Abstract
Aims: Pulmonary embolism (PE) associated with haemodynamic instability has exceedingly high mortality. While intravenous thrombolysis is considered the therapy of choice, percutaneous mechanical thrombectomy may represent an alternative treatment.
Methods and results: The impact of AngioJet® rheolytic thrombectomy (RT) in PE associated with cardiogenic shock was assessed in a single-centre prospective pilot study. Ten consecutive PE patients in cardiogenic shock were included in the study. Six patients had thrombolysis contraindications, eight were intubated before the RT procedure and six had experienced cardiac arrest prior to the RT procedure. The RT procedure was technically successful in all cases. The Miller index improved from 25 to 20 (p=0.002). The shock index decreased from 1.22 to 0.9 (p=0.129). Thrombolytic agents were administered during or after the procedure in four patients because of progressive clinical deterioration. Seven patients died in the first 24 hours: two from multi-organ failure, one from post-anoxic cerebral oedema, and four from progressive right heart failure. The three survivors had favourable outcomes at one year.
Conclusions: This study suggests that the AngioJet® RT procedure may be safely performed in PE patients with cardiogenic shock. However, despite angiographic and haemodynamic improvements, the procedure does not appear to influence the dismal prognosis of these high-risk patients.
Introduction
Pulmonary embolism (PE) is a major cause of mortality, accounting for more than 300,000 deaths worldwide every year1,2. The short-term mortality associated with pulmonary embolism (up to 11%) occurs typically in the first few hours after symptom onset and this rate may further increase to 17% at three months3,4. In patients presenting with PE and cardiogenic shock the in-hospital mortality may be as high as 60%3,5.
Recently, European and American guidelines stratified patients presenting with PE into several categories according to the initial clinical presentation as well as haemodynamic status. Accordingly, high-risk or massive PE implies a haemodynamic instability, defined as systolic hypotension (i.e., systolic blood pressure <90 mmHg) or a >40 mmHg decrease of the baseline systolic blood pressure or requiring inotropic support6,7. Treatment modalities vary widely among the different PE categories, ranging from anticoagulation alone to systemic intravenous (IV) thrombolysis as well as mechanical thrombectomy, either percutaneous or surgical5-8.
Percutaneous mechanical thrombectomy (PMT) procedures are particularly attractive, considering that up to 40% of patients presenting with high-risk PE are either too unstable to undergo emergency surgical embolectomy or may present absolute or relative contraindications to fibrinolysis6,7,9,10. So far, the available data on PMT have been limited to several retrospective and a few prospective series that included patients with different degrees of haemodynamic instability, as well as different types of PMT devices. Consequently, it is difficult to establish which technique or device may be considered the most suitable when treating high-risk PE patients1,11-26.
We have hypothesised that the use of a percutaneous rheolytic thrombectomy (RT) procedure might also be safely performed in patients presenting with high-risk PE and cardiogenic shock as it might improve haemodynamics while decreasing the need for a systemic IV thrombolytic. Accordingly, we have conducted a prospective pilot study evaluating the use of a RT procedure in this highly unstable setting. This pilot study (NCT00780767) was mainly aimed at evaluating the feasibility and safety of the percutaneous RT procedure in patients presenting with high-risk PE and cardiogenic shock.
Material and methods
All patients presenting with a high-risk PE were considered for the study protocol. All patients received IV heparin immediately after PE was suspected. In order to confirm the diagnosis, patients underwent a CT scan, if the haemodynamic status allowed the transfer to the CT scan and the administration of IV contrast medium, or at least a transthoracic echocardiogram (TTE). The echocardiography study had to confirm, or at least be highly suggestive of, PE diagnosis (i.e., right ventricular [RV] dilatation±dysfunction). All consecutive patients remaining in cardiogenic shock (i.e., with a shock index [SI] >1) despite resuscitation measures (fluids, catecholamines) were included in the study.
Very unstable patients (e.g., those in cardiac arrest), where the time delays to transport them to the catheterisation laboratory were judged too long, were excluded from the study in order to allow the immediate administration of IV thrombolysis. Patients presenting with non-high-risk PE, or those responding adequately to the initial fluid resuscitation, were excluded. Finally, patients with a life expectancy of less than three months for other pre-existing medical conditions were also excluded from the study. The primary endpoints of the study were the feasibility (defined as number of successful RT/number of attempted RT) and the safety (defined as the absence of major device-related or procedure-related complications) of the procedure. Secondary endpoints were the improvement of clinical (i.e., NYHA dyspnoea class), haemodynamic (i.e., SI) and angiographic (i.e., Miller index) parameters.
