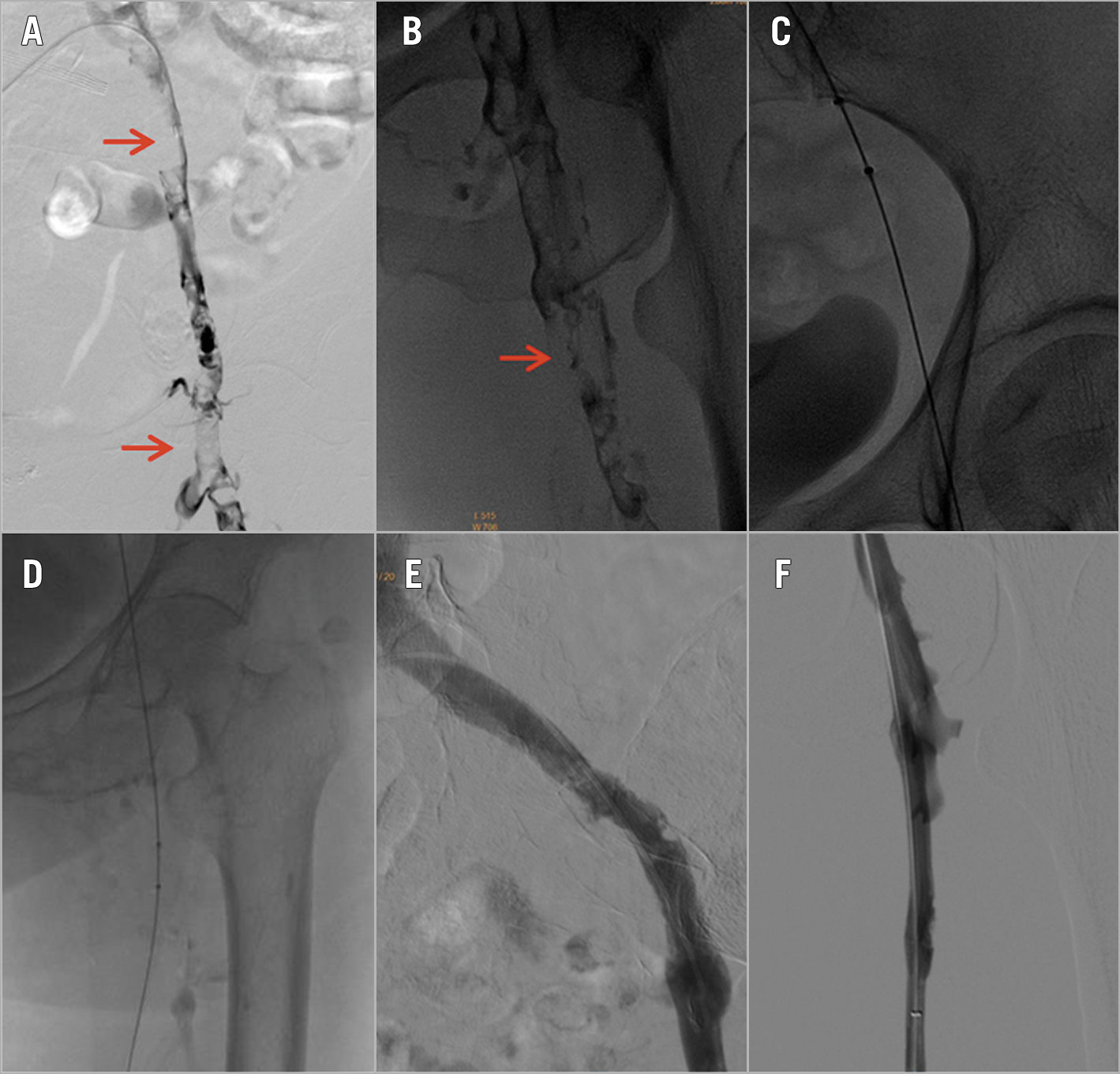
Figure 4. Low extremity venogram images from the same patient as in Figure 1. A) & B) Iliofemoral thrombus (red arrow). C) & D) The AngioJet catheter was introduced to the iliofemoral thrombus vein and ART was performed. E) & F) Post-procedural angiography.
