Abstract
Valvular heart disease (VHD) is one of the most frequent causes of heart failure (HF) and is associated with poor prognosis, particularly among patients with conservative management. The development and improvement of catheter-based VHD interventions have broadened the indications for transcatheter valve interventions from inoperable/high-risk patients to younger/lower-risk patients. Cardiogenic shock (CS) associated with severe VHD is a clinical condition with a very high risk of mortality for which surgical treatment is often deemed a prohibitive risk. Transcatheter valve interventions might be a promising alternative in this setting given that they are less invasive. However, supportive scientific evidence is scarce and often limited to small case series. Current guidelines on VHD do not contain specific recommendations on how to manage patients with both VHD and CS. The purpose of this clinical consensus statement, developed by a group of international experts invited by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Scientific Documents and Initiatives Committee, is to perform a review of the available scientific evidence on the management of CS associated with left-sided VHD and to provide a rationale and practical approach for the application of transcatheter valve interventions in this specific clinical setting.
Introduction
Valvular heart disease (VHD) is among the most frequent causes of heart failure (HF) and is associated with poor prognosis, particularly when managed conservatively12. Acute valvular emergencies comprise approximately 8% of coronary care unit admissions3, but it is unclear how many patients with acute HF develop cardiogenic shock (CS)4567. Transcatheter valve interventions provide treatment options for a subset of patients with VHD at prohibitive or very high surgical risk. Moreover, technological advances have broadened their indication to younger or lower-risk patients and even to less symptomatic or moderate VHD8. Conversely, patients with VHD and CS are generally excluded from randomised controlled trials (RCT) exploring these technologies, and less evidence is available in this setting. Therefore, the 2021 European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS) Guidelines for VHD9 did not include specific sections for VHD patients presenting with CS. Treatment strategies are left to the discretion of multidisciplinary Heart Teams in a case-by-case fashion, weighing risks and benefits to identify those likely to benefit from interventions and avoid futility.
The purpose of this consensus statement, developed by international experts invited by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Scientific Documents and Initiatives Committee, is to provide a practical approach to transcatheter valve intervention use in patients with left-sided VHD and CS, based on the available scientific evidence.
Definition of cardiogenic shock
Cardiogenic shock (CS) is a clinical syndrome characterised by life-threatening organ hypoperfusion, which is caused by low cardiac output (CO) due to primary cardiac pump failure despite adequate volume preload1011121314. Varying definitions of CS exist (Supplementary Table 1). A consistent part of CS evidence stems from patients with acute myocardial infarction (AMI), while evidence for other aetiologies is increasing15. The Society for Cardiovascular Angiography and Interventions (SCAI) recently published a disease severity classification in an effort to make CS patients more comparable for clinical and research purposes1617.
We defined CS associated with VHD as significant VHD combined with systolic blood pressure <90 mmHg for >30 mins OR the need of vasopressors to maintain systolic blood pressure >90 mmHg, elevated serum lactate levels and clinical signs of end-organ hypoperfusion (including cool sweaty extremities, altered mental status, oliguria), corresponding to SCAI stage ≥C.
Clinical scenarios of CS and VHD
Acute onset of new severe VHD
CS may be due to an acute onset of severe VHD, such as ischaemic mitral regurgitation (MR), often related to AMI. Functional MR due to left ventricular (LV) global or regional remodelling or ischaemic papillary muscle dysfunction may resolve after revascularisation and recovery of LV function, or it may persist and require treatment. Acute MR may also be related to chord rupture. Acute severe AR commonly leads to CS1819 and is caused by type A aortic dissection, rupture of a fenestrated aortic valve or endocarditis, typically requiring surgical correction19. Other rare situations are iatrogenic or traumatic aortic valve injury, or AR in left ventricular assist device (LVAD) patients. Acute severe VHD may also be related to bioprosthetic valve failure (BVF)1920.
Deterioration of chronic VHD
Pre-existing moderate to severe clinically stable VHD can turn into acute decompensated HF and CS with various cardiac or non-cardiac triggers.