All patients gave signed informed consent to participate in the study. If the clinical condition did not allow the patient to consent (e.g., patient intubated), a family member was asked to sign the consent. In situations where the time delay to obtain the consent from a family member was judged not acceptable, a physician not involved in the study (for the most part a senior physician of the emergency department) gave written informed consent before the RT procedure.
Since, according to the intrinsic logistics of our hospital, the time delay between the PE diagnosis and the transfer to the catheterisation laboratory does not exceed 30 minutes (day and night time), the protocol discouraged the use of thrombolysis as first line treatment. This approach was favoured in order to include all consecutive patients presenting with a high-risk PE and cardiogenic shock. However, it should be stressed here that one of the few exclusion criteria of the study was the imminent death of the patient (i.e., prolonged or recurrent cardiac arrest despite the adopted aggressive drug support), which would have mandated the administration of IV thrombolysis immediately, without any further transfer delay.
Additionally, in case of clinical deterioration despite the RT (i.e., aggravation of the SI ± increase of the catecholamine drug support), the study protocol allowed the use of full dose fibrinolytic treatment as well as other pharmacomechanical interventions (e.g., surgical embolectomy) immediately or in the first hours after the procedure. This “escalation” therapy was always proposed exclusively on clinical and haemodynamic grounds and was not suggested or imposed by the study protocol.
The characteristics of the 6 Fr AngioJet® Xpeedior® used (MEDRAD, Inc./Bayer HealthCare, Radiology & Interventional, Warrendale, PA, USA) as well as details of the thrombectomy procedure have been previously described23,27. The RT techniques adopted as well as the periprocedural adjunctive pharmacologic treatments are reported in this paper’s Online Appendix. The RT procedure was immediately interrupted once the haemodynamic condition of the patient improved (i.e., improvement of the SI ± decrease of the catecholamine drug support), independently of the pulmonary angiographic result.
The haemodynamic parameters (e.g., systolic blood pressure, systolic pulmonary pressure, heart rate) as well as the pH values and oxygenation at blood gas analysis were collected during the procedure and analysed post procedure. Technical aspects and problems related to the procedure as well as periprocedural and post-procedural complications were also collected.
The study protocol was approved by the Ethics Committee of the Geneva University Hospital (Protocol: 07-038).
FOLLOW-UP
Clinical evaluation of the dyspnoea, the ventilatory status (i.e., gas exchange ratios), as well as the haemodynamic conditions of the patients were evaluated at hospital admission, before and after the RT procedure, and at +1, +6, +12 and +24 hours. Transthoracic echocardiography was obtained in all patients before the RT procedure, at one and 24 hours after the procedure, as well as at one week and at three months.
STATISTICAL ANALYSIS
Continuous variables were expressed as median (minimum-maximum). Categorical data were presented as numbers and percentages. All statistical analyses and exact Wilcoxon tests were performed with the software PASW Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered as statistically significant.
Results
From November 2008 to May 2010, 17 patients were screened for inclusion in the study (Figure 1). Six patients were excluded because they presented with at least one exclusion criterion and one further patient was excluded because the diagnosis of PE was not confirmed at pulmonary angiography. The remaining ten patients were included in the study and the RT procedure was attempted in all of them.
