Abstract
Aims: Mortality of massive pulmonary embolism remains exceedingly high despite thrombolytic therapy. Despite initial encouraging results, rheolytic thrombectomy has not been considered the first choice of treatment in the current European Guidelines for massive pulmonary embolism, even in cases of major contraindication to thrombolysis. Our objective was to assess the efficacy of rheolytic thrombectomy in the specific treatment of massive pulmonary embolism with contraindication to systemic thrombolytic therapy.
Methods and results: Between January 2003 and April 2008 a total of 10 patients with massive pulmonary embolism referred for rheolytic thrombectomy were included. Clinical data including medical history, haemodynamic status, procedural characteristic, in-hospital complications and survival were collected. Seven patients survived after undergoing the procedure, three patients died in during their initial hospitalisation however, two of these deaths were not attributable to the pulmonary embolism or the procedure. Rheolytic thrombectomy resulted in reduction of mean pulmonary artery pressures from 34.6±13.1 mmHg to 26.9±8.2 mmHg immediately following the procedure. Additionally, the Miller index improved from 22.4±2.8 to 9.8±2.7. There were no periprocedural bleeding complications associated with the procedure.
Conclusions: Rheolytic thrombectomy might be an effective and safe treatment for massive pulmonary embolism when systemic thrombolytic therapy is contraindicated. These data form the basis for further clinical investigation of this novel therapy among patients with massive pulmonary embolism.
Introduction
It is estimated that more than 300,000 people die annually in the United States from acute pulmonary embolism (PE)1. The clinical onset of this pathology may occur rapidly, ranging from mildly symptomatic cases, not always easily diagnosed, to massive embolism causing sudden death. Thromboemboli in most cases originate from the deep venous circulation in the legs (>80%).2 Acute anatomical obstruction of the pulmonary circulation with subsequent increase in pulmonary pressure, increase in right ventricle afterload, and right ventricular dilatation and dysfunction may become life-threatening. Thrombolysis is an established treatment for patients with acute massive PE associated with haemodynamic instability and/or cardiogenic shock3,4. The role of catheter-based rheolytic thrombectomy (CRT) in this setting is not clearly defined, although the mechanical removal of the thrombus appears to be an attractive therapy to improve flow in the main branches of the pulmonary arteries. In addition, it may be the first-choice therapy in patients with major contraindication to fibrinolysis5.
The purpose of this report is to present our centre’s experience with the use of CRT in the treatment of massive PE with contraindications to the use of fibrinolysis. We assessed the effectiveness of this therapy and evaluated its role in the management of these patients.
Methods
Between January 2003 and April 2008 all patients with massive pulmonary embolism and a major contraindication to systemic fibrinolysis referred to the Montreal Heart Institute for rheolytic thrombectomy were included. Diagnosis of pulmonary embolism was based on clinical presentation and confirmed by V/Q scintigraphy and/or angio CT-scan. Massive PE was diagnosed in the presence of cardiogenic shock and/or sustained hypotension according to criteria of the guidelines of the American College of Chest Physicians6. Cardiac transthoracic echocardiography was systematically performed to confirm right ventricular dysfunction. Pulmonary angiograms were analysed according to the Miller index7 before and after the procedure, and pulmonary artery pressure was monitored during the procedure.
Briefly the Miller index was calculated by the sum in each patient of both obstruction and perfusion indexes. The obstruction index was calculated as follows: nine major segmental branches were identified in the right pulmonary artery (three in the upper lobe, two in the middle, and four in the lower), and eight major branches were identified in the left pulmonary artery (two in the upper lobe, two in the middle and four in the lower); the presence of filling defects in any of these branches was scored with one point per segment leading to a maximum score of 16. The perfusion index was scored as follows: each lung was divided into three zones (upper, middle and lower) and the flow in each zone was assessed as absent (three points), severely decreased (two points) and mildly decreased (one point) and normal (zero point) leading to a maximum score of 18. Thus the Miller index was computed by the sum of these two scores ranging from zero (best) to 34 (worst).