For BVF, patients in CS should have at least severe haemodynamic valve deterioration (HVD) (i.e., Stage 3)20 for valve-related haemodynamic instability. All causes of BVF may lead to severe HVD, including 1) structural valve deterioration (SVD; i.e., cusp tear); 2) non-structural valve dysfunction (i.e., paravalvular leak [PVL]); 3) thrombosis; or 4) endocarditis (Figure 1).
The primary approach should address the triggering condition. However, transcatheter interventions can be used as a bailout strategy in complex cases or when the trigger, such as pregnancy, persists.
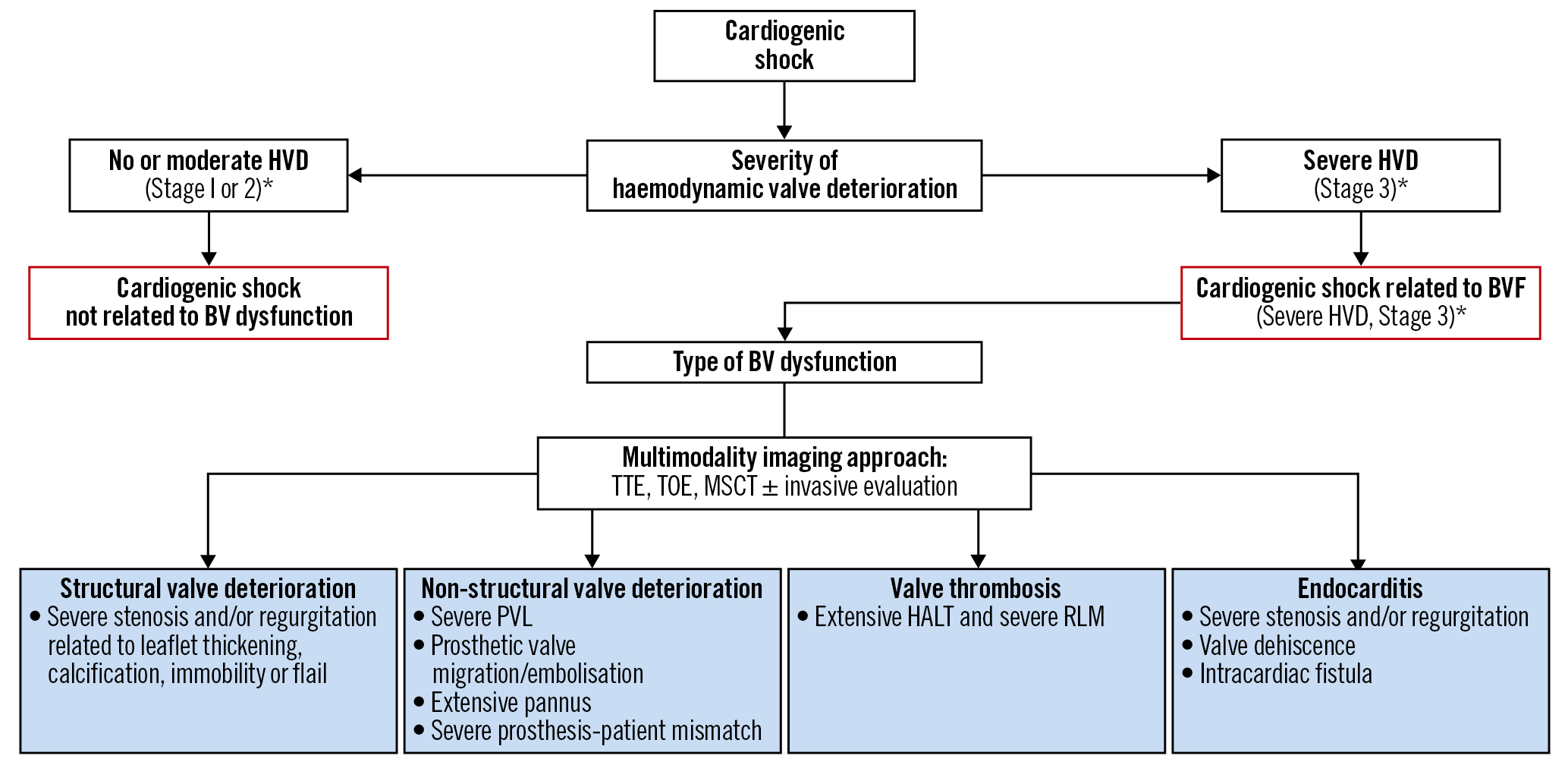
Figure 1. Identification of BVF mechanisms associated with cardiogenic shock. *for HVD severity definition. *Stage 1 HVD definition: evidence of SVD, non-structural valve dysfunction (other than paravalvular regurgitation or prosthesis-patient mismatch), thrombosis, or endocarditis without significant haemodynamic changes. *Stage 2 HVD definition: increase in mean transvalvular gradient ≥10 mmHg resulting in a mean gradient ≥20 mmHg with a concomitant decrease in EOA ≥0.3 cm2 or ≥25% and/or decrease in Doppler velocity index ≥0.1 or ≥20% compared with echocardiographic assessment performed 1-3 months post-procedure, OR new occurrence or increase of ≥1 grade of intraprosthetic AR resulting in ≥moderate AR. *Stage 3 HVD definition: increase in mean transvalvular gradient ≥20 mmHg resulting in a mean gradient ≥30 mmHg with a concomitant decrease in EOA ≥0.6 cm2 or ≥50% and/or decrease in Doppler velocity index ≥0.2 or ≥40% compared with echocardiographic assessment performed 1-3 months post-procedure, OR new occurrence or increase of ≥2 grades of intraprosthetic AR resulting in severe AR20. AR: aortic regurgitation; BV: bioprosthetic valve; BVF: bioprosthetic valve failure; EOA: effective orifice area; HALT: hypoattenuated leaflet thickening; HVD: haemodynamic valve deterioration; MSCT: multislice computed tomography; PVL: paravalvular leak; RLM: reduced leaflet motion; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography
Cardiovascular triggers
a. Atrial fibrillation and other (supra)ventricular arrhythmias: while left-sided VHD precipitates the occurrence of atrial fibrillation, the latter complicates moderate to severe left-sided valvular stenosis. In the SEAS trial, at 4-year follow-up, 6% of patients with mild to moderate aortic stenosis (AS) developed atrial fibrillation21. In AS and mitral stenosis (MS), a rapid heart rate and loss of the atrial contraction limit the filling time of the LV. Restoration of sinus rhythm is crucial, although this is difficult to achieve, particularly in MS.