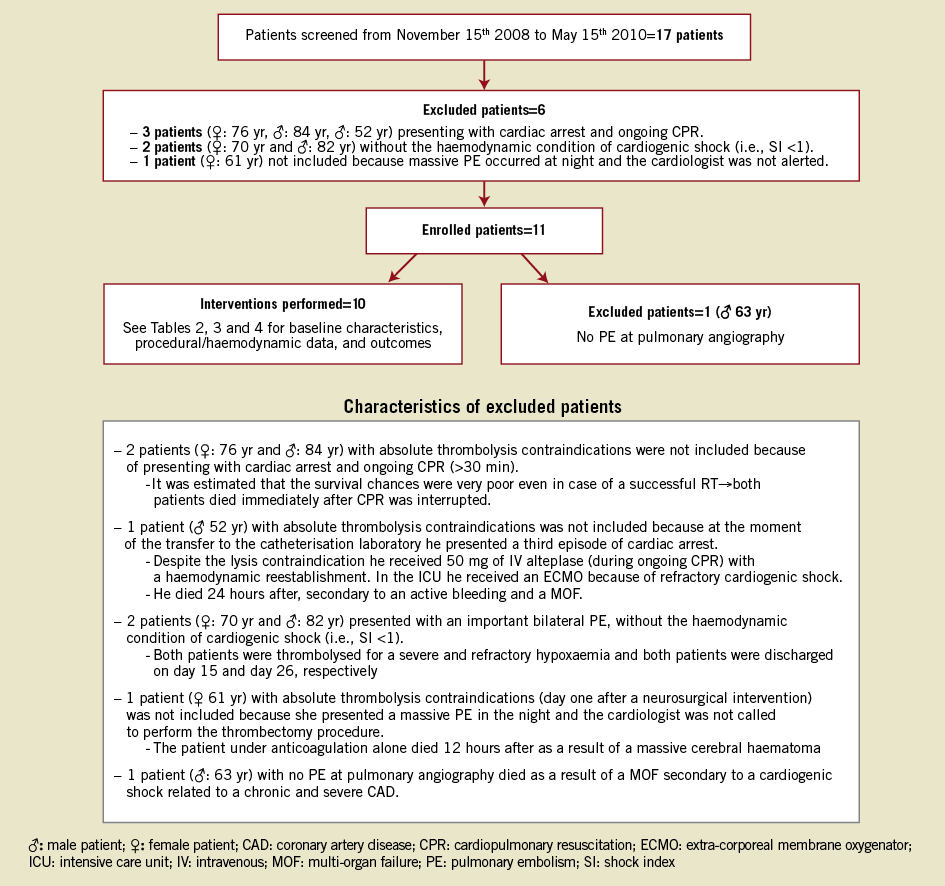
Figure 1. Flow chart of the screened included and excluded patients as well as the excluded patients’ characteristics.
Informed consent was obtained in the following way: one patient (no.4) gave informed consent personally; in two patients (no.1 and no.7) informed consent was obtained from a member of the family and, in the remaining seven patients (the most unstable ones), informed consent was obtained from a physician not involved in the study protocol.
All included patients presented with a high-risk PE and were in cardiogenic shock (i.e., SI >1 despite increasing dose of catecholamine) and had signs of organ hypoperfusion. In three patients, PE diagnosis was based on both TTE and multislice CT scan findings. In the remaining seven patients, due to their unstable haemodynamic situation, PE diagnosis was based solely on clinical presentation, blood gas analysis, arterial lactate level, and TTE. The diagnosis of PE was then confirmed at pulmonary angiography in all patients. Accordingly, in all patients the baseline echocardiographic data have shown: 1) a severely dilated right ventricle (RV/LV ratio = 1.67 [1.4-2.2]); 2) an abnormal septal contraction (two patients with septal flattening, eight patients with D-shape configuration); and 3) a severe right ventricular dysfunction (tricuspid annular plane systolic excursion=10.5 mm [6-20]).
The baseline clinical characteristics of the patients are reported in Table 1. Six patients presented with contraindications to systemic thrombolysis (four absolute and two relative contraindications). Eight patients were mechanically ventilated with positive pressure and six of them had already experienced cardiac arrest requiring at least a few minutes of cardiopulmonary resuscitation (CPR) before initiation of the RT procedure.