COMPARISON BETWEEN HR-PE PATIENTS AND IHR-PE PATIENTS
Compared with the IHR-PE group, the HR-PE group had a significantly worse PESI (107.2±22.0 vs 149.1±35.5, p<0.05) and shock index (0.8±0.12 vs 1.38±0.49, p<0.05) from baseline to endpoint (Table 2). Severity of thromboembolism, as appraised by preprocedural perfusion (10.48±3.1 vs 13.05±2.18), obstruction (11.26±2.68 vs 14.0±1.61), and MI (21.78±5.51 vs 27.05±3.41), was also greater in HR-PE patients than in IHR-PE patients (all p<0.001). At the same time, improvements in shock index, PaO2, and all angiographic endpoints were more obvious in the IHR-PE group (all p<0.05) (Figure 2). However, no difference was observed in mean PAP between the two groups. The ICU length of stay for IHR-PE patients was significantly shorter than for HR-PE patients (1.6±1.5 vs 3.3±2.0 days, p<0.05). Severe thrombocytopaenia was observed in 3 HR-PE patients who were diagnosed before procedure. Renal failure requiring haemodialysis was observed in 1 IHR-PE patient and 5 HR-PE patients. There was no difference in the incidence of clinical events between the two groups (Table 2). HR-PE patients showed a higher in-hospital mortality than IHR-PE patients (28.6% vs 0, p<0.05); in-hospital mortality was significantly higher in patients with shock than in those with hypotension and IHR-PE (45.5% vs 4.2%, p<0.01).
COMPARISON BETWEEN PE+DVT THROMBECTOMY PATIENTS AND PE THROMBECTOMY-ONLY PATIENTS
We compared the outcomes between patients who received the one-stop combined procedure (PE and DVT thrombectomy) and those who underwent only PE thrombectomy (Supplementary Table 2). We found no significant difference in the run times of ART and procedure between the PE thrombectomy-only group and the PE+DVT thrombectomy group, although there appeared to be long run times of ART and procedural time in the PE+DVT thrombectomy group (p>0.05). However, more doses of contrast administration were found in the PE+DVT thrombectomy group than in the PE thrombectomy-only group (p<0.05). There was no significant difference in the incidence of procedure-related complications and serious adverse events (SAE) between the two groups (p>0.05). We identified a low PTS rate (21.1%) in the PE+DVT thrombectomy group and a low major bleeding rate (8%) in the PE thrombectomy-only group during a mean follow-up time of 22.5±9.3 months.
FOLLOW-UP RESULTS
The remaining cases were discharged safely and were followed up for a mean time of 22.5±9.3 months (ranging from 6 to 30 months). All 38 discharged patients were functioning well during follow-up, except for 3 patients (2 HR-PE patients and 1 IHR-PE patient): 2 patients died from progressing cancer at 15 and 24 months, respectively, and 1 patient died from myocardial infarction at 20 months. The survival curve was analysed using the Kaplan-Meier method (Figure 5). All 35 surviving patients did not develop APE recurrence during the follow-up. Echocardiography carried out at six months post procedure showed that the mean PAP had decreased to 24.85±12.36 mmHg (p<0.001) and all 35 patients had normal or only mild right ventricular dysfunction (RVD). CTPA at six months post procedure showed that the majority of thromboemboli in the main pulmonary arteries and the lobar branches were not seen in 26 of 35 patients (74.3%), and residual mural thromboemboli were seen in the segmental branches in 9 of 35 patients (25.7%). Oral anticoagulation with rivaroxaban was being taken by 39 of 44 patients (88.6%), and oral anticoagulation with warfarin was being taken by 5 of 44 patients (11.4%). Among them, in 18 of 44 patients the anticoagulation period (>6 months or no scheduled stop date) was prolonged as they had residual DVT or PE on ultrasound or CT imaging after completing six months of anticoagulant therapy or they were diagnosed with cancer (Supplementary Table 2).
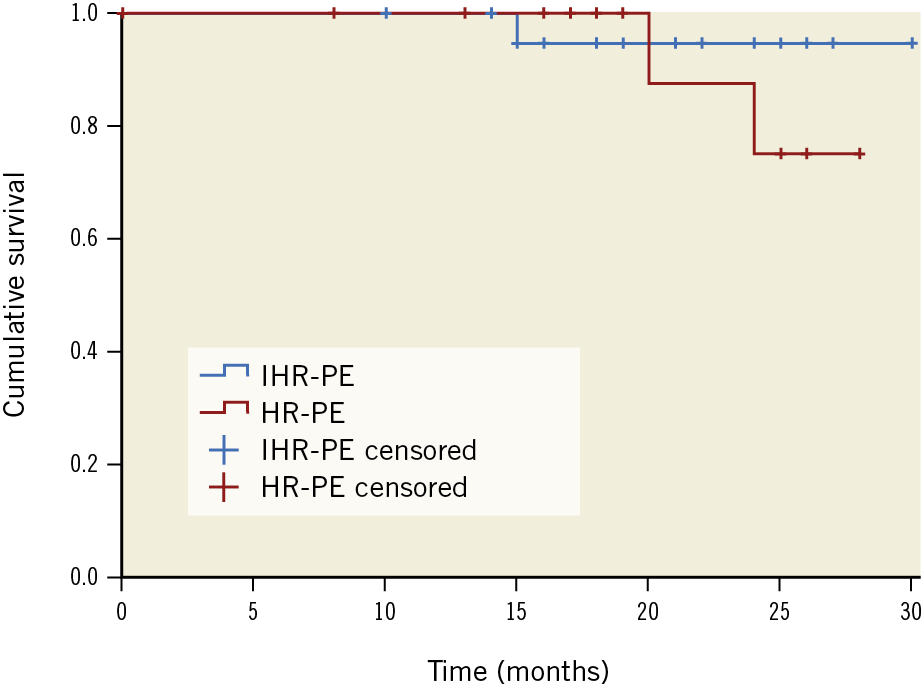
Figure 5. The cumulative survival curve according to categories of pulmonary embolism with high-risk pulmonary embolism (HR-PE) or intermediate-high-risk pulmonary embolism (IHR-PE) (p=0.459), with censored observations (ticks on survival curves).