b. AMI: AS is not uncommon in AMI patients, and this combination is independently associated with short- and long-term mortality22. Impaired ischaemic LV contractility further reduces CO, and AS increases afterload, creating a vicious circle that leads to CS. Treatment is challenging because inotropic drugs and diuretics increase intraventricular pressure, increasing haemodynamic impairment and gradient.
c. Hypertensive crises and rapid volume overload (intravascular intravenous fluid infusion or blood transfusion) can also cause CS in severe VHD but can generally be treated medically.
d. Takotsubo syndrome has been associated with pulmonary oedema in AS23. Moreover, dynamic LV outflow tract obstruction, typical of apical ballooning, may create severe MR through systolic anterior motion of the anterior mitral leaflet, which may result in CS24. As with AMI, medical treatment is challenging and may aggravate haemodynamic impairment and CS in AS. Conversely, cautious use of beta blockers (ideally starting with intravenous, short-acting beta blockers like esmolol) with fluid resuscitation reduces LV outflow tract obstruction by decreasing basal hypercontractility, increasing LV filling and size, and reducing the heart rate, all potentially leading to MR reduction and haemodynamic stabilisation25.
Non-cardiovascular triggers
a. Pregnancy carries a high risk of cardiac decompensation in VHD due to pregnancy-related haemodynamic changes. Stenotic VHD, particularly MS, are generally less tolerated during pregnancy than regurgitant lesions, as increased heart rate, stroke volume and CO increase the transvalvular gradient by approximately 50%, mainly between the first and second trimesters, worsening both the patient’s and foetus’ prognoses2627282930. Accordingly, MS should be treated preconceptionally when diagnosed. Otherwise, transcatheter valve interventions provide a minimally invasive option for an acutely decompensated condition that is not responsive to medical treatment31.
b. Severe infection/sepsis can lead to decreased systemic vascular resistance and hypovolaemia, causing a compensatory increase in heart rate and hypotension despite increased CO, which are poorly tolerated in severe MS or AS patients. Besides, a decreased preload increases valvular gradients, aggravating pre-existing stenosis. Cardiovascular comorbidities are risk factors for septic shock32, while infection is the main cause of non-cardiac deaths (up to 31%) in AS patients333435.