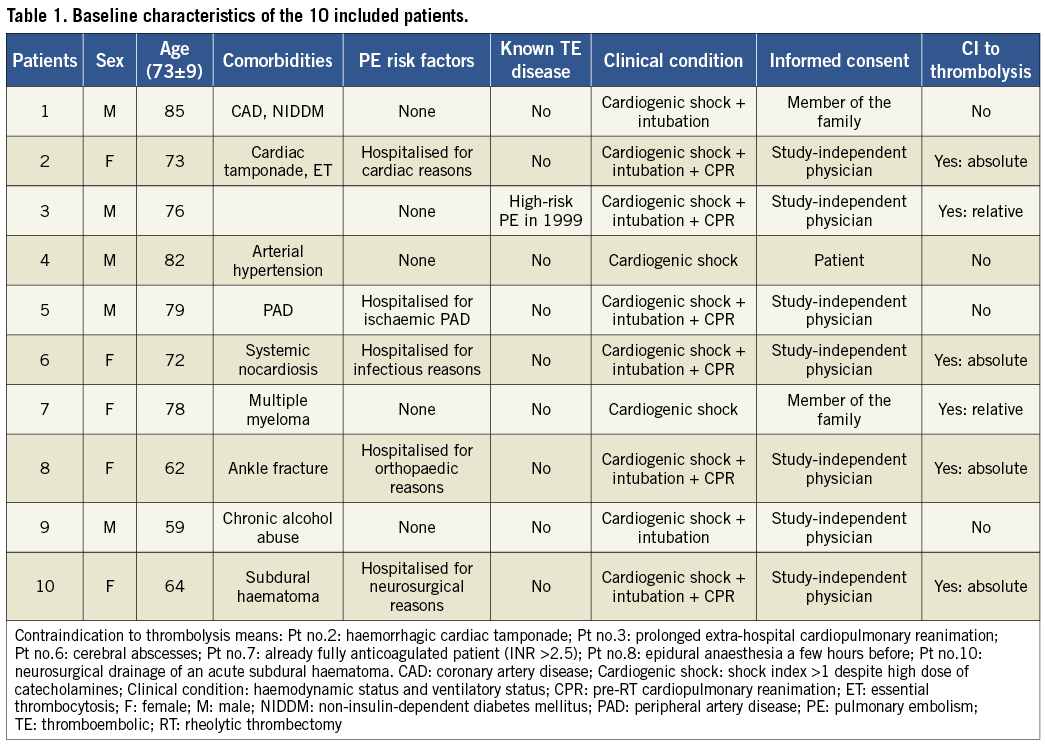
Table 2 summarises the procedural and haemodynamic parameters at the beginning and at the end of the RT procedures. The most frequent finding at pulmonary angiography was the presence of bilateral thrombotic obstructions of multiple lobar arteries, while complete pulmonary artery or pulmonary trunk obstructions were observed in only two cases (patients no.2 and no.9).
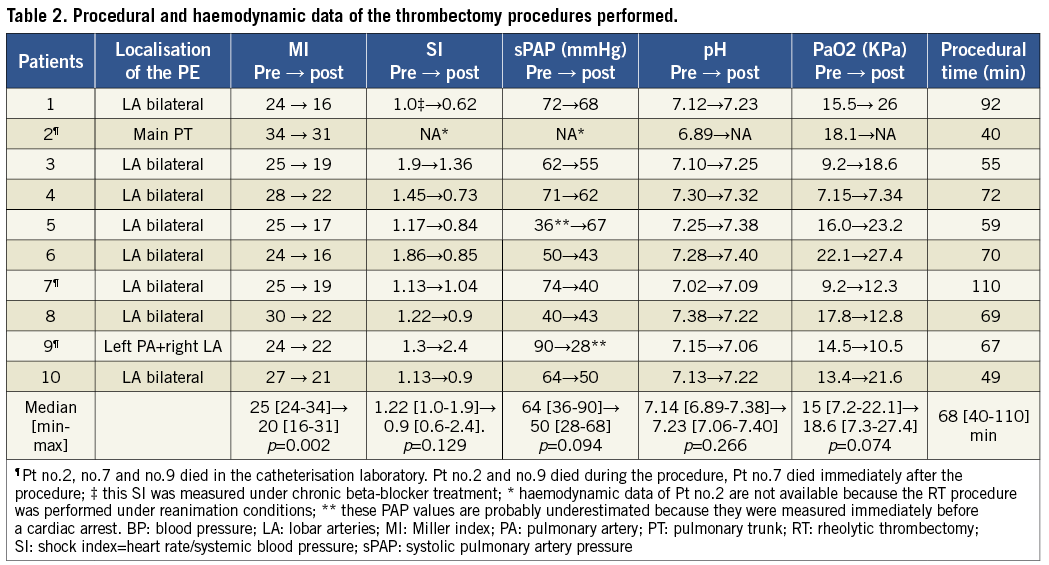
ASSOCIATED THROMBOLYSIS
Six patients received a full or partial thrombolytic regimen before, during, or after the RT procedure (Table 3). Two patients received a full dose IV alteplase (Actilyse®; Boehringer Ingelheim GmbH, Ingelheim, Germany) thrombolysis28 prior to RT and the thrombectomy procedure was performed as rescue strategy due to persistence of shock despite thrombolysis. Two patients received a reduced dose of direct intrapulmonary thrombolysis, while the remaining two patients received a full dose IV thrombolysis in the hours immediately following the RT procedure because of a continuous progressive deterioration of the haemodynamic parameters. Intrapulmonary thrombolysis was administered only in case of imminent death (e.g., during CPR) at the beginning or during the RT procedure.
