Abstract
Aims: This manuscript outlines the treatment of cardiogenic shock (CS) complicating acute myocardial infarction (AMI), focusing on new therapeutic strategies from the interventional cardiologist’s perspective.
Methods and results: CS is a life-threatening complication of AMI occurring in 10% of AMI patients. It can be defined as a state of critical tissue and end-organ hypoperfusion due to reduced cardiac contractility. Early revascularisation is the most important therapeutic measure. Its widespread use has caused a decline in the incidence of CS. However, despite optimal treatment, the mortality rate of CS is still approaches 50%. It is now understood that CS not only involves the heart but the whole circulatory system. In order to increase the survival rates of CS patients, the right decisions have to be taken regarding the optimal revascularisation strategy, treatment with inotropes and vasopressors, mechanical left ventricular support, management of multiorgan dysfunction syndrome, additional intensive care treatment, triage among alternative hospital care levels, and allocation of clinical resources.
Conclusions: CS mortality remains unacceptably high. In the light of very limited evidence regarding most treatment modalities, there is a clear need for adequately designed studies in order to answer the numerous unsettled issues.
Introduction
Due to the widespread performance of early revascularisation, the incidence of cardiogenic shock (CS) in patients with acute myocardial infarction (AMI) has declined slightly over recent years1. Assuming an incidence of infarct-related CS of 5-8%, this translates into approximately 60,000-70,000 patients per year in Europe2. Whilst over recent decades mortality rates of patients with AMI without CS have been reduced markedly, improvements for CS patients have been much less impressive3,4. Many complications are associated with AMI, but none of them has a more devastating prognosis than CS. Despite some therapeutic advances, CS remains the leading cause of in-hospital death of patients with AMI with mortality rates still approaching 50%1,4.
Definition
CS is defined as a state of critical end-organ and tissue hypoperfusion due to a reduced cardiac output. Established criteria for the diagnosis of CS are: systolic blood pressure (SBP) <90 mmHg for >30 minutes or vasopressors required to achieve a SBP ≥90 mmHg; 2) pulmonary congestion or elevated left ventricular filling pressures; 3) signs of impaired organ perfusion with at least one of the following criteria: a) altered mental status; b) cold, clammy skin; c) oliguria; d) increased serum lactate. The diagnosis of CS can usually be made on the basis of easy-to-assess clinical criteria without advanced haemodynamic monitoring.
Causes of cardiogenic shock
Left ventricular dysfunction is the most common cause of CS complicating AMI. In general, a loss of >40% of functional myocardium is required to cause CS. Much less frequent causes of CS secondary to AMI are mechanical complications, such as acquired ventricular septal defect, free wall rupture, and papillary muscle rupture or dysfunction with subsequent acute ischaemic mitral regurgitation. Furthermore, acute right ventricular infarction might cause CS5.
Pathophysiology
Ischaemia induces profound depression of myocardial contractility, which initiates a vicious spiral of reduced cardiac output, low blood pressure, and further coronary ischaemia6,7. The reduction in cardiac output causes severe tissue hypoperfusion and may finally lead to death if the circle is not interrupted by adequate treatment measures. It has been recognised that CS cannot only be attributed to the loss of left ventricular function but that it is the result of derangements in the entire circulatory system. Among other things, development of a systemic inflammatory reaction with capillary leakage and vasodilation contributes to the vicious circle of CS6. Figure 1 gives an overview on the pathophysiology of CS.