In most patients with septic non-CS, VHD is a bystander and does not require specific urgent intervention. However, a valvular intervention might be advisable for selected patients when standard medical therapy fails or when weaning and recovery seem challenging. Emergency percutaneous mitral balloon valvuloplasty (PMBV) has been effective in this context36. Balloon valvuloplasty avoids prosthetic valve implantation in infected patients at risk of endocarditis. However, it carries an acute MR or AR risk. Weighing the risk-benefit ratio is challenging, and the decision should be tailored to the patient’s condition.
a. Other precipitating factors include severe anaemia, acute renal failure, hyperthyroidism and hypoalbuminaemia, all usually improving after treatment and not requiring emergent valve intervention
Emergent diagnostic workup
Non-invasive diagnostic tools
Transthoracic echocardiography
Transthoracic echocardiography (TTE) is the optimal imaging modality in CS37, determining the cause and severity of underlying VHD and whether or not it is responsible, at least partially, for the clinical presentation of the patient. Limited point-of-care cardiac ultrasound focusing on two-dimensional assessment of the LV function, such as left ventricular ejection fraction (LVEF), may miss important VHD lesions38. Discrimination of the severity of VHD using TTE requires high image quality, precise measurement, complex calculations, and integration of multiple criteria. Moreover, the low-flow status of CS should be accounted for, as it might underestimate transvalvular gradients. Conversely, medications used in CS, such as dobutamine, might increase transvalvular gradients, therefore, overestimating the VHD. We herein propose a diagnostic workflow for the assessment of patients presenting with CS. (Figure 2, Table 1).
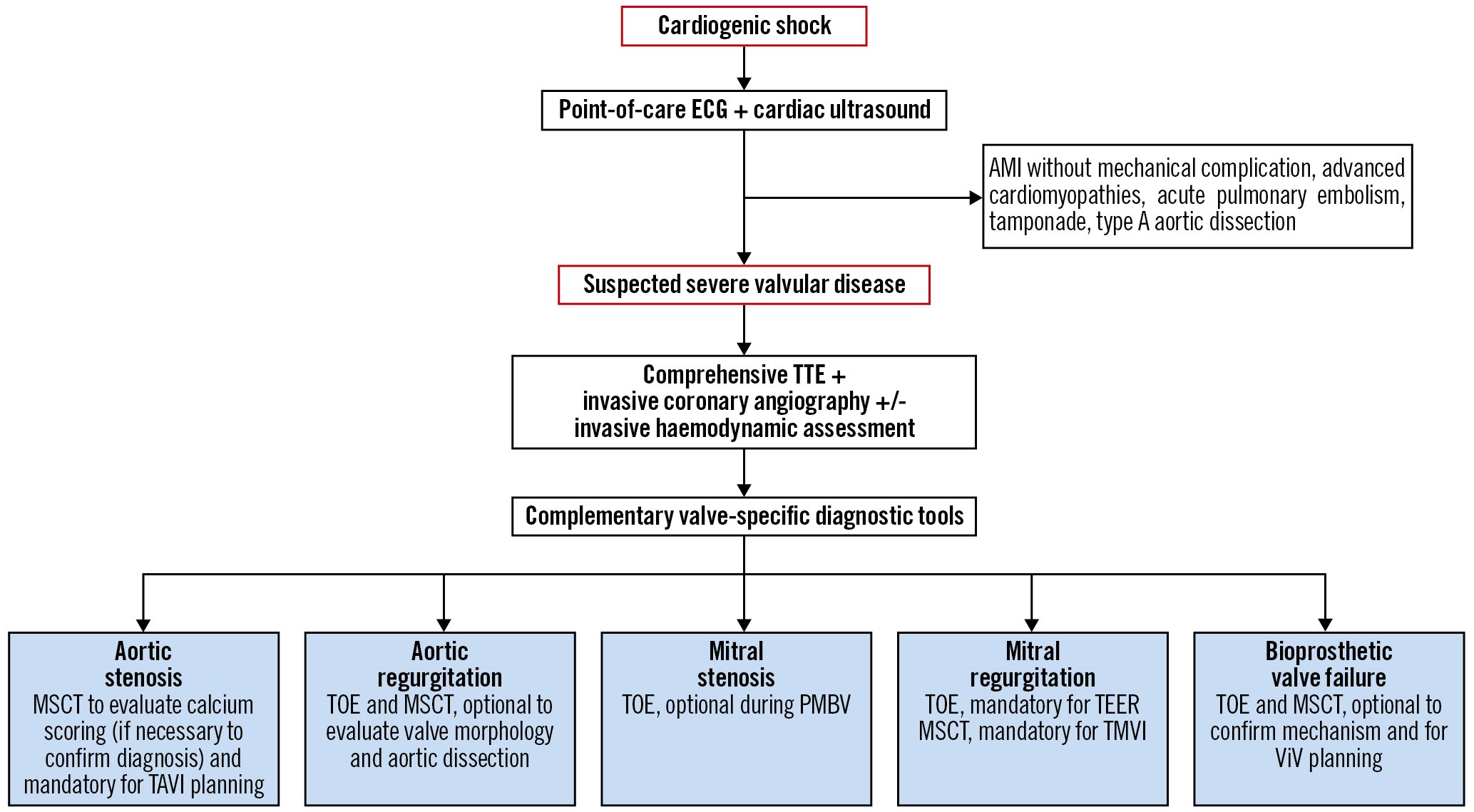
Figure 2. Proposed diagnostic workflow for the assessment of patients presenting with CS. Step 1. Point-of-care cardiac ultrasound. A point-of-care cardiac ultrasound is generally useful as it provides the first clues of severe VHD146. However, it is rarely sufficient. In this phase, ruling out acute myocardial ischaemia, advanced cardiomyopathies, untolerated arrhythmias, acute pulmonary embolism, tamponade