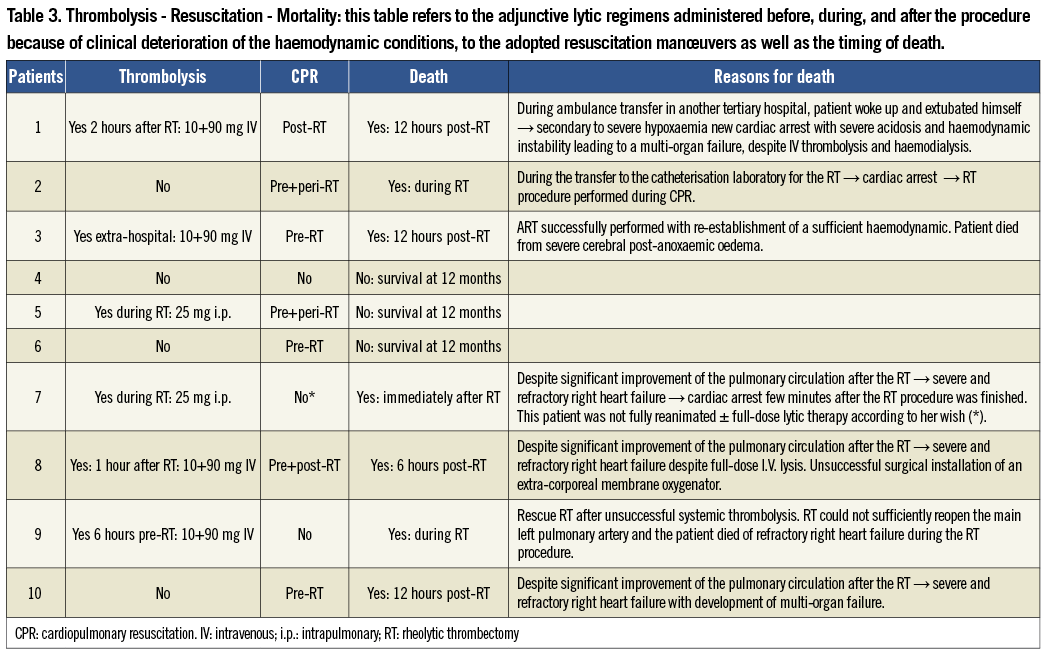
CLINICAL OUTCOMES
Seven of the 10 patients included died in the first 12 hours after the RT procedure (Table 3). Details of the procedure and the reasons and timing of death are reported in Table 3. The two periprocedural deaths observed were: in patient no.2, who presented a cardiac arrest during the transfer to the catheterisation laboratory with the result that the RT procedure was performed under CPR conditions; and in patient no.9, included after a thrombolysis failure, in whom the RT procedure could not sufficiently recanalise the main pulmonary trunk. In this patient, due to the lack of other therapeutic options and the progressive and rapid clinical deterioration, vasopressor drug support escalation was stopped and the patient died a few minutes later in the catheterisation laboratory.
The remaining three patients (no.4, no.5, and no.6) had favourable outcomes after the successful RT procedure. Patient no.4 underwent the procedure under local anaesthesia, while patients no.5 and no.6 were extubated six and 24 hours after the procedure, respectively. The catecholamine drug support was weaned two to 24 hours after the procedure in the three patients and discharge from the intensive care unit occurred at post-procedural day four, day two, and day eight, respectively. Hospital stay ranged from 20 days for patient no.4, who experienced an inguinal haematoma after the procedure, to 32 days for patient no.5, who had to be treated for a previously symptomatic peripheral artery disease, and up to 55 days for patient no.6, who needed a long neurological re-education for an acute ischaemic stroke which occurred simultaneously to the high-risk PE (i.e., paradoxical embolism).
In all three patients a complete regression of the dyspnoea, the RV dilatation and normalisation of RV function was observed at one week post procedure. From a clinical perspective, no PE recurrence or other adverse events occurred up to one year, with the three patients being in NYHA functional class I. Because of a low bleeding risk, the patients were advised to continue long-term oral anticoagulation.
