Abstract
Background: Evidence supporting interventional pulmonary embolism (PE) treatment is needed.
Aims: We aimed to evaluate the acute safety and effectiveness of mechanical thrombectomy for intermediate- and high-risk PE in a large real-world population.
Methods: FLASH is a multicentre, prospective registry enrolling up to 1,000 US and European PE patients treated with mechanical thrombectomy using the FlowTriever System. The primary safety endpoint is a major adverse event composite including device-related death and major bleeding at 48 hours, and intraprocedural adverse events. Acute mortality and 48-hour outcomes are reported. Multivariate regression analysed characteristics associated with pulmonary artery pressure and dyspnoea improvement.
Results: Among 800 patients in the full US cohort, 76.7% had intermediate-high risk PE, 7.9% had high-risk PE, and 32.1% had thrombolytic contraindications. Major adverse events occurred in 1.8% of patients. All-cause mortality was 0.3% at 48-hour follow-up and 0.8% at 30-day follow-up, with no device-related deaths. Immediate haemodynamic improvements included a 7.6 mmHg mean drop in mean pulmonary artery pressure (–23.0%; p<0.0001) and a 0.3 L/min/m2 mean increase in cardiac index (18.9%; p<0.0001) in patients with depressed baseline values. Most patients (62.6%) had no overnight intensive care unit stay post-procedure. At 48 hours, the echocardiographic right ventricle/left ventricle ratio decreased from 1.23±0.36 to 0.98±0.31 (p<0.0001 for paired values) and patients with severe dyspnoea decreased from 66.5% to 15.6% (p<0.0001).
Conclusions: Mechanical thrombectomy with the FlowTriever System demonstrates a favourable safety profile, improvements in haemodynamics and functional outcomes, and low 30-day mortality for intermediate- and high-risk PE.
Introduction
Pulmonary embolism (PE) is a debilitating and potentially fatal disease. A recent US database study reported an overall in-hospital PE mortality rate of 6.5%, which remained stable from 2016 to 20191. The current European Society of Cardiology (ESC) guidelines2 recommend anticoagulation (AC) for intermediate-risk PE patients and systemic thrombolysis for high-risk PE patients. While thrombolytics have been associated with reduced mortality and haemodynamic decompensation compared with AC alone34, this treatment option comes with increased major bleeding risks and is not available to patients with corresponding contraindications25.
Catheter-directed treatment options that do not utilise thrombolytics may have lower associated bleeding risks while also limiting consequent intensive care unit (ICU) monitoring6. Furthermore, mechanical thrombectomy provides the opportunity for rapid relief of right ventricular strain and symptoms following thrombus extraction. Safety and effectiveness data from large-scale clinical studies are needed, however, to determine its role in routine acute PE management.
The FlowTriever System (Inari Medical) is a large-bore catheter-based mechanical thrombectomy device designed to remove thrombus from pulmonary arteries without utilising thrombolytics67891011. The FlowTriever All-Comer Registry for Patient Safety and Hemodynamics (FLASH) was established to evaluate the safety and effectiveness of the FlowTriever System in a real-world population of intermediate- or high-risk PE patients. The purpose of this analysis is to report acute outcomes for the fully enrolled US cohort of FLASH, including immediate changes in haemodynamics, in-hospital safety and effectiveness outcomes, and acute mortality through 30-day follow-up.
Methods
Study design
FLASH is an all-comer, prospective, multicentre registry evaluating real-world outcomes in up to 1,000 PE patients (800 in the US and up to 200 in Europe) treated with the FlowTriever System (ClinicalTrials.gov: NCT03761173). Details of the FLASH registry design have been previously reported11. Investigators obtained Institutional Review Board approval at each site prior to enrolling patients. All patients provided written informed consent, which was obtained before or shortly after the procedure. Key inclusion criteria included patients ≥18 years old with acute intermediate- or high-risk PE (per ESC guidelines2) who underwent mechanical thrombectomy at the discretion of the treating physician or local PE response team. Key exclusion criteria included patients unable to receive AC and those with a life expectancy <30 days (as determined by the investigator). A complete list of inclusion/exclusion criteria is provided in Supplementary Table 1. Follow-up assessments were conducted at 48 hours (±36 hours), 30 days (±15 days), and 6 months (±90 days). The results presented in this analysis include complete in-hospital safety and effectiveness outcomes and mortality outcomes at 30-day follow-up for the fully enrolled US cohort.
Thrombectomy procedure
The FlowTriever System comprises 2 main components: the Triever Catheter (24 Fr, 20 Fr, and 16 Fr), used for aspiration of thromboemboli, and the FlowTriever Catheter, used for mechanical thrombus dislodgement and removal. Thrombectomy is generally performed via femoral venous access. The Triever Catheter is advanced over a preplaced 0.035” guidewire across the right heart into the pulmonary arteries to the location of proximal thrombus. Procedural therapeutic AC with heparin is recommended. After removal of the dilator, the thrombus is extracted by controlled-volume aspiration using a 60 cc large-bore syringe, with multiple aspirations performed as needed. The FlowTriever Catheter may also be used for thrombus removal by deploying the self-expanding nitinol disks to engage the thrombus. A blood return system (FlowSaver; Inari Medical) was introduced in 2021 and was utilised in a limited number of patients in the later phase of enrolment. Several FlowTriever System design iterations occurred during the enrolment period, including improvements to ergonomics, flexibility, and tracking ability and the introduction of new devices into the toolkit, including various luminal diameters between 16 Fr and 24 Fr and a curved 20 Fr aspiration catheter. All procedures were performed with US Food and Drug Administration (FDA)-cleared components.
Investigators determined when to terminate the procedure based on their assessment of residual thrombus and patient status. Haemodynamic parameters, including pulmonary artery pressures, were evaluated pre- and post-thrombectomy per protocol to inform each operator’s decision to determine completion. The type and dosage of anticoagulant prescribed to each patient at discharge was determined by local practice.
Primary endpoint
The primary endpoint is a composite of major adverse events (MAE) within 48 hours of the index procedure, comprising device‐related death, major bleeding, and intraprocedural device- or procedure‐related adverse events. Major bleeding was defined as symptomatic bleeding in a critical area or organ, bleeding causing a haemoglobin drop of at least 5 g/dL, bleeding leading to transfusion of at least 2 units of blood products, or fatal bleeding, similar to Bleeding Academic Research Consortium (BARC) type 3b or greater12. Intraprocedural device- or procedure-related adverse events were specified as device-related pulmonary vascular injury, device-related cardiac injury, and clinical deterioration defined by haemodynamic or respiratory worsening meeting specific thresholds. Components of the composite safety endpoint and relatedness with the study device and/or procedure were adjudicated by an independent medical monitor.
Secondary endpoints
Secondary safety endpoints include the individual components of the composite primary endpoint, major access-site complications requiring open surgical repair, endovascular intervention, or blood transfusion, and device-related serious adverse events (SAE) as defined according to ISO 1415513.