or type A acute aortic dissection potentially responsible for the CS, is crucial147. When a point-of-care cardiac ultrasound reveals hyperdynamic LV function in a patient with severe acute decompensated heart failure or CS, urgent assessment with comprehensive transthoracic echocardiography (TTE) is warranted to exclude VHD emergencies. Step 2. Comprehensive TTE. Comprehensive TTE is generally adequate to accurately investigate valve structure and function. Importantly, increased flow due to sepsis or anaemia can elevate Doppler gradients, potentially leading to overestimation of the severity of stenotic valve lesions. Likewise, volume overload and systemic hypertension often lead to reversible worsening of regurgitant lesion severity. Conversely, low-flow status might underestimate the severity of valvular diseases. Invasive coronary angiography±invasive haemodynamic assessment can give additional information in this step. At this step, an invasive coronary angiography is indicated to rule out concomitant CAD according to guideline criteria9. Alternatively, owing to its high negative predictive value, MSCT may be used in patients who are at low risk of atherosclerosis. Step 3. Complementary valve-specific diagnostic tools include TOE and/or multislice computed tomography. Accurate quantification of VHD severity is essential, as only severe valvular dysfunction can cause CS148. Hence, TOE, including three-dimensional modalities, is useful in the detailed assessment of valve anatomy and function (native or prosthetic)149 and should be systematically performed when TTE is inconclusive. In stabilised patients, MSCT should be performed if required for the planification of the transcatheter heart valve intervention. AMI: acute myocardial infarction; CAD: coronary artery disease; CS: cardiogenic shock; ECG: electrocardiogram; LV: left ventricular; MSCT: multislice computed tomography; PMBV: percutaneous mitral balloon valvuloplasty; TAVI: transcatheter aortic valve implantation; TEER: transcatheter edge-to-edge repair; TMVI: transcatheter mitral valve implantation; TOE: transoesophageal echocardiography; VHD: valvular heart disease; ViV: valve-in-valve
Table 1. Checklist for VHD imaging assessment and eligibility for transcatheter procedures.
