Abstract
Aims: We report the feasibility and outcomes of emergency extracorporeal membrane oxygenation (ECMO) implantation by a cardiac catheterisation team in patients in severe cardiogenic shock or refractory cardiac arrest in a hospital without cardiac surgical facilities.
Methods and results: This prospective cohort study involved 51 consecutive patients who had ECMO implantation (September 2006 - September 2010). Twenty-seven were in severe cardiogenic shock and 24 in refractory cardiac arrest (17 with out-of-hospital cardiac arrest; seven with in-hospital cardiac arrest). Implantations were done via a percutaneous femoral approach by a local interventional cardiologist team, and in collaboration with the nearest cardiac surgical institution. Patients’ mean age was 51±15 years; 38 (74.5%) were men. Stable ECMO implantation was achieved in 26/27 (96.3%) patients in severe cardiogenic shock and in 18/24 (75.0%) patients in refractory cardiac arrest. In-hospital complications occurred in 23/27 cardiogenic shock patients; 13/27 were discharged alive. In patients with refractory cardiac arrest, complications occurred in 20/24; 21/24 were disconnected from ECMO because of brain death or multiorgan failure occurring ≤24 hours; one patient was discharged alive.
Conclusions: Emergency ECMO implantation by an interventional cardiologist in a hospital without cardiac surgical facilities is feasible, with a failure rate concordant with the literature.
Introduction
Transient mechanical circulatory support with extracorporeal membrane oxygenation (ECMO) can improve clinical outcomes for patients with severe cardiogenic shock or cardiac arrest refractory to standard treatments1-16. Owing to the complexity of cardiopulmonary bypass, a well-trained team including a cardiovascular surgeon and clinical perfusionists is needed to perform the procedure; consequently, ECMO is limited to only a small number of specialised centres with cardiac surgery facilities1,3,5,8,10,11,15.
Small studies of interhospital transportation by a mobile surgical team to a site with ECMO facilities have been reported in the literature17-19. However, in all of these the ECMO system and surgeon were dispatched from the referring hospital, leading to long delays in initiating treatment. For some patients in acute refractory shock or cardiac arrest, such delays have fatal outcomes.
As a consequence of advances in the technology for circulatory assistance, percutaneous cannula implantation no longer requires cardiac surgical skills or the involvement of a highly specialised team2,4,7,13. After training and in cooperation with a cardiac surgical hospital, an interventional cardiology team in a local hospital with a high-volume catheterisation laboratory but without on-site cardiovascular surgery facilities would be allowed to implant, prime, and run such devices.
We sought to determine the feasibility of implementing ECMO in patients with severe cardiogenic shock or refractory cardiac arrest in a local hospital without on-site cardiovascular surgery facilities. We report the circumstances, feasibility, in-hospital complications, and outcomes of ECMO implantation by an interventional cardiology team.
Methods
The Centre Hospitalier d’Annecy hosts a catheterisation laboratory available 24 hours a day, seven days a week (24/7), with a team of 12 interventional cardiology nurses and four interventional cardiologists. The hospital catchment area covers a population of 900,000 people, and 1,200 percutaneous coronary interventions (PCIs) are performed annually, but it does not have a cardiovascular surgery department. The cardiac surgical hospital and extracorporeal life-support referral centre is located 100 km away.
A full set of ECMO equipment was transferred from the cardiac surgical hospital to the local hospital. The complete ECMO system and all instruments needed for a vascular approach were placed on two mobile carts to facilitate their transportation both within and out of the local hospital. Patients requiring ECMO implantation should ideally be transported to the cardiac surgical hospital before they become haemodynamically critically unstable. However, when the patient deteriorates extremely rapidly, it was considered better for the local cardiac catheterisation team to implant the ECMO without waiting for the arrival of the cardiac surgical team, after which the patient would be transferred to the cardiac surgery hospital.