Secondary effectiveness endpoints include haemodynamic improvements during the procedure, as described in the following section, and a reduction in the right ventricle (RV)/left ventricle (LV) ratio from baseline to follow-up as measured by echocardiography. Sites could use either computed tomography pulmonary angiography (CTPA) or echocardiography to assess the baseline RV/LV ratio, while follow-up assessments were typically performed with echocardiography per routine clinical practice and to reduce radiation and contrast exposure. The baseline RV/LV ratio is therefore presented as a composite of the modalities, with CTPA values prioritised if both methods were available. To eliminate bias from differences in imaging techniques, longitudinal analysis of the RV/LV ratio was exclusively based on paired echocardiography data. Additional echocardiographic assessments of RV size and function were performed at baseline and follow-up using each site’s standard practice, without specifying blinded readings.
Additional in-hospital measurements include thrombectomy time from the Triever Catheter’s entry into the vasculature until final removal, estimated blood loss, length of post-procedure hospital and ICU stay, and self-reported dyspnoea scores at baseline and 48 hours post-procedure using the modified Medical Research Council (mMRC) dyspnoea scale from 0 (breathless only on strenuous exercise) to 4 (too breathless to leave house, or breathless when dressing/undressing)14.
Haemodynamic calculations
Invasive haemodynamic assessment was performed per protocol via right heart catheterisation both before the Triever Catheter insertion and at least 5 minutes after the Triever Catheter removal. Haemodynamic variables measured pre- and post-thrombectomy include direct measurements of mean right atrial pressure, systolic pulmonary artery pressure (sPAP), and mean pulmonary artery pressure (mPAP), as well as derived measurements of cardiac index (CI) and total pulmonary vascular resistance (TPVR). CI was calculated as cardiac output (CO) divided by body surface area, where CO was estimated using the indirect Fick method. TPVR was calculated as mPAP divided by CO.
Data analysis
Data are presented as percentages for categorical data, and as mean with standard deviation or median with interquartile range (IQR) for continuous variables. The Wilcoxon signed-rank test, and McNemar’s or McNemar-Bowker’s tests were applied to test the changes from baseline for continuous and categorical outcomes, respectively, using available paired values. Multiple linear regression models were applied to assess the associations between baseline characteristics and mPAP reduction post-procedure and dyspnoea improvement at 48 hours. Analyses were performed using SAS 9.4 (SAS Institute) and R v4.1.2 (R Foundation for Statistical Computing)15.
Analysis of patients with severe pulmonary hypertension
A post hoc exploratory analysis was conducted for the subset of patients with severe pulmonary hypertension, as defined by prethrombectomy sPAP ≥70 mmHg16 measured invasively immediately prior to the procedure. This analysis was performed to assess haemodynamic changes and safety outcomes including MAE and mortality at 48-hour and 30-day follow-up in this patient population with higher risk for mortality and morbidity.
Results
Baseline characteristics and patient disposition
Between December 2018 and December 2021, 800 patients across 50 US sites were enrolled in the FLASH registry. This analysis included 799 patients, with 1 patient excluded post-enrolment for meeting 1 of the exclusion criteria in effect at the time (RV/LV ratio <0.9). The mean age was 61.2±14.6 years, and 54.0% of patients were male (Table 1). Risk stratification using ESC guidelines showed 7.9% of patients had high-risk (massive) PE, and the remaining 92.1% had intermediate-risk (submassive) PE, with most (83.2%) of those patients being categorised as intermediate-high risk. The baseline composite RV/LV ratio was 1.50±0.46. Nearly two-thirds (65.0%) had concomitant deep vein thrombosis (DVT) and the mean simplified Pulmonary Embolism Severity Index (sPESI) score was 1.6±1.1. Furthermore, 32.1% were determined by the treating physician to have either an absolute or relative contraindication to thrombolytic drugs (Supplementary Table 2). Additional baseline characteristics are included in Table 1.
At 30-day follow-up, patient status was known for 734 (91.9%) patients, 46 (5.8%) had withdrawn from the study, and 19 (2.4%) had unknown status due to missing data entry.
Table 1. Demographics and clinical presentation.
| Demographics and medical history | n (%) or mean±SD | N | ||
|---|---|---|---|---|
| Age, years | 61.2±14.6 | 797 | ||
| Male | 431 (54.0) | 798 | ||
| BMI, kg/m2 | 34.5±8.4 | 796 | ||
| Race* | American Indian or Alaskan Native | 3 (0.4) | 780 | |
| Asian | 5 (0.6) | 780 | ||
| Black or African American | 192 (24.6) | 780 | ||
| Native Hawaiian or Pacific Islander | 1 (0.1) | 780 | ||
| White | 571 (73.2) | 780 | ||
| Other | 9 (1.2) | 780 | ||
| Hispanic or Latino ethnicity | 18 (2.5) | 717 | ||
| History of DVT | 143 (17.9) | 797 | ||
| History of PE | 85 (10.7) | 798 | ||
| History of PHTN | 77 (9.7) | 793 | ||
| History of cancer | 165 (20.7) | 798 | ||
| Active cancer | 65 (8.1) | 798 | ||
| Clinical presentation | n (%) or mean±SD | N | ||
| Duration of current PE symptoms, days | 2.6±6.6 | 784 | ||
| Failed prior therapy for current PE† | 40 (5.2) | 771 | ||
| Anticoagulation | 36 (90.0) | 40 | ||
| Thrombolysis, systemic | 4 (10.0) | 40 | ||
| Thrombolysis, catheter-directed | 2 (5.0) | 40 | ||
| Other mechani cal thrombectomy | 3 (7.5) | 40 | ||
| Lytics contraindication | 256 (32.1) | 797 | ||
| Absolute | 31 (12.2) | 255 | ||
| Relative | 224 (87.8) | 255 | ||
| PE risk stratification | High-risk PE | 63 (7.9) | 797 | |
| Intermediate-risk PE | 734 (92.1) | 797 | ||
| Intermediate-high | 611 (83.2) | 734 | ||
| Intermediate-low | 59 (8.0) | 734 | ||
| Intermediate (not further classified) | 64 (8.7) | 734 | ||
| sPESI score | 1.6±1.1 | 751 | ||
| sPESI = 0 | 123 (16.4) | 751 | ||
| sPESI ≥1 | 628 (83.6) | 751 | ||
| PE location at screening | Saddle PE | 319 (40.0) | 798 | |
| Unilateral PE | 68 (8.5) | 798 | ||
| Bilateral PE | 411 (51.5) | 798 | ||
| Concomitant DVT | 512 (65.0) | 788 | ||
| Proximal only | 152 (29.9) | 508 | ||
| Distal only | 266 (52.4) | 508 | ||
| Both | 90 (17.7) | 508 | ||
| Positive biomarker(s)‡ | 720 (94.6) | 761 | ||
| RV/LV ratio (CTPA) | 1.56±0.47 | 624 | ||
| RV/LV ratio (echo) | 1.23±0.36 | 582 | ||
| RV/LV ratio (CTPA or echo composite§) | 1.50±0.46 | 756 | ||
| Elevated RV/LV ratio >0.9 | 734 (97.1) | 756 | ||
| Depressed cardiac index (<2.0 L/min/m2) | 176 (25.9) | 680 | ||
| Severe PHTN (sPAP ≥70 mmHg) | 99 (12.7) | 781 | ||