| FOR DIAGNOSIS | FOR ELIGIBILITY FOR TRANSCATHETER PROCEDURES | ||
|---|---|---|---|
| AV disease | TTE | Confirm AV disease severity Evaluate valve morphology Check for associated VHD, LV/RV function, PASP |
|
| TOE | Confirm diagnosis (optional) | Confirm annular sizing (3D evaluation, only if MSCT not available) | |
| MSCT | Confirm VHD severity (calcium score) in LFLG AS Rule out CAD in selected cases |
Confirm annular sizingEvaluate valve morphology and calcium distributionGeneral aortic root assessment (sinus of Valsalva and STJ dimensions, coronary ostia height)Evaluate aorta and vascular access | |
| ICA | Rule out CAD | ||
| L-RHC | Confirm disease severity in selected cases | ||
| MV disease | TTE | Confirm MV disease severity Evaluate valve morphology and mechanism of VHD Check for associated VHD, LV/RV function, PASP |
|
| TOE | Confirm diagnosis in selected cases | Evaluate valve morphology and mechanism of MR for TEER and TMVI Rule out LAA and LA clots |
|
| MSCT | Rule out CAD in selected cases | Evaluate annulus size for TMVI eligibility Predict LVOT obstruction for TMVI eligibility |
|
| ICA | Rule out CAD | ||
| L-RHC | Confirm disease severity in selected cases | ||
| BVF | TTE | Confirm BVF severity Evaluate valve morphology and mechanism of failure Check for associated VHD, LV/RV function, PASP |
|
| TOE | Confirm mechanism of bioprosthetic valve dysfunction (SVD, non-SVD, thrombosis, endocarditis) Discriminate between PVL and intravalvular AR |
Rule out LAA and LA clots in case of planned valve-in-valve in mitral position Identify PVL location to select the most appropriate vascular access for transcatheter occlusion |
|
| MSCT | Confirm mechanism of bioprosthetic valve dysfunction (SVD, non-SVD, thrombosis, endocarditis) Rule out CAD in selected cases |
Confirm valve sizeIdentify PVL location to select the most appropriate vascular access for transcatheter occlusionEvaluate vascular access+ IN AORTIC POSITION: General aortic root assessment (sinus of Valsalva and STJ dimension, coronary ostia height) Check for interference with coronary ostia Evaluate aorta and vascular access+ IN MITRAL POSITION:Predict LVOT obstruction |
|
| ICA | Rule out CAD | ||
| L-RHC | Confirm disease severity in selected cases | ||
| 3D: three-dimensional; AR: aortic regurgitation; AS: aortic stenosis; AV: aortic valve; BVF: bioprosthetic valve failure; CAD: coronary artery disease; ICA: invasive coronary angiography; LA: left atrial; LAA: left atrial appendage; LFLG: low-flow low-gradient; L-RHC: left-right heart catheterisation; LV: left ventricular; LVOT: left ventricular outflow tract; MSCT: multislice computed tomography; MV: mitral valve; PASP: pulmonary artery systolic pressure; PVL: paravalvular leak; RV: right ventricular; STJ: sinotubular junction; SVD: structural valve deterioration; TOE: transoesophageal echocardiography; TEER: transcatheter edge-to-edge repair; TMVI: transcatheter mitral valve implantation; TTE: transthoracic echocardiography; VHD: valvular heart disease | |||
Transoesophageal echocardiography
Transoesophageal echocardiography (TOE) may increase diagnostic accuracy, especially in case of a poor acoustic TTE window, and is relatively straightforward to perform in patients under mechanical ventilation. TOE may be able to identify BVF aetiology, differentiate PVL from valvular regurgitation, and help in suspected endocarditis. It is mandatory for transcatheter mitral valve therapies to evaluate eligibility and guide interventions. It may be used for aortic valve sizing if a computed tomography (CT) scan is not available (Table 1).