Patient population
Patients had to fulfil one of the following criteria to be considered for ECMO implantation in situ:
1) Have had a witnessed refractory cardiac arrest, occurring either outside or inside the hospital. The expected no-flow time (between collapse and efficient cardiac massage) had to be ≤5 minutes and the expected low-flow time (cardiac massage before ECMO implantation) ≤100 minutes20;
2) Have severe cardiogenic shock and be at high risk of early death in the absence of ECMO21-23.
Exclusion criteria were: physiological advanced age (threshold dependent upon the clinical situation), terminal malignancy, previous irreversible brain damage, patients with a known do-not-resuscitate policy, and patients without a witnessed cardiac arrest. Consent to include information in the database was obtained from the patient’s next of kin or family whenever possible.
Definitions
Definitions for cardiac arrest and cardiogenic shock followed guidelines21-23. Cardiac arrest was considered as refractory if no return of spontaneous circulation was obtained after 30 minutes of cardiopulmonary resuscitation. ECMO implantation in refractory cardiac arrest was performed under cardiac massage. Severe cardiogenic shock was defined as systolic blood pressure <90 mmHg despite treatment with high-dose catecholamine (inotropic and vasopressor agents).
Initial successful ECMO implantation was defined as achievement of a mean blood pressure ≥60 mmHg and a flow ≥3 L/min for ≥30 minutes13.
Major bleeding was defined as a blood loss requiring transfusion or re-intervention or resulting in death.
Procedure
Cardiopulmonary resuscitation and advanced life support were performed according to standard recommendations16,22,23. The hardware for the cardiopulmonary circulation comprised a Biomedicus portable bypass system (Medtronic, Inc., Minneapolis, MN, USA). A circuit incorporating a portable centrifugal pump with a membrane oxygenator (Jostra-Maquet, Orleans, France) and percutaneous cannulae (Medtronic) was used.
In most cases, ECMO implantation was performed at the site of collapse in patients who developed in-hospital refractory cardiac arrest, and in the catheterisation laboratory in patients who developed out-of-hospital refractory arrest or in the case of severe cardiogenic shock.
The interventional cardiologist implanted the cannulae while the two nurses assembled and primed the circuit with normal heparinated saline. The femoral artery was cannulated according to the Seldinger technique with a 14-17 Fr cannula (depending on the size of the patient) and the femoral vein with a 21 Fr cannula. Puncture was echo-assisted when required, especially in the case of refractory cardiac arrest due to the absence of a femoral arterial pulse. Heparin 50-100 IU/kg was administered intravenously to the patient immediately before cannulation of the vessels.
In some cases distal femoral arterial perfusion was performed using a 5 Fr sheath, with an echo-guided antegrade puncture2. This arterial shunt was introduced between the side port of the arterial cannula and a point located some centimetres distally in the superficial femoral artery (Figure 1).
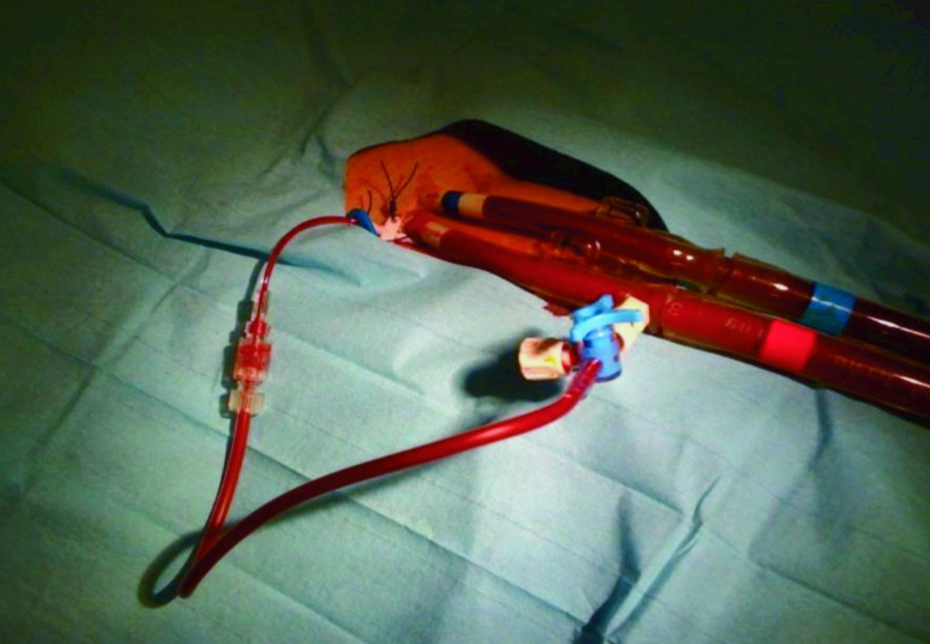
Figure 1. Mechanical circulatory support with extracorporeal membrane oxygenation.