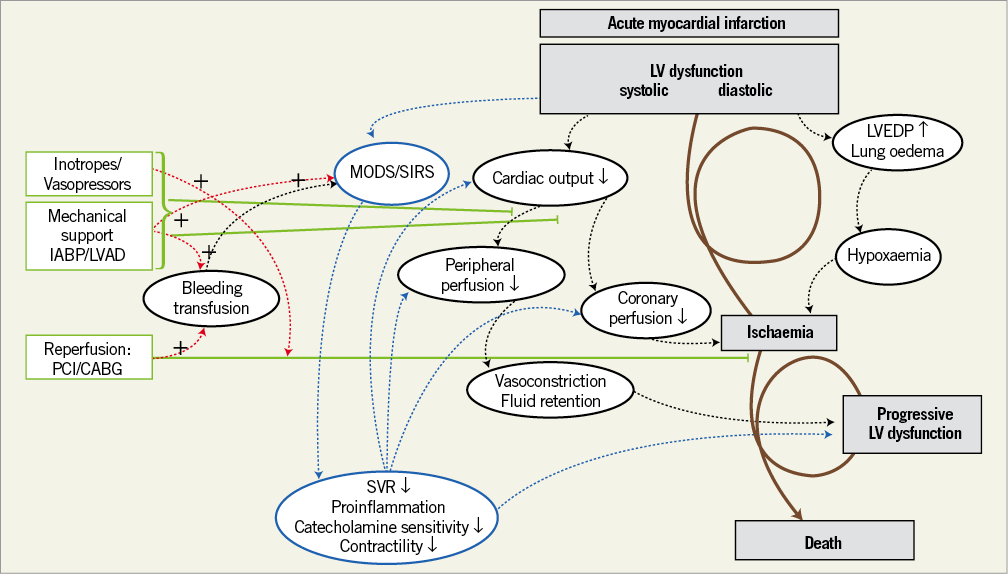
Figure 1. The pathophysiological concept of the expanded shock spiral. Treatment options, such as 1) revascularisation, 2) mechanical support, and 3) inotropes or vasopressors to reverse the shock spiral, are shown on the left-hand side in red. Potential drawbacks of therapeutic interventions including bleeding complications and influence on systemic inflammation are shown on the right-hand side in black. (Reproduced with permission7). LV: left ventricular; LVAD: left ventricular assist device; LVEDP: left ventricular end-diastolic pressure; MODS: multiorgan dysfunction syndrome; SIRS: systemic inflammatory response syndrome; SVR: systemic vascular resistance; TNF: tumour necrosis factor
Treatment
REVASCULARISATION
THE BENEFIT OF EARLY REVASCULARISATION
The SHOCK trial is one of the most important randomised trials in CS. It compared the effects of early revascularisation by either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) versus initial medical stabilisation on the clinical outcome of CS patients8. Although only a statistical trend in favour of revascularisation was observed with respect to the primary endpoint of 30-day mortality8, there was a significant reduction of mortality after six months in the revascularisation arm9 which persisted at long-term follow-up10. Since the publication of the SHOCK trial, numerous registries have confirmed the beneficial effect of revascularisation. Based on the available evidence, the ESC revascularisation guidelines give a strong recommendation (class I B) to perform emergency revascularisation in CS patients. Even though application of early revascularisation has increased markedly in today’s clinical practice, real-world revascularisation rates are still unsatisfactory1,4,11. Therefore, even more efforts are needed to convince clinicians to recognise the benefit of revascularisation even if the associated risk is high, as for example in elderly patients.
REVASCULARISATION STRATEGY
CABG VERSUS PCI
The issue about the optimal revascularisation strategy in CS remains unresolved, because the published randomised trials did not specify the reperfusion type, i.e., PCI or CABG8,12,13. According to the evidence from observational studies comparing PCI versus CABG, the type of revascularisation did not affect the outcome of CS patients14,15. The ESC guidelines give a general recommendation to perform revascularisation by either PCI or CABG, as appropriate. In the recent IABP-SHOCK II trial and registry, only 4% of patients underwent immediate CABG16. Presumably, this reflects current clinical practice. Due to its limited efficacy, fibrinolysis is reserved for those patients in whom PCI cannot be performed within 120 minutes17,18. Emergent surgery after the failure of PCI or fibrinolysis is only indicated in patients with persistent instability or life-threatening ventricular arrhythmia (class 1 C recommendation)18.