Discussion
This small prospective pilot study suggests that a percutaneous RT procedure may be performed in highly unstable patients presenting with high-risk PE and cardiogenic shock. Indeed, the RT procedure was performed in all 10 patients in the absence of cardiopulmonary procedure-related complications. The main pulmonary finding at angiography was that of occlusions of multiple bilateral lobar arteries responsible for the poor haemodynamic status of the patients. Despite the good angiographic results and the trend for haemodynamic improvements after the RT procedure, the observed 30-day mortality rate remained exceedingly high (70%).
CURRENT RECOMMENDATIONS FOR TREATING HIGH-RISK PE
In high-risk PE the treatment of choice is IV thrombolysis (bolus and infusion), with a class 1 level of evidence (LOE) A6-7, 9. Recently, percutaneous mechanical thrombectomy procedures in this setting were graded in the European guidelines (ESC) as class 2b LOE C indication6, in the American ones as class 2a LOE C (AHA guidelines)7 and as class 2C indication according to the ACCP guidelines9. These procedures may be classified into aspiration thrombectomy, fragmentation thrombectomy, and rheolytic thrombectomy, which differ from other thrombectomy procedures in several respects29,30. First of all, one of the principal advantages of RT is that this procedure, by using its high-velocity saline jets, fragmentises and simultaneously aspirates, through the Venturi effect, thrombotic material, thus minimising the deleterious distal embolisation phenomenon frequently observed with other thrombectomy procedures31,32.
CURRENT AVAILABLE EVIDENCE OF ANGIOJET® RT IN HIGH-RISK PE
Rheolytic thrombectomy procedures have already proved their efficacy and safety in coronary33,34 as well as in extra-coronary domains, such as peripheral arterial and venous diseases35,36. However, its use in case of high-risk PE has not so far been prospectively evaluated.
Accordingly, the AngioJet®-related evidence in the PE setting consists solely of sporadic case reports and a few small retrospective series19,20,22,23,25,26,37. Accordingly, Zeni et al in 2003 applied the AngioJet® RT in 17 patients presenting with PE. In this series, the AngioJet® RT procedure was generally associated with the administration of an intrapulmonary thrombolysis, ultimately masking the sole effect of the RT procedure19. Similarly, the retrospective study by Chechi et al suggested the safety and efficacy of this specific RT procedure in 51 PE patients23. More recently, Nassiri et al have reported the AngioJet® RT ± power-pulse spray technique in 15 patients presenting mainly with non-high-risk PE (93% of the included sample)25.
The main difference between our patient population and those included in Zeni’s, Chechi’s and Nassiri’s series is that in these series patients presenting with non-high-risk PE (i.e., sub-massive PE) were also included. Accordingly, only two thirds of the patients included in Chechi’s series, which is the largest PE series using AngioJet® technology, presented with high-risk PE, defined as a Miller index ≥17 at angiography. Thus it is not surprising that the median Miller index of 19 described in Chechi’s study, which included <30% of patients in cardiogenic shock, was six points lower than the one calculated for our population (median Miller index 25). This probably explains why the mortality rates (Zeni’s series: 11.8%; Chechi’s series: 15.7%; Nassiri’s series: 0%) in these series were far lower than the ones we observed, as well as the ones reported in the literature of high-risk PE3,5,25.
Of note, we did not consider the Miller index to be a valuable parameter to define high-risk PE. Accordingly, we exclusively considered haemodynamic parameters, such as the SI, as inclusion criteria for the study. Indeed, patients presenting with PE and solely right ventricular dysfunction or with hypotension that could rapidly be managed with aggressive fluid supplement in the absence of catecholamine support were not considered eligible for the study. The direct consequences of these selective inclusion criteria were that patient enrolment was relatively slow (i.e., one patient every two months) and that the included patients were all in very unstable condition: 100% in cardiogenic shock; 80% mechanically ventilated for severe respiratory distress; and 60% having already experienced a short episode of cardiac arrest necessitating CPR before the RT procedure.
Accordingly, the likely explanation for the fact that the positive angiographic effects of the RT procedure did not translate into a survival benefit is probably that the patients were already in an irreversible stage of right ventricular failure at the time of the procedure. This right heart failure finally led to the development of fatal multi-organ failure in the majority of the included patients. Indeed, it is well known that highly unstable PE patients, especially those experiencing cardiac arrest, are associated with the worst outcomes38,39.