| *One patient was multiracial (Black and Native Hawaiian or Pacific Islander). †In some patients, more than one prior therapy was attempted. ‡Positive biomarkers include elevated brain natriuretic peptide and/or cardiac troponins. §Composite used either CTPA or echo measurements, with CTPA prioritised if both were available. BMI: body mass index; CTPA: computed tomography pulmonary angiography; DVT: deep vein thrombosis; echo: echocardiogram; LV: left ventricle; PE: pulmonary embolism; PHTN: pulmonary hypertension; RV: right ventricle; SD: standard deviation; sPAP: systolic pulmonary artery pressure; sPESI: simplified Pulmonary Embolism Severity Index | ||||
Procedural characteristics and hospital stay
Procedural characteristics are presented in Table 2. The majority of patients (86.6%) received local anaesthesia with sedation. Most access sites (99.5%) utilised the femoral or common femoral vein. There were no major access-site complications that met the secondary endpoint definition. The median thrombectomy time was 43 minutes (IQR: 29-62). Nineteen patients (2.4%) received adjunctive treatment for PE, 18 (2.3%) with catheter-directed thrombolysis (CDT) and 1 (0.1%) with an alternate percutaneous mechanical thrombectomy device. Post-procedure extracorporeal membrane oxygenation (ECMO) was used in 3 (0.4%) of patients. The median estimated blood loss per procedure was 225 mL (IQR: 95-400). The FlowSaver blood return system was used in 82 (10.3%) patients, following its availability in July 2021, with an estimated median blood loss of 100 mL (IQR: 50-200) in these patients compared with 250 mL (IQR: 100-400) in patients treated without FlowSaver. There were no escalations to open surgical thrombectomy. Following the procedure, 473 (62.6%) patients did not have an overnight ICU stay. Among the 283 who did, the median postprocedural ICU stay was 1 night (IQR: 1-2). The median length of post-procedure hospital stay was 3 nights (IQR: 2-5). Anticoagulants prescribed at 48-hour follow-up included a new/direct oral anticoagulant in 55.3% of patients, a vitamin K antagonist in 5.3% of patients, low molecular weight heparin in 14.3% of patients, unfractionated heparin in 30.0% of patients, and other anticoagulant agents in 2.1% of patients.
Table 2. Procedural characteristics.
| Characteristic | n (%) or median [IQR] | N | |
|---|---|---|---|
| Time from diagnosis to procedure, days | 0.82 [0.38–1.21] | 788 | |
| Femoral or common femoral vein access site | 794 (99.5) | 798* | |
| Ultrasound-assisted access site puncture | 702 (88.0) | 798* | |
| Access site closure | Stitching | 495 (62.2) | 796* |
| Closure device | 182 (22.9) | 796* | |
| Manual | 79 (9.9) | 796* | |
| Other | 40 (5.0) | 796* | |
| Major access site complications | 0 (0.0) | 788 | |
| Location of treated PE† | Central only | 234 (29.3) | 798 |
| Lobar only | 100 (12.5) | 798 | |
| Both | 464 (58.1) | 798 | |
| Anaesthesia‡ | General anaesthesia | 18 (2.3) | 799 |
| Local anaesthesia | 95 (11.9) | 799 | |
| Local anaesthesia with sedation | 692 (86.6) | 799 | |
| Spinal anaesthesia | 1 (0.1) | 799 | |
| Thrombectomy time, min | 43.0 [29.0–62.0] | 753 | |
| Total procedure time, min | 66.0 [51.0–92.0] | 757 | |
| Use of FlowSaver blood return system | 82 (10.3) | 799 | |
| Estimated blood loss, mL | 225.0 [95.0–400.0] | 721 | |
| With FlowSaver blood return, mL | 100.0 [50.0–200.0] | 79 | |
| Without FlowSaver blood return, mL | 250.0 [100.0–400.0] | 642 | |
| Activated clotting time, seconds, preprocedure | 179.0 [150.0–230.5] | 652 | |
| Activated clotting time, seconds, post-procedure | 241.0 [213.0–272.0] | 607 | |
| Post-procedure ECMO use | 3 (0.4) | 799 | |
| Adjunctive PE therapy | 19 (2.4) | 799 | |
| Catheter-directed thrombolysis | 18 (2.3) | 799 | |
| Other mechanical thrombectomy | 1 (0.1) | 799 | |
| Hospital overnights post-procedure | 3.0 [2.0–5.0] | 745 | |
| ICU overnights post-procedure | 0.0 [0.0–1.0] | 756 | |
| No overnight ICU stay post-procedure | 473 (62.6) | 756 | |
| One overnight ICU stay post-procedure | 153 (20.2) | 756 | |
| Site-reported reason for ICU stay | Standard of care | 161 (57.3) | 281 |
| Need for additional care§ | 120 (42.7) | 281 | |
| *Access-site sample size is the total number of access sites. †Central, defined as main PA, left main PA, and/or right main PA, or lobar, defined as left lobar PA and/or right lobar PA, as reported by treating physician. ‡More than one type of anaesthesia may have been used. §Reasons included adjunctive thrombolysis monitoring, ongoing critical care for an unrelated condition, and haemodynamic instability. ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; IQR: interquartile range; PA: pulmonary artery; PE: pulmonary embolism | |||
Mortality and safety outcomes
Acute mortality outcomes are shown in Table 3. There were no intraprocedural deaths. There were 2 deaths at 48-hour follow-up (0.3% all-cause mortality), which were each adjudicated as unrelated to the study device. One intermediate-risk PE patient died due to cardiopulmonary arrest on the second day post-thrombectomy. The patient experienced haemodynamic decline on the second day, became pulseless, and was unable to be resuscitated. The second death occurred in an intermediate-risk PE patient who experienced a new PE 1 day after thrombectomy and expired. There were an additional 4 deaths at 30-day follow-up (0.8% all-cause mortality), all adjudicated as unrelated to the study device. These 4 deaths were due to pre-existing cancer, septic shock secondary to pneumonia, septic shock secondary to an ischaemic bowel, and deterioration during orthopaedic surgery that involved a new PE and cardiac arrest. The 30-day all-cause readmission rate was 6.2% (1.4% related to PE treatment and 4.8% unrelated).
There were 14 MAE (1.8%) at 48 hours (Table 3), including 11 major bleeds (1.4%) and 3 intraprocedural MAE (0.4%). Two intraprocedural events were clinical deteriorations (one from cardiac injury due to extracorporeal membrane oxygenation and the other from hypotension). The third intraprocedural event was a cardiac injury involving the tricuspid valve. While no injury to the valve was evident during the procedure, tricuspid regurgitation was noted by echocardiography 2 months post-procedure, though it was unknown whether this was related to the study device. None of the MAE were adjudicated by the independent medical monitor to have a confirmed relationship with the study device. Eight of the 11 major bleeds involved a haemoglobin drop ≥5.0 g/dL between screening and 48-hour follow-up; none of these had utilised the FlowSaver blood return system during thrombectomy. Of the remaining major bleeds, one involved a retroperitoneal haematoma, one involved transfusion of 2 units of packed red blood cells, and the final major bleed involved transfusion of 7 units of packed red blood cells following placement of a bilateral percutaneous nephrostomy tube 2 days post-index procedure. No major bleed involved intracranial haemorrhage.