Unless a non-coronary cause of haemodynamic instability was recognised, patients underwent coronary angiography, and a PCI when considered necessary.
The ECMO system was run to stabilise the patient. A systemic artery catheter was positioned to monitor blood pressure. Fluid repletion, vasopressors and inotropes were used to obtain a mean blood pressure ≥60 mmHg. Intra-aortic balloon pumps inserted before ECMO implantation were left in place. Rhythm to maintain a pulsatile flow through the native heart was restored, and low doses of an inotrope infusion were used to unload the left ventricle when the diameter became too wide or the wedge pressure too high.
After ECMO implantation, patients were transferred by ambulance to the cardiac surgery hospital, with an on-board emergency physician from the local hospital trained in ECMO management. The patient’s post-resuscitation neurological status was assessed on the first day, with a clinical examination, electroencephalogram, transcranial Doppler, and injected computed tomography, to determine whether to continue ECMO.
Data collection and analyses were performed in the cardiac interventional unit of the local hospital. A clinical research nurse, in charge of the data collection, prospectively collected clinical and procedural information and recorded in-hospital outcomes. Data were reviewed and analysed retrospectively.
Statistical analysis
The patient population was divided into two groups according to the patient’s condition at the time of implantation: patients implanted in refractory cardiac arrest, and patients implanted in severe cardiogenic shock. Comparisons between the two groups included in-hospital complications (including implantation failure) and in-hospital outcome.
Categorical variables are expressed as frequencies. Continuous variables are expressed as mean (SD), except for time intervals, which are expressed as median (interquartile range [IQR]). Comparisons between groups were performed using the Chi-square test and Fisher’s test if the groups were <5.
Results
Between September 2006 and September 2010, 51 consecutive adults (≥18 years) underwent ECMO implantation by the local team. The annual rate of implantations increased during the course of the study (Figure 2). Fifteen patients had severe cardiogenic shock without previous cardiac arrest (Figure 3). Thirty-six patients presented in cardiac arrest: 12 had had a transient episode of cardiac arrest and then recovered spontaneous circulation, but subsequently experienced haemodynamic deterioration leading to severe cardiogenic shock; the remaining 24 patients had refractory cardiac arrest (Figure 3). Among these 24 patients, 17 had experienced an out-of-hospital cardiac arrest and seven an in-hospital cardiac arrest. An ECMO was implanted in one patient with acute respiratory distress syndrome, who was included in the cardiogenic shock population.
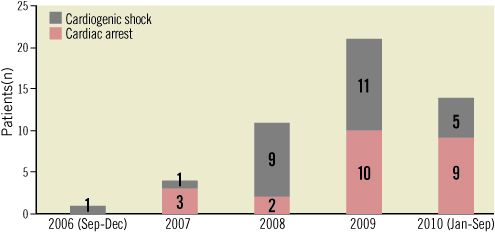
Figure 2. Referral of patients to the Centre Hospitalier d’Annecy for ECMO from September 2006 to September 2010.