Thrombectomy was performed using an Angiojet® Catheter (Possis Medical, Inc., Minneapolis, MN, USA), which removes thrombus by means of the Bernoulli principle. The CRT was performed by percutaneous transfemoral approach, using either the Xpeedior (vessel size between 4-12 mm) or the SF XVG (vessel size between 3-8 mm) AngioJet® catheters (Figure 1).
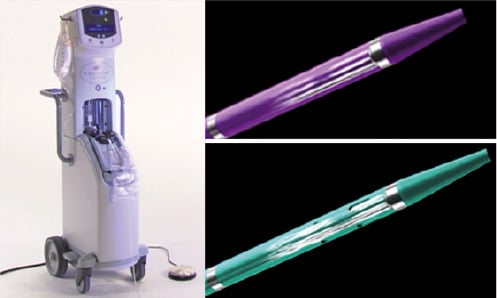
Figure 1. Components of AngioJet®: On the left, the Angiojet® Ultra Console. On the right, the two different aspiration catheters used in our study: on the top, the Xpeedior aspiration catheter and on the bottom. the SF XVG aspiration catheter.
The catheter was advanced over the guide wire in a 8 Fr guiding catheter most commonly a multipurpose catheter or a Judkins right (JR4), with the pump unit activated during slow catheter passages across the thrombus in a distal-to-proximal or proximal-to-distal direction under fluoroscopic guidance. The aim was to fragment and aspirate as much thrombus as possible. In two patients, local perfusion of fibinolytic therapy (FBL) (tenecteplase 25 mg in 1L of normal saline) through the Angiojet® infusate was administered during the procedure. Available clinical history, risk factors predisposing PE, adjunctive medical therapy and in-hospital complications were systematically recorded.
Continuous variables are expressed as mean ±SD and categorical variables are represented as number (percentage). Since continuous variables did not present a normal distribution, non-parametric tests were used for comparison. Two-tailed statistical significance was set at the 0.05 level. All computations were performed with SPSS 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
We collected data on 10 consecutive patients admitted to the Montreal Heart Institute from January 2003 to April 2008 with massive PE. There were seven women and three men, with mean age of 43.7±18.8 (Table 1).
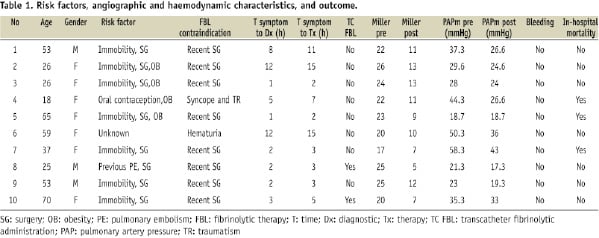
Deep venous thrombosis was diagnosed by Doppler echography in six of them. Of the 10 patients, eight had undergone recent surgery. One patient had an active neoplasia (bladder carcinoma with active haematuria) and one patient had a cranial trauma secondary to syncope and was judged to be contraindicated for systemic thrombolysis.
All patients presented with acute onset of dyspnea with one patient presenting additional syncope. Typical ECG pattern of sinus tachycardia and S1Q3T3 was observed in nine patients, one patient had a right bundle branch block.
Diagnosis was confirmed with non-invasive imaging techniques in all patients. Six patients had a diagnostic angio-CT, while four had a positive V/Q scan. All patients showed moderate to severe right ventricle dilatation with severe dysfunction on echocardiography. Estimated average pulmonary artery pressure was of 60.7±8.3 mmHg. Massive PE was confirmed on the basis of haemodynamic instability and presence of cardiogenic shock. The mean peak of troponin T levels was of 1.1 UI/L±0.7. Average time from symptom onset until final diagnosis was of 4.8 hours (range 1-12) and time from symptom onset until thrombectomy was of 6.6 (range 2-15).