A total of 35 SAE in 34 (4.3%) patients were reported over the first 48 hours, none of which were adjudicated as related to the study device. The SAE types are listed in Supplementary Table 3.
Table 3. Safety and mortality outcomes.
| Safety outcomes at 48 hours | % (n/N) |
|---|---|
| Major adverse event composite | 1.8 (14/788) |
| Device-related death | 0.0 (0/788) |
| Major bleeding | 1.4 (11/788) |
| Intraprocedural device- or procedure-related AE | 0.4 (3/788) |
| Clinical deterioration | 0.3 (2/788) |
| Pulmonary vascular injuries | 0.0 (0/788) |
| Cardiac injuries | 0.1 (1/788) |
| Serious adverse event | 4.3 (34/791) |
| All-cause mortality | % (n/N) |
| At 48-hour follow-up | 0.3 (2/794) |
| At 30-day follow-up | 0.8 (6/734) |
| AE: adverse event | |
Immediate haemodynamic outcomes
Haemodynamic and vital outcomes are summarised in Table 4. Immediately following thrombectomy, the mPAP decreased from 32.6±9.0 to 24.9±8.9 mmHg (–7.6 mmHg mean change [–23.0%]; p<0.0001). The change in distribution of patients with a normal (<25 mmHg), mildly, moderately, or severely elevated mPAP from pre- to post-procedure is shown in Figure 1, with a significant increase in the proportion of patients with a normal mPAP following thrombectomy (p<0.0001). The sPAP also decreased on-table from 53.2±14.5 to 40.4±14.1 mmHg (–12.8 mmHg mean change [–23.4%]; p<0.0001). The pre-thrombectomy CI was depressed (<2.0 L/min/m2) in 25.9% of patients. Immediately following thrombectomy, the CI increased in these patients from 1.64±0.26 to 1.93±0.58 L/min/m2 (0.29 L/min/m2 mean change [18.9%]; p<0.0001). Immediately following thrombectomy, the TPVR decreased from 6.65±3.21 to 4.99±2.76 mmHg·min/L (–1.67 mmHg·min/L mean change [–20.1%]; p<0.0001) and heart rate decreased from 101.5±16.3 to 89.5±16.9 bpm (–12.0 bpm mean change [–11.2%]; p<0.0001). There were no significant differences in haemodynamic improvements for patients with high-risk PE versus those with intermediate-risk PE.
Table 4. Immediate changes in haemodynamics and vitals following thrombectomy.
| Haemodynamic/vital value | Preprocedure (mean±SD) | Post-procedure (mean±SD) | Mean change (%) | p-value |
|---|---|---|---|---|
| mPAP, mmHg | 32.6±9.0 n=779 | 24.9±8.9 n=767 | −7.6 (−23.0) n=767 | <0.0001 |
| sPAP, mmHg | 53.2±14.5 n=781 | 40.4±14.1 n=769 | −12.8 (−23.4) n=769 | <0.0001 |
| Baseline sPAP ≥70mmHg | 78.9±12.1 n=99 | 60.9±15.2 n=97 | −18.1 (−22.4) n=97 | <0.0001 |
| Mean right atrial pressure, mmHg | 11.6±5.8 n=726 | 9.0±5.4 n=657 | −2.6 (−16.9) n=657 | <0.0001 |
| Heart rate, bpm | 101.5±16.3 n=798 | 89.5±16.9 n=779 | −12.0 (−11.2) n=778 | <0.0001 |
| CI, L/min/m2 | 2.56±0.82 n=680 | 2.56±0.82 n=614 | 0.02 (4.4) n=614 | 0.5819 |
| Baseline CI <2.0 L/min/m2 | 1.64±0.26 n=176 | 1.93±0.58 n=159 | 0.29 (18.9) n=159 | <0.0001 |
| TPVR, mmHg·min/L | 6.65±3.21 n=683 | 4.99±2.76 n=621 | −1.67 (−20.1) n=621 | <0.0001 |