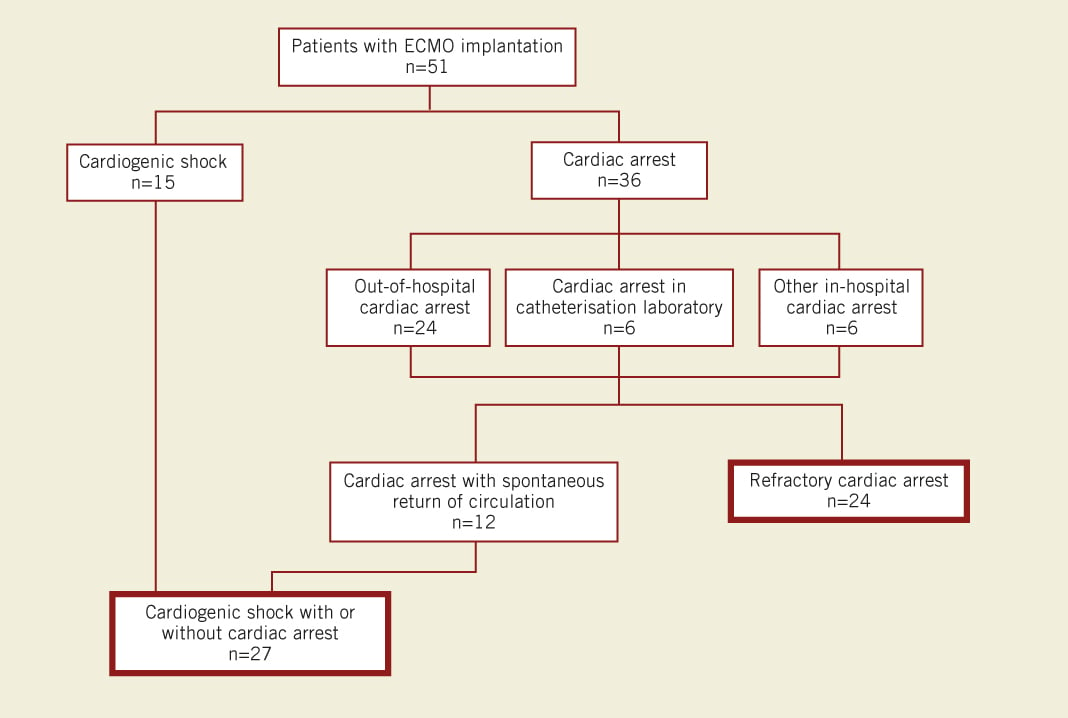
Figure 3. Patient disposition.
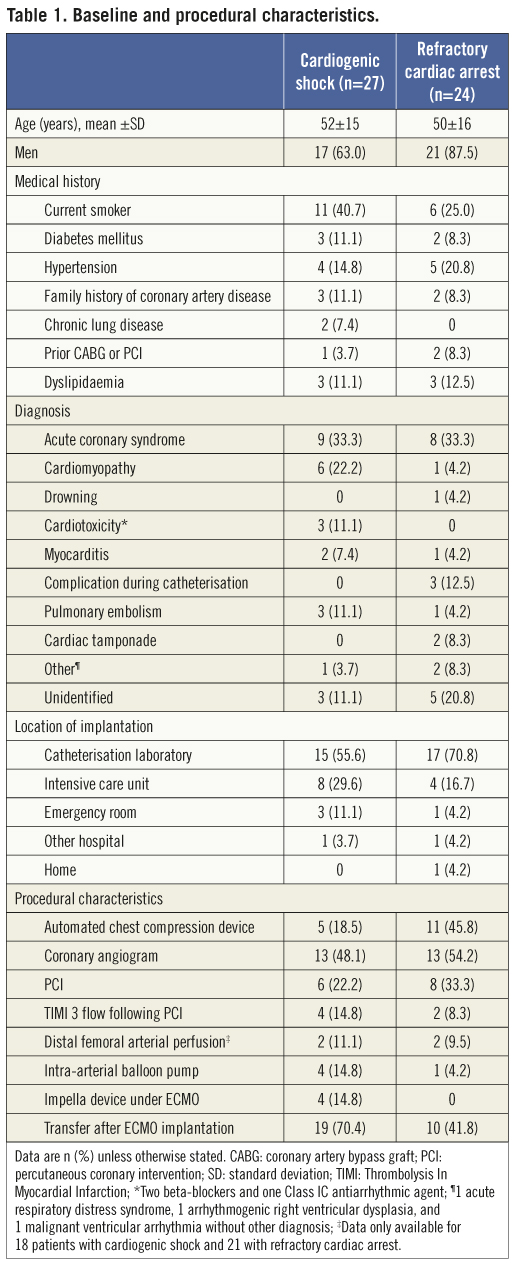
The characteristics of the study population are shown in Table 1. ECMO implantation was done in the catheterisation laboratory in six patients who developed cardiac arrest or cardiogenic shock during an interventional procedure. Three of these patients developed iatrogenic left main or proximal left anterior descending (LAD) artery occlusive dissection or extensive LAD artery thrombosis during angiography or PCI, and three developed spontaneous refractory cardiac arrest or severe cardiogenic shock after admission to the catheterisation laboratory for acute coronary syndrome because of their poor condition.
Two patients, who were considered too ill to be transported before implantation, underwent ECMO implantation in local neighbourhood hospitals, located 45 km and 50 km away. These two patients were subsequently transferred directly to the cardiac surgical hospital. In a 40-year-old man with known dilated cardiomyopathy, refractory cardiac arrest, and persistent ventricular fibrillation requiring multiple electric shocks, ECMO was implanted at home. In this case, the patient was