MULTIVESSEL PCI VERSUS CULPRIT LESION ONLY PCI
There are no randomised data available comparing multivessel versus culprit lesion only PCI in CS. However, this is an important open question, since more than 70% of CS patients present with multivessel and/or left main disease16. Current guidelines recommend a multivessel approach in CS with PCI of all critical stenoses or highly unstable lesions in addition to the culprit lesion (class IIa B recommendation)17,18. Due to the lack of prospective data, these recommendations are based mainly on pathophysiological considerations. Interestingly, they are also in contrast to those for haemodynamically stable STEMI patients. Notably, as shown in Figure 2, most registries comparing multivessel PCI versus culprit lesion only PCI showed an increased mortality for the multivessel approach19-24. Taken together, the current clinical evidence does not support an immediate multivessel intervention. Therefore, it is not surprising that treatment modalities for multivessel disease in CS differ widely among different institutions and operators. Overall, multivessel PCI is performed in approximately one third of CS patients with multivessel disease15,16. Since non-randomised observational studies and registries are prone to treatment-selection bias, there is an urgent need for randomised data. To answer the unsolved issue and to fill the apparent gap of evidence regarding the optimal revascularisation therapy in CS patients with multivessel disease, the prospective, randomised CULPRIT-SHOCK trial (ClinicalTrials.gov: NCT01927549) was designed to investigate immediate multivessel PCI in comparison to culprit lesion only PCI with potential staged PCI afterwards. This trial has currently started recruiting patients with the final aim of randomising 706 patients. The primary endpoint is defined as mortality and/or renal failure requiring renal replacement therapy within 30 days.
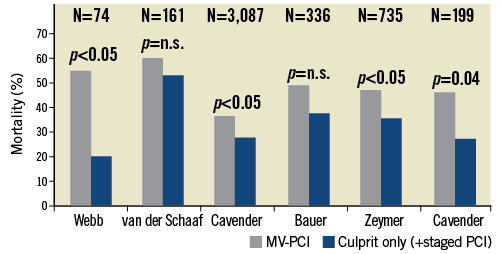
Figure 2. Overview of registry studies comparing multivessel PCI versus culprit-only PCI in cardiogenic shock with respect to mortality19-24.
ADJUNCTIVE TREATMENT
CATECHOLAMINES
If CS persists after restoration of euvolaemia, treatment with catecholamines becomes inevitable in order to maintain adequate circulation. Despite beneficial effects on haemodynamics and organ perfusion, there are no randomised data showing a prognostic benefit. Notably, many observational data suggest that the use of catecholamines is even associated with an increased risk of mortality25,26. Due to these safety concerns, guidelines strongly suggest using catecholamines only as long as necessary and at the lowest possible dose.
The choice of the catecholamine is mainly based on individual experience, institutional policy, and pathophysiological considerations. The mode of action of different inotropes and vasopressors has been reviewed previously26. Vasopressors are given to raise blood pressure and to redistribute blood volume to the vital organs. In a randomised clinical trial enrolling 1,679 patients with shock of different causes including a predefined subgroup of 280 CS patients, a reduction in mortality in the norepinephrine compared to the dopamine group was observed27. Therefore, according to the ESC STEMI guidelines, norepinephrine should be preferred over dopamine with a class IIb B recommendation17. Norepinephrine should be titrated until the systolic arterial pressure rises to at least 80 mmHg. Dobutamine should be the agent of choice if inotropic support is inevitable to improve cardiac contractility via β1-adrenergic stimulation. If additional vasopressor support is necessary, it should be combined with norepinephrine17.
LEVOSIMENDAN
Levosimendan acts as an inotrope by increasing the susceptibility of myofilaments to calcium and exerts vasodilatory properties28. The use of levosimendan and the underlying evidence have been reviewed in more detail previously29. The evidence of levosimendan in the setting of true CS is very limited with no randomised data being available. In view of its vasodilatory effects with subsequent blood pressure lowering, it has to be used with caution. ESC guidelines recommend its use with a weak (IIb C) indication in selected patients with shock. Further studies will be necessary to clarify the clinical role of levosimendan in patients with CS.