| p-values are based on available paired assessments using Wilcoxon signed-rank tests. bpm: beats per minute; CI: cardiac index; mPAP: mean pulmonary artery pressure; PA: pulmonary artery; SD: standard deviation; sPAP: systolic pulmonary artery pressure; TPVR; total pulmonary vascular resistance | ||||
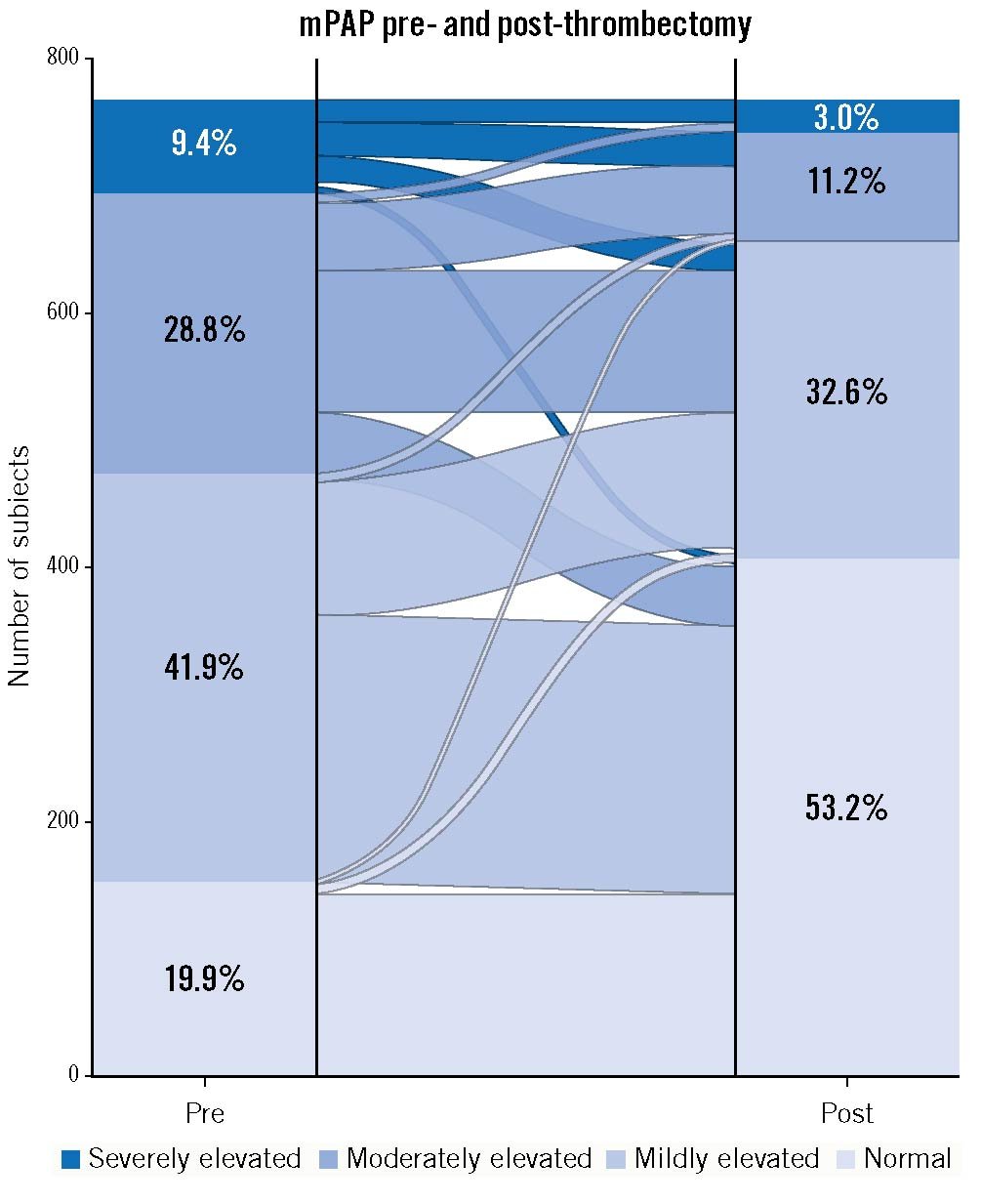
Figure 1. On-table improvements in mean pulmonary artery pressure. Alluvial graph of the number and proportion of patients with normal (<25 mmHg), mildly elevated (≥25 and <35 mmHg), moderately elevated (≥35 and <45 mmHg), and severely elevated mPAP (≥45 mmHg) pre- and post-thrombectomy, showing a significant change (p<0.0001). The lines flowing between the columns indicate the movement of patients from their prethrombectomy mPAP category to their post-thrombectomy category, with the width of the lines proportional to the number of patients. mPAP: mean pulmonary artery pressure
Outcomes in patients with severe pulmonary hypertension
Ninety-nine (12.7%) patients presented with severe pulmonary hypertension as defined by an sPAP ≥70 mmHg16. Immediately following thrombectomy, the sPAP decreased in this subset from 78.9±12.1 to 60.9±15.2 mmHg (–18.1 mmHg mean change [–22.4%]; p<0.0001) as shown in Table 4. Three MAE (3.1%) occurred in this patient subset, including 2 major bleeds and 1 intraprocedural cardiac injury with an unknown relationship to the study device. There was a single death in this patient subset through 30-day follow-up.
RV function and dyspnoea outcomes
RV echocardiographic outcomes are shown in Figure 2A-Figure 2C. At 48 hours, the echocardiographic RV/LV ratio decreased from 1.23±0.36 to 0.98±0.31 (p<0.0001 for paired values), and RV systolic pressure decreased from 48.9±14.9 to 38.8±14.8 mmHg (–13.4 mmHg mean change [–22.9%]; p<0.0001). RV function also improved significantly from baseline to 48 hours, with the proportion of patients with severe dysfunction decreasing from 29.0% to 4.7%, and the proportion with no or mild dysfunction increasing from 33.9% to 74.5% (p<0.0001).
As shown in Figure 2D, dyspnoea significantly improved following thrombectomy, with the mMRC score improving from 2.7±1.4 at baseline to 1.1±1.2 at 48 hours (–1.7 point mean change [–61.2%]; p<0.0001). The proportion of patients with severe dyspnoea (mMRC score 3 or 4) decreased from 66.5% at baseline to 15.6% at 48 hours (p<0.0001). Similarly, supplemental oxygen requirements improved for 87.4% of patients by 48 hours (e.g., improving from nasal cannula to room air or reducing litre-per-minute requirements) (Figure 2E), and the proportion of patients on room air increased significantly from 10.5% prethrombectomy to 71.2% at 48 hours (p<0.0001) (Figure 2F). Key effectiveness outcomes are summarised along with safety outcomes in the Central illustration.
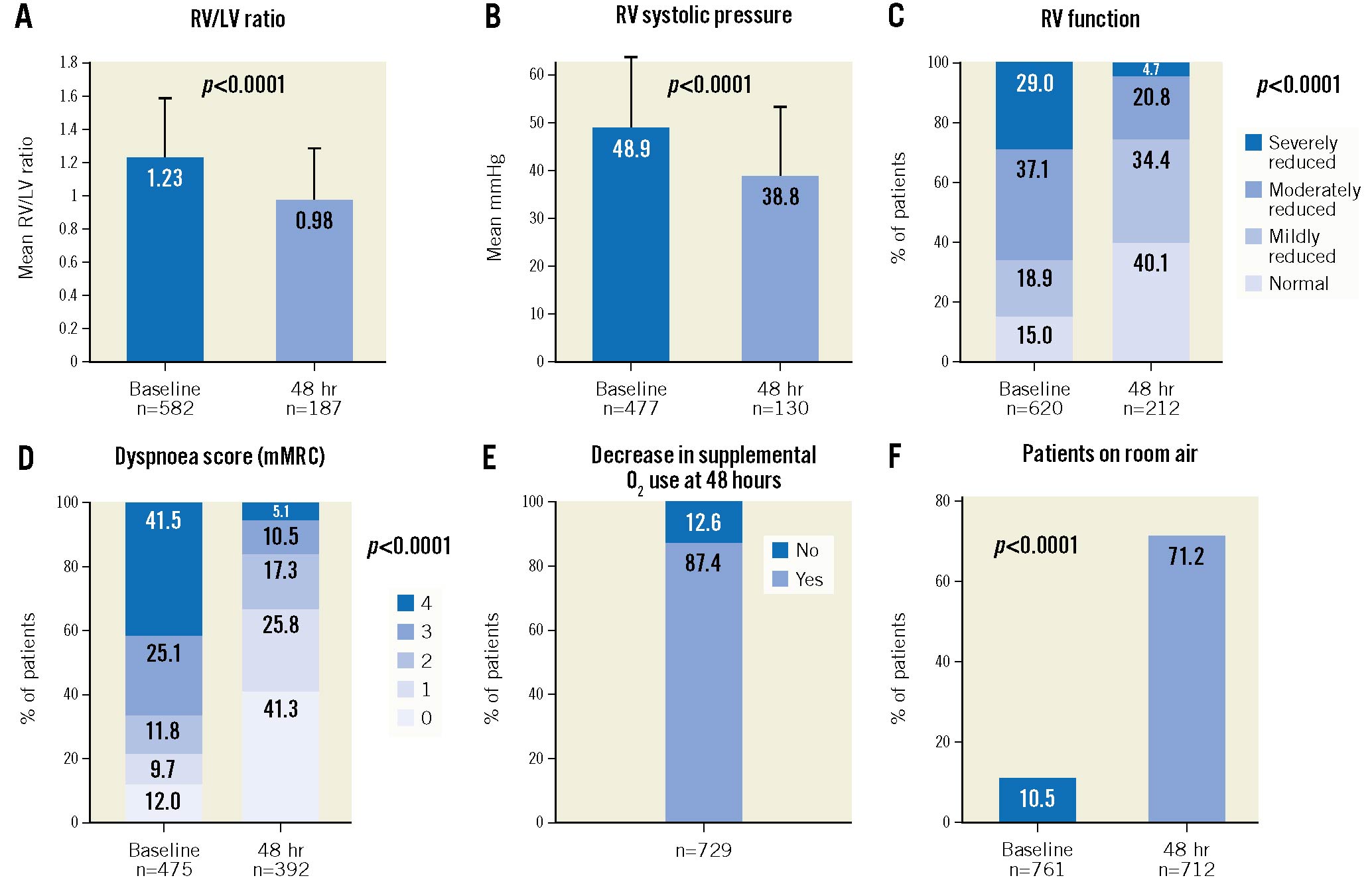