deemed unable to be transferred. The ECMO team travelled by helicopter to the patient’s home, thereby minimising the delay to implantation. Despite successful implantation, the patient died the following day from brain death. The no-flow time for this patient was 0 minutes and the low-flow time was 119 minutes.
Among patients with refractory cardiac arrest, the median no-flow time was 0 (IQR 0-5) minutes and the median low-flow time was 87 (IQR 57-119) minutes (Table 2). Seventeen patients with refractory cardiac arrest were implanted with ECMO in the catheterisation laboratory. Median time from the start of cardiopulmonary resuscitation to arrival at the catheterisation laboratory was 81 (IQR 53-94) minutes, and from arrival at the catheterisation laboratory to ECMO implantation was 25 (IQR 15-40) minutes. As shown in Figure 4, 13/24 patients with refractory cardiac arrest and 6/17 with out-of-hospital refractory cardiac arrest fulfilled the predefined inclusion time criteria of no flow ≤5 minutes and low flow ≤100 minutes.
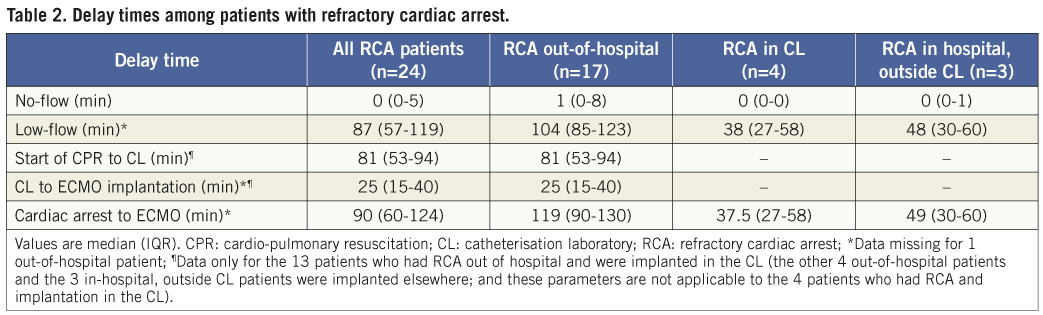
Figure 4. Patients with refractory cardiac arrest who met the criteria for enrolment in terms of no-flow and low-flow times.