Another aspect that may have influenced the poor outcome of our patients is that, in contrast to the coronary arteries, the thrombus burden present in the pulmonary arteries in the case of massive PE is much more important and much more difficult to fragment and aspirate correctly. Furthermore, still in contrast with the coronary arteries where the AngioJet® RT is very efficacious for the treatment of platelet rich thrombi33, the thrombus in the pulmonary arteries arises from the venous circulation (i.e., a more fibrin-rich thrombus), suggesting that it may be older (i.e., days or weeks), more organised, and therefore again more difficult to fragment and aspirate.
In order to solve these issues at least partially, many operators nowadays prefer, if the haemodynamic of the patient allows it, to use the power-pulse technique of the AngioJet® RT37. Accordingly, recently Hubbard et al, Nassiri et al and Ferrigno et al have obtained a very encouraging in-hospital mortality rate of 9.1%, 0%, and 6.3%, respectively, by using this power-pulse technique in case of massive and sub-massive PE. It is clear that these reports have included patients with a lesser degree of instability than those included in our study; however, the concept of powerfully injecting a small amount of thrombolytics directly in the thrombus (i.e., 10-20 mg of alteplase), which will soften the thrombus, deserves further evaluation, but may finally be a possible solution to improve patient outcomes40,41.
POTENTIAL ANGIOJET®-RELATED COMPLICATIONS
Despite the several advantages of the rheolytic fragmentation of the pulmonary emboli, there are still concerns regarding the potential complications related to the use of this technology in the setting of high-risk PE.
Accordingly, fragmentation of the clot induces significant haemolysis which may be associated with a massive release of neurohormonal substances such as adenosine and bradykinin at the pulmonary vasculature level14,42. This phenomenon, associated with the concomitant activation of stretch receptors in the pulmonary arteries, is considered to be the leading cause of procedure-related bradyarrhythmias and hypotension42,43. Measures to counterbalance these effects include the placement of a transvenous temporary pacemaker wire either at the beginning or during the procedure44, as well as by administration of IV medication such as catecholamine and aminophylline.
Another issue related to the haemolysis induced by ART is the occurrence of severe hyperkalaemia and haemoglobinuria45. Hyperkalaemia may contribute to worsening the electrical instability, finally leading to severe ventricular arrhythmias, while haemoglobinuria causes further deterioration of renal function, which is often already impaired by the concomitant severe low cardiac output which occurs during high-risk PE.
STUDY STRENGTHS AND LIMITATIONS
The main limitations of the present study are the limited sample size, the single-centre design and the absence of a comparison group. However, our study was designed as a pilot study and its main aim was to establish the technical feasibility of RT in a very highly unstable setting, as well as to confirm the absence of any device-related major complications. These limitations are at least partially compensated for by the prospective nature of the study and the stringent inclusion criteria allowing the selection of the highest risk PE patients, namely those with cardiogenic shock1. Additional strengths are that the RT procedure was standardised and always performed by the same experienced operators (RFB, MRo).
The protocol allowed for RT as the initial reperfusion strategy, independent of the presence of thrombolysis contraindications. This was allowed by our ethics committee because it was considered that up to 30 minutes transfer delay for the RT was acceptable for the majority of patients. As nine out of 10 patients were included during day time (i.e., <15 minutes for the transfer to the catheterisation laboratory) and the only patient included at night (≈ 30 minutes for the activation of the interventional team) was included after a thrombolysis failure, we do not think that this time delay has influenced the poor outcome of the included patients.
Finally, the ethical issue of starting a novel treatment modality, instead of a proven regimen (i.e., lysis) in unstable patients was analysed in depth by the authors at the time of the study concept and during the entire enrolment period as well as by our local ethics committee. Indeed, if clinically indicated, thrombolysis was administered at any time, before, during or after the RT procedure. This is corroborated by the fact that six out of 10 of the included patients received some sort of thrombolytic regimen. Moreover, in the remaining four patients treated solely with the RT procedure, two survived without the need of lysis (patients no.4 and no.6) and two had absolute lysis contraindications (patients no.2 and no.10) (Table 3).
Conclusions
This pilot study evaluating the feasibility and safety of the AngioJet® rheolytic thrombectomy procedure in patients with PE and cardiogenic shock shows that this procedure may also be safely performed in this highly unstable setting. Concerning efficacy, the procedure reduced the thrombus burden in the pulmonary arteries in nine out of 10 of the patients, as was confirmed by the significant reduction in the Miller index. However, the high mortality rate observed, despite this angiographic improvement, suggests that the patients included were already experiencing an irreversible stage of right ventricular failure at the time of the RT or that RT on its own is ineffective and should be combined with some form of thrombolysis.