Figure 2. Right ventricular echocardiographic parameters, dyspnoea, and supplemental oxygen use at baseline compared to 48 hours post-thrombectomy. A) Change in RV/LV ratio (p<0.0001 for available paired assessments; McNemar’s test). B) Change in RV systolic pressure (p<0.0001 for available paired assessments; McNemar’s test). C) Change in the distribution of patients’ RV function (p<0.0001 for available paired assessments; McNemar-Bowker’s test). D) Dyspnoea was assessed at baseline and at 48 hours using the modified Medical Research Council (mMRC) assessment tool (higher score=worse dyspnoea). The proportion of patients with each score (0-4) is presented, showing a significant change in score distribution (p<0.0001 for available paired assessments; McNemar-Bowker’s test). E) The proportion of patients whose use of supplemental oxygen decreased from baseline to 48 hours is presented. A decrease was defined as a reduction in the oxygen volume used, or in the type of supplemental oxygen required (types of supplemental oxygen were ranked as a reduction if a patient moved from one type to another type in order as follows: intubation, face mask, nasal cannula, room air). F) The proportion of patients on room air at baseline and 48 hours post-thrombectomy is presented (p<0.0001 for paired assessments; McNemar’s test). hr: hours; LV: left ventricle; RV: right ventricle
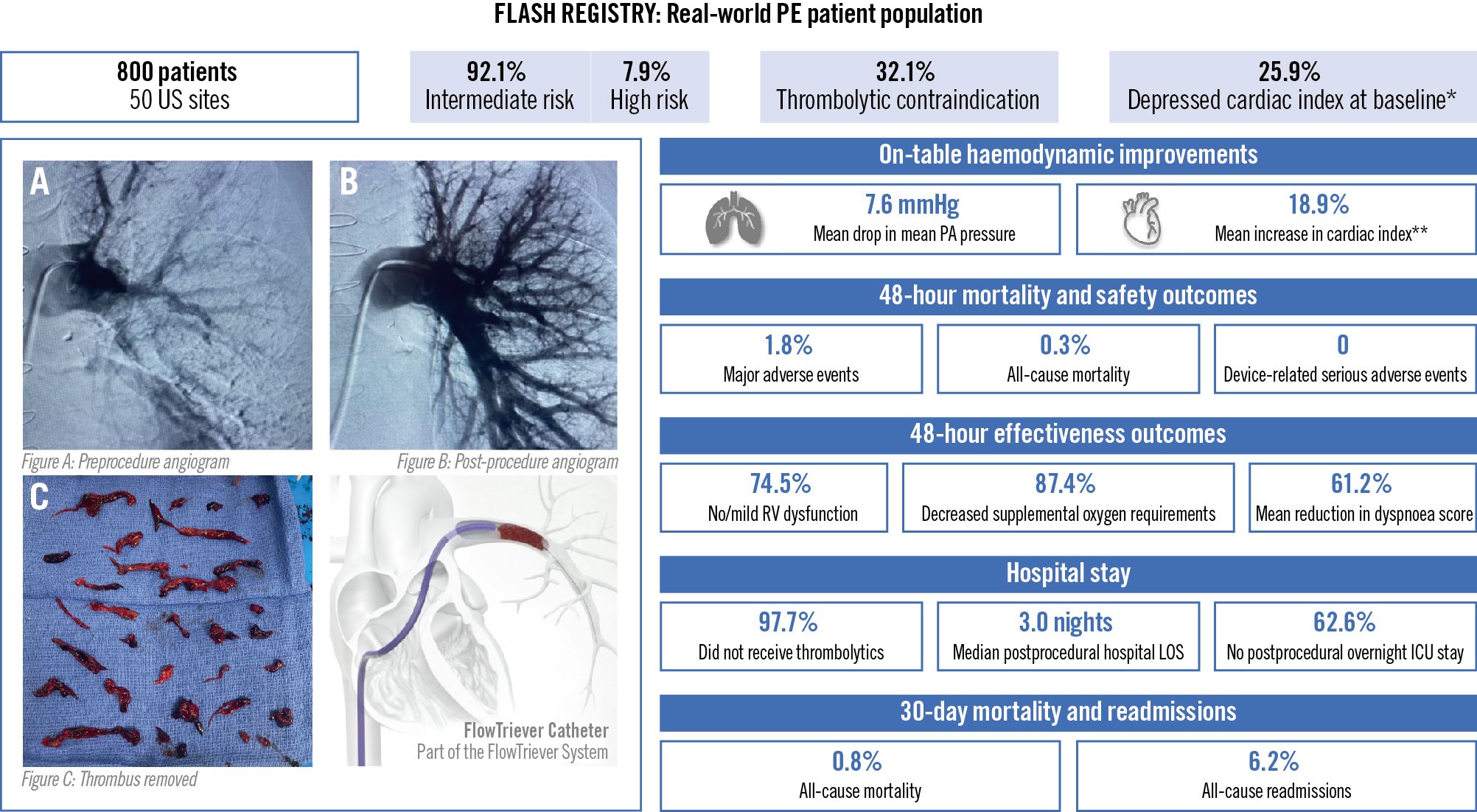
Central illustration. Acute outcomes of the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. Baseline characteristics, in-hospital safety and effectiveness outcomes, and acute mortality for the fully enrolled US cohort of the FLASH registry in pulmonary embolism. Between December 2018 and December 2021, 800 patients with acute pulmonary embolism were enrolled across 50 US sites in the FLASH registry for mechanical thrombectomy using the FlowTriever System. Patient characteristics, immediate changes in haemodynamics, in-hospital safety outcomes including major adverse events, effectiveness outcomes including RV function recovery and dyspnoea symptom improvement, hospital stay details, and all-cause mortality and readmissions through 30-day follow-up are reported. Images provided by Dr. Michael Brown, Missouri Cardiovascular Specialists, Columbia, MO, USA. *Depressed cardiac index is defined as <2 L/min/m². **In patients with depressed cardiac index at baseline. ICU: intensive care unit; LOS: length of stay; PA: pulmonary artery; PE: pulmonary embolism; RV: right ventricle
Baseline characteristics associated with a reduction in mPAP and dyspnoea score
Results from multiple linear regression modelling (Figure 3) indicate that concomitant DVT involving both proximal and distal veins was associated with a greater mPAP reduction (p<0.001) compared to patients without concomitant DVT. PE location also affected mPAP reduction following thrombectomy. Compared with unilateral PE, bilateral PE or saddle PE were both associated with a greater mPAP reduction (p=0.027 and p=0.010, respectively). In contrast, a prethrombectomy sPAP ≥70 mmHg and higher body mass index were associated with a more modest reduction in mPAP following thrombectomy (p<0.001 and p=0.008, respectively). Furthermore, a longer duration of PE symptoms prior to thrombectomy was associated with a more modest reduction in the dyspnoea score at 48 hours (p=0.007).