Of the 10 patients treated with the Angiojet® Catheter all were initially treated with unfractioned heparin (70 UI/Kg). In addition, two patients received in-situ FBL (25 mg of tenecteplase) during the procedure. Both these case presented with significant thrombus burden, the benefit of fragmenting the thrombus and facilitating aspiration was judged to be superior to the risk of bleeding. Both these patients had undergone recent abdominal surgery, one for a leiomyosarcoma seven days prior to the PE and the other an emergency appendectomy five days prior to the precipitating event. Tenecteplase was administered directly into the pulmonary artery prior to aspiration. None of the patients presented bleeding complications.
Mean values for the Miller index before and after the procedure were 22.4±2.8 to 9.8±2.7 respectively (p=0.005). Thrombus elimination from the main pulmonary artery and major branches was achieved in all cases. A representative case treated with CRT, in which perfusion is re-established after the procedure, is demonstrated in Figure 2.
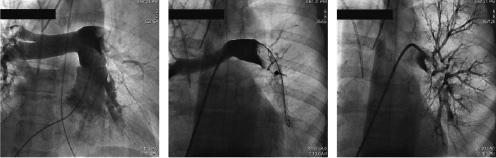
Figure 2. Catheter based rheolytic thrombectomy: A. The perfusion defect on the left pulmonary artery confirms the pulmonary embolism. B. The AngioJet® catheter is advanced to perform rheolytic thrombectomy. C. The thrombus is removed from the main branches and blood perfusion is recovered.
The mean pulmonary artery pressure decreased from 34.6±13.1 to 26.9±8.2 mmHg after the procedure (p=0.008). A significant haemodynamic improvement was observed in nine patients, leading to a reduction of inotropic drugs and to an increase in systolic blood pressure of 16.5±12 mmHg. In one patient, no changes were observed in pulmonary and systemic pressures after the procedure.
Neither major nor minor bleeding complications were described. None of the patients presented bradycardia or required temporary pacemaker implantation during or after thrombectomy. There were not any other periprocedural complications reported. Three patients died during hospital stay. Death was attributed to pulmonary embolism in one patient. This patient showed no haemodynamic improvement after thrombectomy despite improvement in his Miller Index. The other two patients died from causes not directly related to the PE, the first from sepsis related to his recent abdominal surgery, the second after surgical wound dehiscence compounded with intra-abdominal sepsis. Neither of these patients received thrombolysis. The remaining seven patients survived and were discharged from hospital. Two patients had a subsequent inferior vena cava filter implanted and all patients received oral anticoagulant therapy. The long-term follow up of this cohort is not available since the patients were sent back to their reference centre.
Discussion
The management of acute PE remains a challenge, especially because of major concomitant co-morbidities. Mortality remains exceedingly high, particularly in those patients with massive PE, even if they have been treated with fibrinolysis (52.4%). In fact, the clinical impact of fibrinolysis on mortality or recurrent PE has not been clearly established beyond 90 days8.
Moreover FBL is contraindicated in a sizeable portion of patients, and increases bleeding complications (17.6% in patients with massive PE)8. Surgical embolectomy is an alternative therapy for selected patients with massive PE in whom fibrinolysis is contraindicated or has failed. Mortality rates are extremely variable ranging from 11 to 30% in available reports and only when performed by experienced operators in specialised centres9,10. Most surgeons remain reluctant to intervene due to a high rate of major complications including death.
The recent European Guidelines for management of PE11 consider CRT an alternative to surgical treatment in high-risk patients when thrombolysis is contraindicated or has failed. Indeed, CRT presents clear advantages when compared to other therapies: First, the thrombus is removed by an anatomically localised therapy. Intra-pulmonary fibrinolytic can be added if deemed necessary and assuming that it does not induce a systemic fibrinolytic state. In most cases the thrombus is already organised and the association of local lysis with the thrombolytic agent, mechanical fragmentation with the device and aspiration have the potential to offer good results. In our series two patients were treated with tenecteplase and no bleeding complications were observed. Secondly, the guiding catheter can be steered to the affected territories with supra-selective aspiration. Recovery of flow can be ascertained during the procedure and pulmonary pressure is continuously monitored. Thirdly, CRT can be performed immediately after a diagnostic pulmonary angiogram. Finally, a wider window of opportunity to treat patients may be possible with CRT relative to FBL. In randomised trials on FBL in PE, patients were excluded when the onset of symptoms was beyond 96 hours. It is likely that the greater the treatment delay is, the more organised the thrombus will be with lower chances for successful FBL. However, there is no available data on the effectiveness of CRT treatment according to the time of treatment for PE. In addition, bleeding complications can be substantially reduced with CRT. Extrapolating data from MI trials confirm lower rates of major bleeding in patients treated with percutaneous interventions when compared to those who receive FBL12. Previous series with the Angiojet® catheter confirm our results with successful procedures and low rates of bleeding5. Moreover, the venous approach appears to present fewer local bleeding complications when compared historically to the commonly used arterial access of coronary interventions. Finally, the CRT system is a relatively user friendly device with a very rapid learning curve.