Further studies are needed to define the respective roles of mechanical thrombectomy, fibrinolytic therapy, and possibly the association of these two strategies (e.g., the power-pulse technique with the AngioJet®), in high-risk PE patients with a lesser degree of haemodynamic compromise.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Technical aspects of the AngioJet® rheolytic thrombectomy procedure
ANGIOJET® CATHETER CHARACTERISTICS AND THROMBECTOMY PROCEDURE.
The AngioJet® catheter (MEDRAD, Inc./Bayer HealthCare, Radiology & Interventional, Warrendale, PA, USA) is a double lumen catheter percutaneously introduced through the common femoral vein into the main pulmonary trunk or the affected pulmonary artery, respectively. One lumen serves to deliver high-pressure saline jets (1200 psi) into the thrombus and the other effluent lumen serves for clot removal utilising a localised negative pressure region (Venturi effect) that attracts the fragmented thrombus into a collector bag.
The AngioJet® RT procedure was performed in the presence of a full anaesthesiology team in addition to the cardiac catheterisation personnel, independently of whether the patient was conscious or mechanically ventilated.
After the insertion of a venous 8 Fr sheath, an 8 Fr multipurpose guiding catheter was advanced with telescoping technique over a 125 cm long 6 Fr Judkins right diagnostic catheter and a steerable 0.035” guidewire was positioned in one of the main pulmonary arteries. A selective pulmonary angiogram was then performed with 20 cc of contrast medium injected at 10 ml/sec and 600 psi of pressure. Once the diagnosis of PE was confirmed by angiography, the guiding catheter was positioned just before the visualised thrombus and the RT procedure was initiated.
The 6 Fr AngioJet® Xpeedior®, mode of action 1, was the only AngioJet device used because, according to the protocol, RT was strictly forbidden in vessels smaller than 6 mm (e.g., segmentary pulmonary arteries)27. The AngioJet® catheter was activated for 10-20 seconds and slowly advanced over the thrombus and pulled back for clot removal. According to the study protocol, the prophylactic endovenous pacemaker insertion in the RV was left to the operator’s discretion. However, following severe bradycardia and hypotension observed during the first case, a prophylactic 4 Fr pacemaker insertion at the beginning of the procedure was performed in all subsequent patients. After two to three passes of 10-20 seconds of the AngioJet® catheter in each occluded lobar artery, a pulmonary angiogram was obtained. In case of significant thrombus reduction without any significant haemodynamic improvement, the AngioJet® catheter was then positioned in the controlateral pulmonary artery.
The RT procedure was immediately interrupted once the haemodynamic condition of the patient had improved (i.e., improvement of the SI ± diminution of catecholamine drug support ± PaO2 – pH improvements), independently of the angiographic result. The pre-RT and the post-RT Miller indices were then calculated.
PERIPROCEDURAL ADJUNCTIVE PHARMACOLOGIC TREATMENT
Unfractionated heparin (UFH) according to current practice was immediately administered once the diagnosis of high-risk PE had been suspected. During the RT procedure, further UFH boluses were applied in order to obtain an activated clotting time of ≈ 300 sec. Special attention was given to the pre-RT potassium level, because of a possible haemolysis-induced hyperkalaemia, and glucose-insulin infusion ± calcium gluconate were administered accordingly. Before each AngioJet® activation small boluses of catecholamines (e.g., 100 µg of noradrenalin) were given in order to avoid or at least reduce the RT-induced neurohormonal-related hypotension. The right ventricular pacemaker was programmed 10-20 bpm less than the intrinsic heart rate. Whenever possible, the patient was maintained in mild sedation and not intubated, in order to avoid the pre-load reduction related to the positive end-expiratory pressure generated by the mechanical ventilation.
Systemic or intrapulmonary thrombolysis was administered as strict bailout only in case of clinical deterioration, and this independently of the result obtained with the RT procedure. Accordingly, in case of clinical deterioration during the RT procedure or immediately after, a full-dose thrombolysis, in the absence of absolute contraindications, was administered (i.e., alteplase 10 mg bolus + two hours 90 mg perfusion [Boehringer Ingelheim GmbH, Ingelheim, Germany])28. The bolus was administered selectively inside the pulmonary artery if the patient was still on the catheterisation table46, or IV if already transferred to the intensive care unit. In case of imminent death, thrombolysis was administered independently of any possible contraindication.