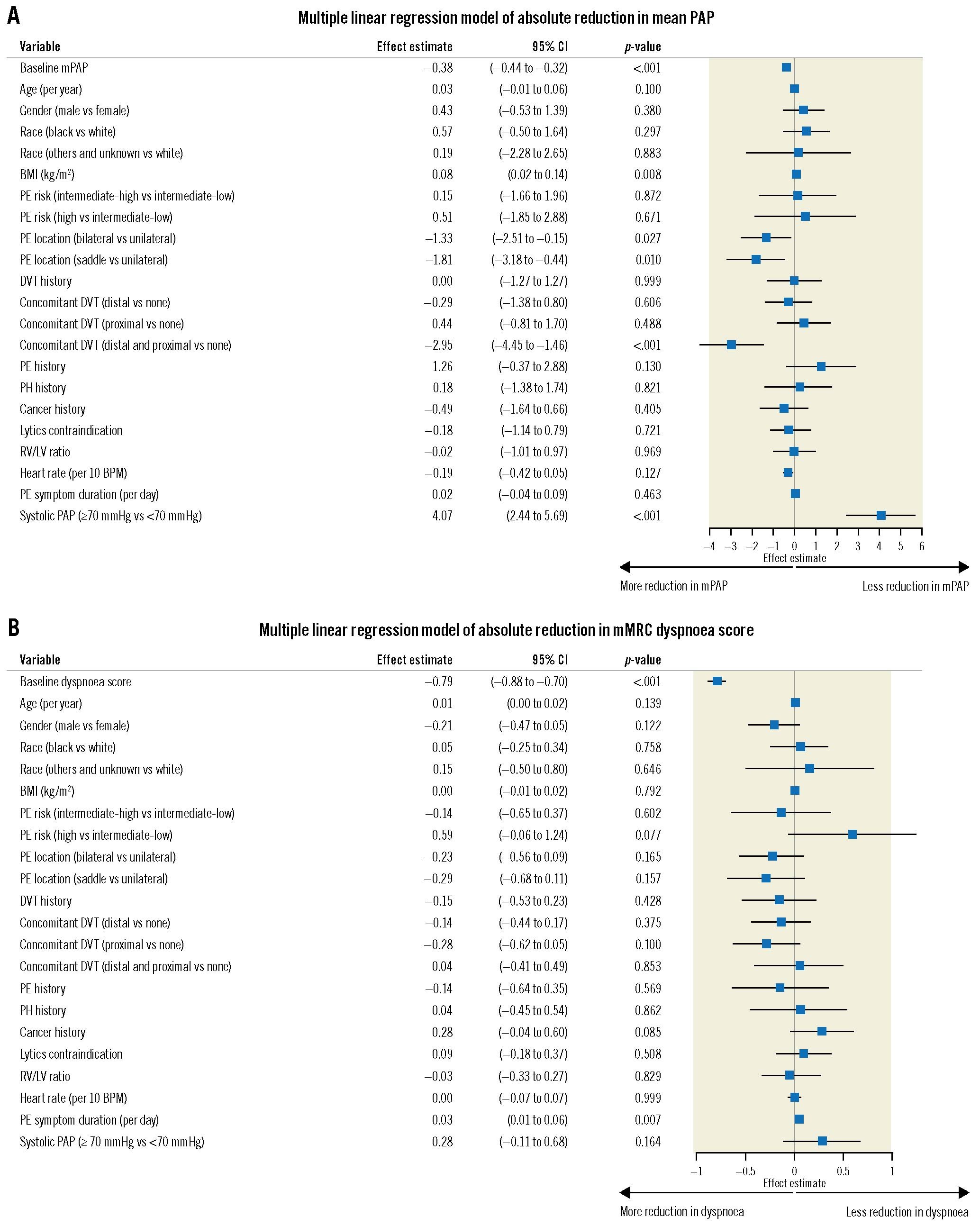
Figure 3. Multiple linear regression models of absolute reduction in mean PAP and dyspnoea score. A) Multiple linear regression was performed to identify baseline characteristics associated with absolute reduction in mPAP measured immediately prior to and following thrombectomy. B) Multiple linear regression was performed to identify baseline characteristics associated with absolute reduction in self-reported mMRC dyspnoea score measured at baseline and 48 hours post-procedure. BMI: body mass index; BPM: beats per minute; CI: confidence interval; DVT: deep vein thrombosis; LV: left ventricle; mMRC: modified Medical Research Council; mPAP: mean pulmonary artery pressure; PAP: pulmonary artery pressure; PE: pulmonary embolism; PH: pulmonary hypertension; RV: right ventricle
Discussion
This is the largest prospective study of an interventional treatment for pulmonary embolism to date, with 800 patients enrolled across 50 centres. The favourable safety profile of the FlowTriever System in this large cohort is highlighted by a 1.8% MAE rate despite an all-comer patient population with significant comorbidities. Furthermore, procedural effectiveness was broadly demonstrated across 50 centres including a mix of interventional specialties, with significant improvements observed in patient haemodynamics, cardiac function, and dyspnoea symptoms. These results reaffirm the earlier findings reported from the FLARE study6 and a recent interim analysis of FLASH11.
Societal guidelines for the treatment of PE recommend AC for the majority of intermediate-risk patients due to a lack of data showing benefit of catheter-based therapies over conservative medical management. In contemporary clinical practice, treating physicians must weigh the risk-benefit balance of achieving reperfusion via advanced therapy, which historically was dependent on thrombolytics, versus the bleeding risks associated with thrombolytic drugs. In this study, the primary endpoint of MAE through 48 hours was reached in only 1.8% of patients and there were no device-related SAE through 48 hours. Even patients with severe pulmonary hypertension, who have historically been excluded from PE studies due to their higher risk profile61718, had favourable safety outcomes with a low 3.1% MAE rate and a single death through 30-day follow-up. These results are encouraging, as they suggest that mechanical thrombectomy may shift the risk-benefit calculation for physicians towards intervention for PE management, though further studies are needed to confirm this.
An important advantage of mechanical thrombectomy resides in its applicability to high bleeding risk patients as well. Systemic thrombolysis carries a clear risk of major bleeding31920, which is only partially mitigated by local catheter-based administration of the drug42122. The SEATTLE II study of the EKOS ultrasonic thrombolysis catheter (Boston Scientific) demonstrated significant improvements in the RV/LV ratio and sPAP, but patients also experienced a 10% major bleed rate18. Many PE patients have a high bleeding risk23 and are therefore excluded from receiving these therapies, limiting the study populations of these treatment options to only those without thrombolytic contraindications. In this study, 32.1% of patients had an absolute or relative contraindication to thrombolytics, yet the major bleeding rate remained low at 1.4% with most events qualifying based on haemoglobin drop. This may be related, at least in part, to large-volume aspiration, which is now routinely compensated by use of a blood return system.