After ECMO implantation, distal femoral arterial perfusion was performed in four patients. The procedure was done by the interventional cardiologist just after ECMO implantation in two patients, and by the surgeon after transportation to the cardiac surgical hospital in the other two patients.
ECMO implantation was successful in 44/51 patients (86%) (Table 3). The success rate was higher in patients with cardiogenic shock than in those with refractory cardiac arrest (96% vs. 75%; p=0.042). Of the seven implantation failures, six were due to catheterisation failure and one to centrifugal pump failure (breakdown). Catheterisation failure was observed in four of the first 25 patients and in two of the last 26 patients.
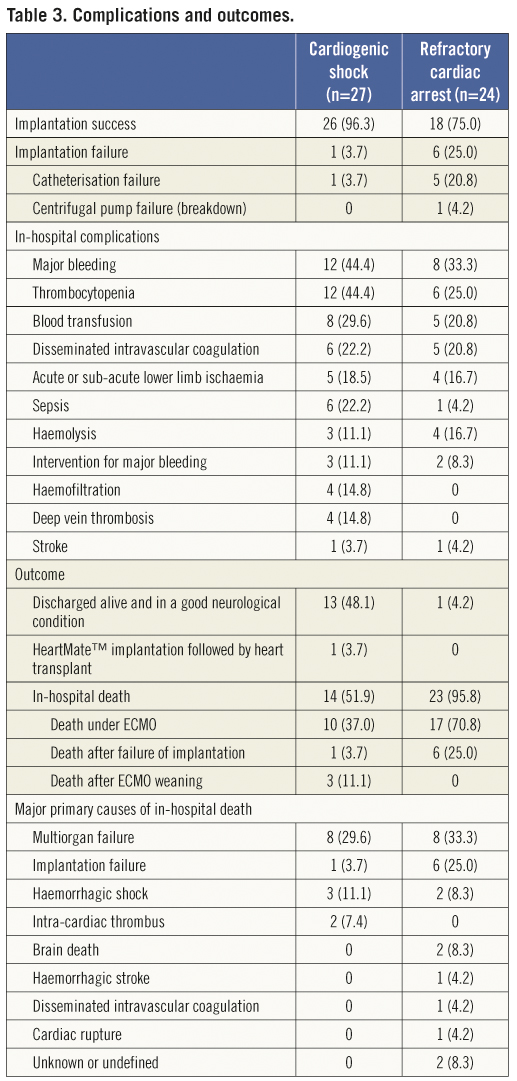
After implantation, 29 patients were transferred to the cardiac surgical hospital and 22 remained in the local hospital. Among these 22 patients, three had cardio toxicant poisoning as the indication for ECMO (with a favourable resolution and weaning after 2, 3, and 4 days), seven experienced ECMO-implantation failure, and 12 patients with an anticipated high rate of early death died during the first 24 hours on ECMO.
In-hospital complications occurred in 43 of the 51 patients (Table 3). Sixteen patients experienced one complication and 27 patients experienced two or more. The most frequent complication was major bleeding (in 20 patients): 12 on cannula, four in the lung, and four from an unknown site (detected by haemoglobin drop and requiring blood transfusions). Nine cases of lower limb ischaemia were recorded: one patient required surgery, but none underwent amputation.
Overall, 14/51 patients (27%) were discharged alive and in a good neurological condition: 13/27 (48%) patients with cardiogenic shock and 1/24 (4%) in refractory cardiac arrest (Table 3). The cause of refractory cardiac arrest in the single patient who survived was an iatrogenic extensive thrombus in the LAD artery during PCI.