PHOSPHODIESTERASE (PDE) INHIBITORS
PDE inhibitors exert positive inotropic effects via an increase of cyclic AMP. Patients pre-treated with a beta-blocker might benefit more from PDE inhibition compared to dobutamine therapy30. However, a significant increase in malignant arrhythmias has been observed under treatment with PDE inhibitors. In a post hoc analysis from the OPTIME-CHF study investigating the effect of milrinone in patients with acute decompensated heart failure, the subgroup of patients with coronary artery disease had a higher event rate (death and rehospitalisation) compared to patients receiving placebo31. Thus, its use might be problematic in patients with infarct-related CS. A small, randomised trial comparing enoximone with levosimendan showed improved survival rates in the levosimendan group32. Current guidelines state that levosimendan or a PDE inhibitor may be considered to reverse the effect of beta-blockade if beta-blockade is thought to be contributing to hypoperfusion, giving both a class IIb C recommendation33.
THERAPY OF MULTIORGAN DYSFUNCTION SYNDROME
An optimal treatment of multiorgan dysfunction syndrome (MODS) is a main cornerstone in the treatment of CS patients since it has an important impact on the prognosis of the patients. An important measure is early lung-protective ventilation, as described for the treatment of acute respiratory distress syndrome (ARDS) in the Surviving Sepsis Guidelines34. Analgosedation should be monitored34. Urinary production should be measured and continuous renal replacement therapy should be initiated early in case of acute renal failure with clinical signs of uraemia, hydropic decompensation, metabolic acidosis and refractory hyperkalaemia35. Moreover, optimal, multidisciplinary intensive care treatment including nutrition, glycaemic control, blood transfusions, thromboembolism and stress ulcer prophylaxis should be provided.
Percutaneous mechanical support
INTRA-AORTIC BALLOON PUMP
The intra-aortic balloon pump (IABP) improves peak diastolic pressure and lowers end-systolic pressure, thereby improving coronary perfusion and reducing afterload. However, effects on cardiac output are only modest36. Until recently, the American and ESC guidelines recommended IABP use in CS with a class I level of evidence B and class I level of evidence C recommendation, respectively18,37,38. Based on a systemic meta-analysis, these recommendations have been downgraded to IIb B in the 2012 ESC guidelines and to IIa B in the 2013 American guidelines17,39. Due to a lack of randomised trials, only registries were included in this analysis and its results were conflicting: in the pre-fibrinolytic and in the fibrinolytic era, risk reductions for mortality in favour of the IABP were seen40. In contrast, in the PCI era, the use of IABP was associated with an increase in mortality40. In the large, randomised multicentre IABP-SHOCK II trial, 600 patients with CS complicating AMI who underwent early revascularisation were randomised to either IABP or conventional treatment41. No difference was seen between the two treatment groups with regard to the primary endpoint of 30-day mortality6. One limitation of the trial was the slightly lower mortality rate compared to other randomised trials, which might hamper a generalisation of the results to patients with the most severe CS. Furthermore, as in all negative trials, a type II error cannot be definitely excluded. However, the lack of benefit for any of the investigated secondary study endpoints and the neutral results in all subgroup analyses argue against a clinically meaningful beneficial effect of the IABP. The 12-month follow-up analysis confirmed these negative findings42. The influence of these results on guideline recommendations and on clinical practice needs to be determined in the future.