Mechanical thrombectomy with the FlowTriever System led to an immediate improvement in haemodynamic parameters, including a decrease in PA pressures, an increase in CI, and a drop in TPVR (which probably best reflects the degree of vascular obstruction and RV afterload). CDT treatment was used as an adjunctive therapy in 2.3% of the FLASH cases, which were performed immediately following the thrombectomy at the discretion of the treating physician, for example, when this was part of local practice or when the thrombectomy result was deemed incomplete, with the majority of these cases (77.8%) being limited to 4 sites. The 12.8 mmHg mean on-table reduction in sPAP compares favourably to the EXTRACT-PE study of the Indigo aspiration system (Penumbra), which showed a 4.3 mmHg mean on-table reduction in sPAP17. While CDT studies have also reported significant haemodynamic improvements24, these treatments require hours to days to achieve thrombus resolution. In contrast, the haemodynamic benefits from FlowTriever treatment were evident while the patient was still in the procedure room. The rapid thrombus extraction and the lack of thrombolytic usage led to minimal ICU usage in this study, as nearly two-thirds of patients did not spend a night in the ICU following thrombectomy. Recent experience with the COVID-19 pandemic has highlighted the critical constraints on ICU bed availability25, which, coupled with rising pressures for more efficient hospital resource utilisation, create a tangible advantage for more rapid PE resolution without the need for an ICU stay.
It is plausible that the rapid reperfusion possible with mechanical thrombectomy may change the course of the disease and potentially impact its mortality. Despite the inclusion of 63 high-risk PE patients, a patient population with a reported all-cause mortality in other studies of 27-35% at 30 days2627, the all-cause mortality in this all-comer cohort was only 0.3% at 48-hour follow-up and 0.8% at 30-day follow-up. In comparison, a recent meta-analysis of over 9,000 mostly intermediate-risk PE patients from 12 studies demonstrated an in-hospital mortality rate of 6.3% and a 30-day mortality rate of 10.0% for patients receiving AC only4. Other recent studies reporting data from single-centre Pulmonary Embolism Response Team (PERT) databases with patient populations receiving either AC or advanced therapies show in-hospital mortality rates of 6.4%28 and 8.3%26 and 30-day rates of 12.8%28 and 11.8%26. A recent single-centre retrospective study directly compared outcomes of intermediate-high and high-risk PE patients treated with either FlowTriever mechanical thrombectomy or routine care, showing significantly lower in-hospital mortality in patients treated with mechanical thrombectomy (3.6% vs 23.3%, respectively; p<0.05)10. Although comparisons across studies are difficult, the key message from FLASH is that mortality and procedure-related complications were low in this registry, underscoring the excellent safety profile of this procedure.
Identifying patients most likely to benefit from advanced therapy may guide better outcomes and more efficient resource utilisation. The large size of the FLASH registry allowed for initial exploration into some of these factors for mechanical thrombectomy. Since only 2 in-hospital deaths occurred in this current study, it was impractical to assess the associations between baseline characteristics and mortality risk. Larger proximal clot burden, presence of both proximal and distal DVT (possibly another marker of acute disease), and shorter duration of symptoms were associated with better short-term surrogate outcomes, including mPAP reduction and dyspnoea improvement. Importantly, markers of disease severity such as the RV/LV ratio did not correlate with response, in contrast with prior analyses of CDT29. A post hoc analysis of the SEATTLE-II study found that patients with higher baseline heart rates experienced less improvement in both the RV/LV ratio and sPAP, and those with hepatic or renal insufficiency also had less sPAP improvement following ultrasound-facilitated CDT29.
Limitations
There are certain limitations to this analysis. The FLASH registry is a single-arm study, so direct comparisons to other treatment options cannot be made. However, the ongoing PEERLESS randomised controlled trial (ClinicalTrials.gov: NCT05111613) will provide the first definitive evidence directly comparing 2 interventional approaches, mechanical thrombectomy with the FlowTriever System and CDT, for the treatment of intermediate-high risk PE. The patient population reported in this study was all-comer, based on patients chosen by each treating physician to be good candidates for mechanical thrombectomy, which therefore may limit the applicability of the results to the general PE population. Another limitation is the bias that may have been introduced due to the allowance of post-procedure consenting, which provided flexibility to participating sites to capture as many of their patients treated with the FlowTriever System in the study as possible, especially due to the emergent nature of some PE patients and the effects of COVID-19 on clinical research. In addition, as with most registries, a detailed treatment protocol was not specified. As a result, procedures and lab testing were not standardised, so procedural and outcome variability will likely be higher compared to an investigational trial with formally prescribed procedural requirements. Finally, due to the effects of COVID-19 on clinical research and the fact that the 48-hour echocardiogram was not required at follow-up throughout a portion of the study enrolment period, some follow-up assessments were only available in a subset of patients.
Conclusions
The results from this largest prospective interventional dataset in PE highlight the robust safety profile of the FlowTriever System, with low rates of MAE (1.8%) and all-cause mortality at 48-hour and 30-day follow-up (0.3% and 0.8%, respectively) that compare favourably to previous studies of intermediate- and high-risk PE patients. Immediate improvements in haemodynamic parameters were observed following thrombectomy. Furthermore, within 48 hours, patients experienced a significant reduction in dyspnoea symptoms, supplemental oxygen needs, and physiologic indicators of RV strain. Longer-term follow-up through to 6 months in addition to geographic expansion to European centres should further enrich the strength and broad application of these data.
Impact on daily practice
Guidelines for the treatment of pulmonary embolism (PE) reserve advanced interventions, including catheter-based therapy, for patients who decompensate and/or have absolute contraindications to thrombolytics. In the largest prospective interventional study in the field of PE, mechanical thrombectomy with the FlowTriever System showed that patients with intermediate- and high-risk PE can be safely treated (MAE rate of 1.8%) and derive immediate benefits in haemodynamics, vitals, and symptoms. The strong safety profile of the procedure, effective thrombus removal, lack of bleeding concerns, and low mortality rates suggest that mechanical thrombectomy may shift the risk-benefit balance towards a more aggressive treatment algorithm for PE patients.
Acknowledgements
The authors acknowledge all FLASH principal investigators who enrolled patients in the US cohort (Supplementary Table 4). The authors acknowledge biostatistical support from Yu-Hsiang Shu, PhD, and writing assistance from Linda Hansen, PhD, and Jessica Parsons, PhD.
Funding
This study was funded by Inari Medical, which was involved in the design of the study and in the collection, analysis, and interpretation of the data in collaboration with the Principal Investigators.
Conflict of interest statement
C. Toma is a consultant to Medtronic and Philips. W.A. Jaber is a consultant to Inari Medical. M.D. Weinberg is a consultant to Boston Scientific, Magneto Thrombectomy Solutions, and Medtronic. M.C. Bunte receives institutional grant support from Inari Medical and is a consultant to Inari Medical, Abbott Labs, and Shockwave Medical. B. Stegman is a consultant to Edwards Lifesciences, Medtronic, Boston Scientific, and Forge Medical. R. Amin is a consultant to Inari Medical. H. Kado is a consultant to Inari Medical. M.A. Brown is a speaker for Inari Medical. M. Savin reports owning Inari Medical stock. J.M. Horowitz is a consultant to Inari Medical and Penumbra. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