The in-hospital survival rate in patients with out-of-hospital refractory cardiac arrest was 0% (0/17), compared with 14% (1/7) in those with in-hospital refractory cardiac arrest. The rate of in-hospital survival among patients with cardiogenic shock and no prior transient cardiac arrest was significantly lower than that in patients with cardiogenic shock and transient cardiac arrest (73% [11/15] vs. 17% [2/12]; p=0.003; Figure 5).
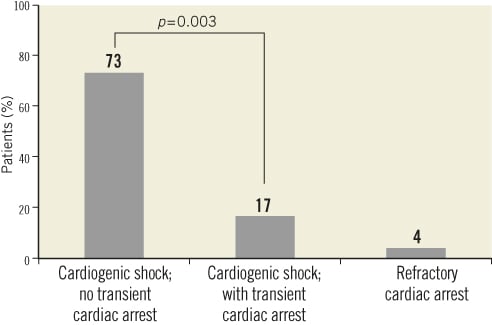
Figure 5. In-hospital survival by patient group.
Of the 37 deaths that occurred in hospital, seven occurred during ECMO implantation. Of the remaining 30 deaths, 23 occurred during the first 24 hours (Figure 6). Twenty-seven patients died under ECMO and three after weaning off the device and before hospital discharge (Table 3). The neurological condition of all of the surviving patients was good. Impella devices were implanted into four patients: two died under ECMO; one patient was weaned at day 13 after ECMO implantation and was alive at 14 months; and the last of these four patients was implanted with a left ventricular assist device afterwards, and was transplanted 25 months later.
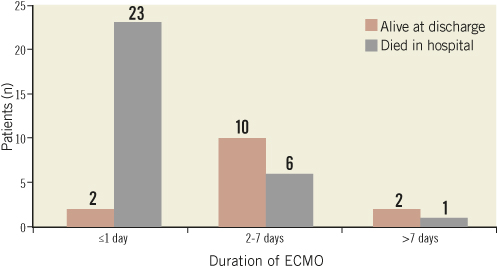
Figure 6. Clinical outcomes by duration of ECMO (n=30; 7 patients died during ECMO implantation procedure).
The exact causes of in-hospital death were difficult to diagnose as they may have been multifactorial. Multiorgan failure was the most frequent primary cause of death (Table 3); however, most of these patients also had brain death. Bleeding (haemorrhagic shock or stroke) was the primary cause of death in six patients (Table 3).
All 14 patients discharged alive were still alive after a median of 17 (IQR 13–26) months. One patient underwent heart transplantation.
Discussion
We report our initial experience of ECMO implantation by an interventional cardiologist team in a centre without on-site cardiac surgery facilities, and the first case of ECMO implantation at a patient’s home. The rate of initial implantation success was high, at 86%. In this study, patients were subsequently transferred to a cardiac surgery hospital after ECMO implantation when judged necessary, i.e., those patients with neither a very low nor a very high anticipated rate of death within 24 hours.
Most published reports do not mention rates of ECMO implantation failure3,5,8,10,12,14,15. For example, in the Extracorporeal Life Support Registry of approximately 29,000 implantations, patients were included only after successful ECMO implantation7. In other reports, the rate of successful initial implantation ranged from 82% to 93% depending on the population studied1,4,9,11,13, and our results are consistent with this. Implantation failure is largely due to the absence of a femoral pulse in cardiac arrest, leading authors to consider the surgical approach as indispensable in such a situation. With higher rates of use of echo-guided femoral catheterisation and placement of thin sheaths in the femoral vein and artery in patients with cardiac arrest, the implantation failure decreased during the course of our experience of ECMO implantation.
Not surprisingly, ECMO is associated with a myriad of possible complications (107 complications among the 51 patients in our report). This equates to 2.1 complications/person studied, which is consistent with the published literature (0.7-2.9 complications/person studied3,4,8,10,12,13). Again, the wide variation in complication rates may be explained by heterogeneity in the populations studied10.