Discussion
Due to the high mortality rate and the limited time available for thrombolysis, the first hour after symptom onset for haemodynamically unstable APE patients is called the “golden hour”7,15. Intravenous thrombolysis has been recommended to improve haemodynamic stabilisation and survival rates in HR-PE patients4,10. However, thrombolytic therapy not only increases the risk of major bleeding, but is also not applicable for patients with thrombolytic contraindications. Furthermore, the benefit of thrombolytic therapy in IHR-PE patients has not been well proven. Surgical thrombectomy is effective in highly selected APE patients16,17. Several studies have indicated that the endovascular approach for the management of severe APE can relieve clot burden quickly and improve pulmonary blood circulation effectively without increasing major bleeding2,4,10. However, previous studies have been restricted mainly to different types of PMT devices and patients from different subgroups of APE3. ART can be performed in a relatively easy and rapid manner and can effectively extract central and peripheral emboli with a wider therapeutic window5,6. However, previous studies reported a high rate of procedure-related complications during ART12. The cause of these complications is believed to be secondary to the haemolysis with a massive release of neurohormonal substances and the subsequent occurrence of severe hyperkalaemia and haemoglobinuria. We agree that procedural modifications can minimise or eliminate these complications12. For instance, a short run time seems to avoid these complications, and not using ART in vessels <3 mm could decrease vessel wall damage. We used the Solent Omni AngioJet device in all subjects because it can provide more effective clot retrieval and fewer complications due to its unique design and modified procedure than other older-generation catheters6,12. This procedure was well tolerated without major complications in most of the patients. In our series, there was a statistically significant improvement in all angiographic endpoints. Moreover, we obtained marked improvements in shock index and mean PAP post procedure.
It remains under debate whether PMT should be followed by local thrombolysis3. It has been emphasised that major haemorrhagic complications are associated with thrombolytic agents18. Reducing the dose of fibrinolytic agents and a shorter thrombolytic time are linked to less major bleeding. When using the suction rheolytic mode of the AngioJet, more than 90% of the fibrinolytic agents are aspirated back. On the other hand, this spray mode of the AngioJet not only mechanically fragments clot, but also delivers fibrinolytic agents into the interstices of thrombus masses, and potentially activates more plasminogen for more rapid thrombolysis12. Intraprocedural powerful injection of thrombolytics with the power pulse of the AngioJet will soften the thrombus for effective removal of thrombus, leading to better patient outcomes than only rheolytic thrombectomy and other PMT devices19. In our series, 30 (68.2%) patients showed relative contraindications to fibrinolytic therapy, and adjunctive local fibrinolytic therapy was performed in all patients except for four patients with absolute contraindications to thrombolysis. Significant improvements in angiographic endpoints and clinical results as well as a low rate of major bleeding (4.5%) were observed. The major bleeding rate of 4.5% with ART is considerably lower than that with fibrinolytic therapy: this is an acceptable bleeding rate, especially in patients with IHR-PE20. As a result, local thrombolysis can be preferred over ART for a low bleeding risk patient with relative contraindications or no contraindications to thrombolysis if thrombectomy only is inadequate.
Limitations
The current study has obvious limitations due to its retrospective nature and its relatively small sample size. Prospective studies in multiple centres or large randomised controlled trials are needed to confirm and expand our observations. The risk-benefit balance of local infusion thrombolytics as well as ART in APE patients with relative contraindications to systemic fibrinolytic therapy is also not clear.
Conclusions
In conclusion, ART plus local thrombolysis of the pulmonary artery for HR-PE or IHR-PE is feasible and does not appear to be associated with safety concerns. Patient response to treatment was encouraging in our experience. Prospective, controlled studies are warranted to investigate comparative efficacy compared to existing treatment protocols for the management of these acutely unwell patients.
|
Impact on daily practice Pulmonary ART can maximise efficacy and reduce complications in the treatment of APE. It can also provide a prompt clinical response in the acute setting with the limited time available for systemic thrombolysis. ART for the simultaneous treatment of APE and LEDVT can effectively prevent long-term sequelae of pulmonary hypertension and PTS and reduce the recurrence rate of VTE. With more studies coming up, ART should be considered as the first-line treatment for severe APE. |
Funding
This study was supported by the National Natural Science Foundation of China (grant number 81301328) and the Henan Provincial Medical Science and Technology Research Program (grant number 201602216).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