Multislice computed tomography
Multislice CT (MSCT) is complementary to TTE. Calcium scoring using non-contrast MSCT can confirm AS severity (likely severe if >2,000 Agatston units [AU] in men and >1,200 AU in women) in discordant AS grading (aortic valve area <1 cm2 and mean gradient <40 mmHg) related to low CO. MSCT with contrast injection is the gold standard for feasibility studies and planning of valvular interventions, such as transcatheter aortic valve implantation (TAVI). MSCT acquisition protocol should include a contrast-enhanced electrocardiogram (ECG)-gated or triggered heart and aortic root scan and a non-ECG-gated vascular bed scan from the subclavian arteries to superficial femoral arteries, reconstructed at a slice thickness of 1.0 mm or less for accurate multiplanar evaluations (at least 64-detector technology).
High spatial resolution of MSCT by multiplanar and three-dimensional volume reconstructions provides an accurate analysis of valve morphology (tricuspid vs bicuspid, calcium distribution), the aortic root anatomy, vascular access route, and coronary arteries, the latter being challenging in CS due to tachycardia and low CO. Contrast-enhanced MSCT is also useful in BVF to discriminate SVD, thrombosis, pannus, and infective endocarditis and in planning valve-in-valve procedures (Table 1).
Invasive diagnostic tools
Invasive coronary angiography
Coronary artery disease (CAD) is diagnosed in 20-80% of symptomatic severe AS patients, according to age group, and increases operative risk39. In addition, among patients with CS undergoing TAVI, 10% and 20% present with significant left main CAD and proximal left anterior descending artery stenosis, respectively40. The coexistence of CAD and secondary MR is much more frequent; more than two-thirds of patients undergoing transcatheter edge-to-edge repair (TEER) have relevant CAD41. Therefore, invasive coronary angiography (ICA) is mandatory to rule out CAD. The objectives of ICA are to 1) identify treatable CAD that is aggravating the CS; 2) perform myocardial revascularisation when needed; and 3) obtain safe and adequate arterial access for percutaneous mechanical circulatory support (MCS). Except for CS in AMI patients, there is a lack of robust scientific evidence about the optimal therapeutic strategy for patients with coexisting CAD requiring revascularisation and significant VHD. For this reason, the time sequence of events – placement of MCS, revascularisation and emergency balloon valvuloplasty/TAVI or TEER – depends on team experience, patient clinical status and VHD (Table 1).
Invasive right heart catheterisation
Alongside diagnosing pulmonary hypertension, right heart catheterisation was previously broadly used for haemodynamic monitoring and treatment adjustment. However, several registries reported considerable complications related to its routine use for treatment monitoring, and, despite conflicting registry evidence4243, the only RCT demonstrated no additional benefit compared to TTE4445. Therefore, right heart catheterisation is not recommended for daily monitoring. It can be useful, alone or in combination with left heart catheterisation for decision-making or, in selected cases, during the peri-interventional phase, in experienced hands (Table 1). Moreover, selective use of right heart catheterisation can be considered to guide medical decisions in CS, particularly in patients considered for or supported by MCS4647.
Therapeutic strategies: general concepts
Both the 2021 ESC/EACTS Guidelines for the management of VHD9 and the 2021 ESC Guidelines for diagnosis and treatment of acute and chronic HF11 contain few recommendations on VHD management in CS patients. CS is time-sensitive with rapidly increasing mortality, for which diagnosis and management should start as early as possible. Early identification and treatment of the underlying cause, along with haemodynamic stabilisation and management of organ dysfunction, are key.
Medical treatment and ancillary procedures
After initial fluid challenge (if appropriate), pharmacological management of CS consists of intravenous (I.V.) vasoactive agents to improve organ perfusion by increasing CO and blood pressure1148. The selection of pharmacological agents is largely empirical, and they must be used with caution, starting at low doses and up-titrating with close monitoring194849. Norepinephrine is the vasopressor of choice, despite a potentially harmful increase in stroke volume and transvalvular gradient in cases of AS and an increase in LV afterload11. Accordingly, medical stabilisation is often difficult in the presence of VHD, and a rapid escalation to other strategies (mechanical support and/or intervention) is strongly advisable.