PERCUTANEOUS LEFT VENTRICULAR ASSIST DEVICES (LVADs)
Active percutaneous LVADs have been used not only in patients with refractory CS not responding to standard treatment including fluids, catecholamines and IABP but also as first-line treatment. However, the clinical experience and the available evidence are very limited43. Currently available devices include the TandemHeart™ (Cardiac Assist, Inc., Pittsburgh, PA, USA) and the Impella® 2.5, 5.0 and CP (ABIOMED Europe GmbH, Aachen, Germany). Figure 3 shows the different devices and Table 1 provides an updated overview of current percutaneous LVAD features. The mode of action of different devices and the underlying evidence for the treatment of CS have been summarised previously44. A meta-analysis reported the results of three randomised trials comparing percutaneous LVADs versus IABP43. Overall, only 100 patients were investigated. Two trials compared the TandemHeart™ and one the Impella® 2.5 against IABP45-47. Compared to IABP patients, patients treated with active LVADs demonstrated improved haemodynamics as shown by higher cardiac index, higher mean arterial pressure, and lower pulmonary capillary wedge pressure. On the other hand, bleeding complications and inflammation were more frequent with LVAD therapy. However, no difference was seen with regard to 30-day mortality7. Current ESC guidelines recommend considering the use of an LVAD if the patient continues to deteriorate and remains in low-output despite optimal treatment (class IIa C recommendation). However, routine use of an LVAD is clearly discouraged (class III recommendation) based on the above-mentioned limited evidence18. An ongoing Danish randomised multicentre trial (DanShock, NCT01633502) will compare the newly introduced Impella® CP with standard treatment with or without IABP. The Impella® CP can be inserted via a femoral approach and is able to deliver up to 3.7 l/min. It is planned to enrol a total of 360 patients, and the primary endpoint will be mortality.
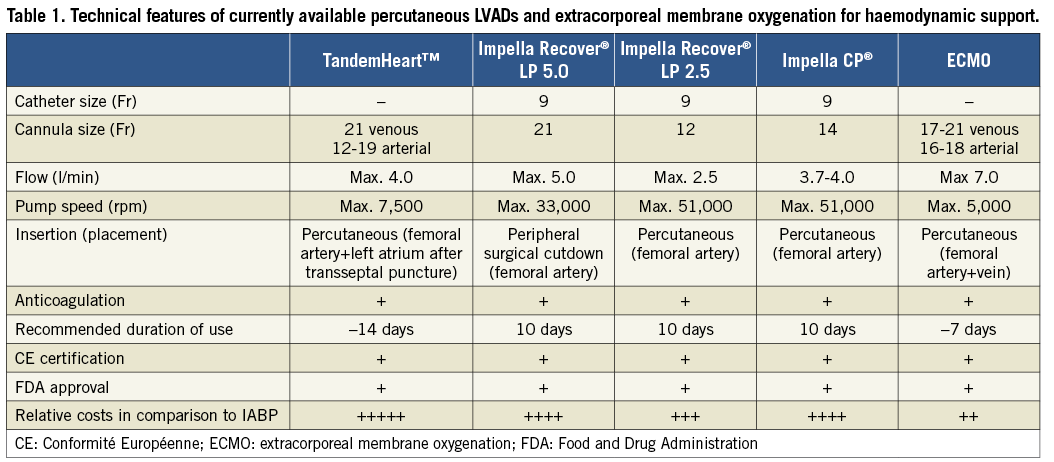
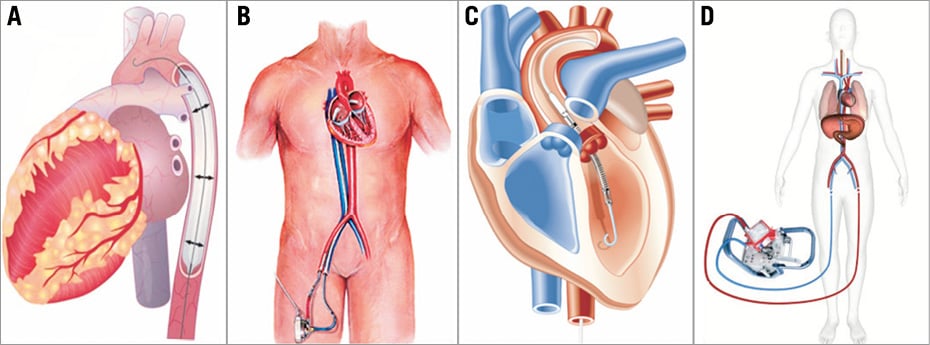
Figure 3. Schematic drawings of current percutaneous mechanical support devices. A) Intra-aortic balloon pump; B) TandemHeart™; C) Impella®; D) ECMO.
EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO)
Integral features of ECMO devices are a blood pump and an oxygenator. The main drawbacks of these devices are large cannula sizes causing lower limb ischaemia, the requirement for perfusionists, lack of direct unloading of the left ventricle, rise in afterload, and a limited support time48. There are only limited experiences in CS with one single-centre, non-randomised retrospective analysis showing improved survival rates with ECMO support in comparison to a historical control group49. In a recent prospective report, in-hospital mortality of ECMO patients was as high as 63.2%. The groups of patients older than 62 years and with prior cardiopulmonary resuscitation were even characterised by a mortality of 100%. These data question the unselective use of ECMO50.
Mechanical circulatory support with LVADs or ECMO is pathophysiologically appealing and it may give some additional time to allow recovery of the ischaemic myocardium. On the other hand, their use might promote the development of a systemic inflammatory response syndrome (SIRS). A second, potentially deleterious effect of extracorporeal circulation might be the promotion of disseminated intravascular coagulation causing severe bleeding complications. Currently, there are no randomised data showing an improved clinical outcome of CS patients with the use of mechanical support devices. Therefore, guidelines clearly state that percutaneous LVAD treatment should not be used as a first-line treatment for CS. Their use should be restricted to patients with refractory CS, with the aim of stabilising the patient to allow early revascularisation and/or as bridge-to-recovery, bridge-to-transplant, or also as a bridge to surgical LVAD. In case none of these options is achievable, an implantation of an active LVAD is futile. In patients with equivocal neurology, LVAD therapy may also be performed as a bridge to decision. In this case, implementation of the assist device gives time to assess neurologic function, evaluate the patients’ situation and talk to the relatives for further therapeutic decision making. Additional clinical investigation and randomised trials are needed for a more complete assessment of the role of different circulatory supportive strategies in CS.
An often used argument is also to insert an LVAD or IABP prior to revascularisation because some animal data support an infarct size reduction by left ventricular unloading51,52. However, no human data are available. Therefore, any decision making should be based on the criteria for LVAD implantation described above.
Summary and future perspectives
CS is the major cause for in-hospital mortality of patients with AMI. Due to the more rapid and widespread use of revascularisation, the incidence of CS has slightly decreased over recent years. However, mortality rates of CS patients are still unacceptably high. Cooperation in a multidisciplinary team within a specialised centre is crucial in order to improve the clinical outcome of CS patients. If patients are treated according to the guidelines with the use of emergent revascularisation and an optimal intensive care treatment, mortality of CS may be reduced to 40%, as shown in a recent randomised trial16. Currently, there are many unresolved issues, for example concerning the strategy of reperfusion (culprit lesion only PCI versus multivessel PCI), the optimal inotrope or vasopressor support, the role and potential treatment options of concomitant inflammation, patient selection and timing of mechanical support with LVADs, the type of LVAD, optimal mechanical ventilation, and treatment of bleeding complications. Some of these open questions are being addressed by ongoing trials such as the CULPRIT-SHOCK trial or the Danish shock trial53. Randomised clinical trials in CS are difficult to perform and are often more costly than trials in other clinical conditions. Therefore, many believe that conducting a randomised study in this critically ill population may not be possible. However, as AMI is a frequent event and as CS represents a devastating complication, any intervention reducing mortality is likely to have major public health implications and should therefore be thoroughly tested in adequately designed randomised trials.
Conflict of interest statement
S. Desch has received lecture fees from Maquet Cardiovascular. H. Thiele received institutional unrestricted grants from Maquet Cardiovascular and Teleflex Medical. J. Poess has no conflicts of interest to declare.