Many devices have been tested for the treatment of pulmonary embolism, some using the aspiration technique, others fragmenting the thrombus and finally devices such as the Angiojet® that use a rheolytic system. The ideal device should have a large area of aspiration at the tip with the smallest external diameter. The best technique and device for mechanically treating PE remains to be determined since experience with different devices is limited.
Use of the Greenfield aspiration catheter has been associated with a high rate of major bleeding (17%). This may possibly be related to its relatively large size (10 Fr)13. Other major complications have been described with the Greenfield catheter, such as right ventricular perforation and tricuspid regurgitation, possibly due to the size and catheter rigidity13. Mechanical complications and severe bradycardia during the aspiration seem to be the main and most frequent complications that limit the use of this kind of device. However, some aspirations devices such as the Aspirex (Straub Medical, Wangs, Switzerland), have shown promising results with low complication rates (11.1%), and one death among a series of 18 patients, where 11 were treated with this device14. Similarly, our Angiojet® data are encouraging with both high survival rate and low complication rates (Table 2).
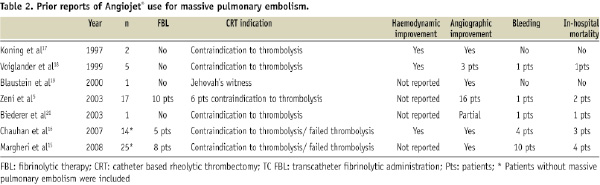
In a study using the Angiojet Catheter by Zenit and colleagues, similar positive results were reported, however little data concerning the haemodynamic status of the patients are available. Massive pulmonary embolism was defined only by angiographic criteria. This could explain the low mortality reported in this series. Furthermore, it is not clear how many patients presented with cardiogenic shock. Two other groups have presented their results with the Angiojet Catheter in a heterogeneous group of patients, some with massive PE and some others with moderate to severe PE15,16. Comparable results were presented, with exception given to the study by Magheri and colleagues in which the bleeding complications were elevated. In this study, most patients were administered concomitant FBL.
The aim of our study was to provide more data in an attempt to confirm the effectiveness of CRT in the treatment of massive EP in the presence of contraindication to FBL. Our study presents two main limitations: first the small sample size is limited and thus, conclusions from this data have to be interpreted with caution. Secondly, our study was limited to patients with contraindications to systemic fibrinolytic therapy. As a consequence, our results can only be applied to this subset of patients. Since only patients with massive PE and haemodynamic compromise are referred to our institution, our selection is biased to this high risk subgroup of patients. In all series, including ours, a reduction in mortality has been observed with the use of CRT and contra-indications to FBL. In our study, mortality was often related to pathologies other than PE. Hence CRT could be considered a first choice therapy, when available, for patients with massive PE with contraindication to thrombolysis. This indication could be possibly extended to patients without contraindications for FBL and those with a late presentation (>96 hours after symptom onset).
To date, all data concerning these devices come from small observational studies. Therefore, this technique still remains experimental. Only a randomised trial comparing CRT to systemic thrombolytic therapy may determine the best management for treating critically-ill patients with pulmonary embolism. In the absence of such a trial, CRT is a feasible option to treating critically ill patients with PE.