The main complication encountered in our study was major bleeding (39%), which is consistent with previous reports where the rate of major bleeding varied from 20% to 49%.4,6,7 ECMO is well known to cause coagulopathy; systematic heparinisation is still advisable because of the risk of end-organ damage from microthrombus, but low doses of heparin are now advocated and strict adherence to an activated partial thromboplastin time of 50-70 seconds is reasonable. Megarbane et al13 advised no unfractionated heparin bolus, whereas Massetti et al8 used a 50 IU/kg bolus instead of the 100 IU/kg bolus used by Chen et al and Vanzetto et al3,4. Attempts should be made to minimise bleedings regarding restriction and attention to deep venous access, being more restrictive with the use of fibrinolysis before ECMO implantation and glycoprotein IIb/IIIa inhibitors, and using echo-guided catheterisation. We report four instances of pulmonary haemorrhage (fatal in two patients) in patients in whom an automated chest compression device was used. The use of such devices is still the subject of debate23.
The development of nine (18%) cases of acute limb ischaemia in our cohort of patients suggested the need for a higher rate of systematic implantation of a femoral shunt (for distal leg perfusion) or more caution in the selection of cannulae diameter or puncture, by angiography when possible. The rate of this complication is consistent with previous reports, which range from 2.5% to 19%4,8,12,24. Chen et al5 suggests the insertion of a shunt when an ECMO is implanted if the mean pressure of the superficial femoral artery is <50 mmHg.
Our 27% survival rate at hospital discharge is consistent with the Extracorporeal Life Support Registry7, in which 543 of 29,000 adults who underwent ECMO implantation with a cardiac indication had a survival rate of 33%.
Previous reports of ECMO in out-of-hospital cardiac arrest were encouraging, with survival rates at discharge ranging from 4% to 25%9-11,15. However, the lack of survival of patients with out-of-hospital refractory cardiac arrest not due to poisoning or hypothermia in our report is consistent with the latest report by Le Guen et al11, with a 4% survival in such a population. Our results suggest greater restriction of ECMO implantation for patients with out-of-hospital refractory cardiac arrest and particularly in those with short no-flow and low-flow times. Efforts are needed to decrease transportation time to the place of ECMO implantation in these patients. It appears that peripheral venous oxygen saturation, serum lactate concentration, and end-tidal carbon dioxide at admission would help best to identify ECMO indications for a patient in out-of-hospital refractory cardiac arrest11,13,25.
In patients with refractory cardiac arrest, transfer to hospital would be hazardous and subject to delays. In one patient, ECMO was implanted at the patient’s home, in order to decrease the low-flow time. To the best of our knowledge, this is the first report of home ECMO implantation. In this case, out-of-hospital implantation was feasible, and deserves further investigation.
In accordance with prior reports4,9, all of the patients in our study who were alive at discharge remained alive after long-term follow-up.
The high rate of complications in our study, similar to that in other reports, warrants further research in order to improve devices and strategies for these patients. Today, however, ECMO implantation appears to be the “last option” for patients in a very poor clinical condition.
Limitations
The sample size was relatively small and involved our early experience. Nevertheless, the results enable us to alert the medical community to the potential role of hospitals with a high-volume catheterisation laboratory but without on-site cardiac surgical facilities in undertaking ECMO implantation.
Conclusions
Our experience suggests that ECMO implantation performed by an experienced interventional cardiology team is a feasible treatment approach for patients with severe cardiogenic shock or refractory cardiac arrest at high early risk of death, with a favourable long-term outcome among survivors. Further research, with longer-term follow-up and involving a larger number of patients and interventional centres, is needed to confirm the safety and efficacy of this approach.
Acknowledgements
We would like to thank S. Rushton for her help in writing and H. Madiot and C. Ricard for their help with data management and statistics.
Conflict of interest statement
The authors have no conflicts of interest to declare.