Triggering factors must be recognised and treated immediately. In cases of acute coronary syndrome, urgent revascularisation is required regardless of VHD. Nishino et al showed that a shorter symptom onset-to-reperfusion time was an independent predictor of early MR improvement in AMI patients50. Out of 51 patients from the TAVI-shock registry, 33% had CAD, but only 1 (2%) presented with AMI51.
Other causative factors include valve thrombosis, especially within 12 months of prosthetic valve implantation, when it is the most common cause of valve dysfunction5253. Anticoagulation using vitamin K antagonists and/or unfractionated heparin is the first-line treatment of biological valve thrombosis. Fibrinolysis is an option (streptokinase was the most commonly used fibrinolytic agent, followed by tissue plasminogen activator and urokinase − all at standard recommended doses) in obstructive, especially mechanical, valve thrombosis54. However, considering the risks of bleeding, systemic embolism and recurrent thrombosis, emergency surgical valve replacement is recommended over fibrinolysis if immediately available and not contraindicated9.
Mechanical circulatory support devices
Evidence regarding outcomes of MCS in CS patients with VHD remains scarce, deriving mainly from small case series or registries515556575859, and there are no published guidelines for short-term MCS in this setting. Hence, unselected use of MCS is not supported and requires multidisciplinary expertise for device selection, implantation, and management. In persistent severe haemodynamic deterioration and CS despite medical support and removal of the triggering factor, early MCS could increase CO and end-organ perfusion as a bridge-to-recovery, bridge-to-destination or bridge-to-bridge111946. Different temporary MCS are currently available, including intra-aortic balloon pump (IABP), venoarterial extracorporeal membrane oxygenation (VA-ECMO), the Impella (Abiomed), the TandemHeart percutaneous system (LivaNova) and implantable LVAD. Device selection requires an in-depth understanding of anatomy, physiology, and the pathology of VHD194647.
In severe AS, most MCS options may be used47. VA-ECMO may increase LV afterload and, in some cases, concomitant LV unloading is mandatory, requiring unloading devices such as a microaxial flow pump device, if not contraindicated, or through atrial septostomy.
In MS, where LV end-diastolic pressure is generally low, the optimal device would be the TandemHeart (with direct left atrial [LA] drainage). However, especially in RV failure and hypoxaemia, VA-ECMO would be best, with the preferred use of the LA VA-ECMO modality.
In patients with CS due to AMI with papillary muscle rupture and acute MR, IABP may be considered, according to ESC Guidelines1160, to decrease afterload, to support adequate mean arterial pressure and to potentially decrease MR, despite minimal CO augmentation. ECMO can better support CO but is less commonly used alone, since it may increase the total peripheral vascular resistance, potentially worsening MR. More frequently, in MR physicians should consider the LA VA-ECMO modality to unload the LA or the Impella device, alone or with ECMO (i.e., ECPella), to directly unload the LV (caution is needed in papillary muscle rupture-related MR)194661.
Given AR pathophysiology, most (if not all) MCS are relatively contraindicated, especially in the presence of concomitant aortic dissection47. In fact, elevated diastolic blood pressure during IABP inflation and increased afterload due to VA-ECMO may both increase AR and contribute to LV distention. Similarly, the use of continuous flow implantable LVAD, and Impella (precluding aortic valve coaptation) may worsen AR and recirculation, reducing the device’s forward flow. If MCS is absolutely necessary in severe AR, TandemHeart or LA VA-ECMO could be considered because of their capacity for concomitant LA unloading194647.
MCS including ECMO, IABP, Impella and TandemHeart have also been used in high-risk transcatheter valve procedures during CS626364. This use varies widely depending on institutional practice and expertise, but it was demonstrated that a “standardised team-based approach” with predefined algorithms for early MCS implant and close monitoring of clinical signs, invasive haemodynamics and biochemical markers may translate into improved survival656667.
Valvular intervention
On top of pharmacological and organ-specific support, valvular intervention can be considered when VHD is the primary cause or an aggravating factor in CS (Central illustration). Significant VHD is associated with increased in-hospital mortality in CS patients68, and early treatment is advocated because delay between CS onset and valvular intervention predicted poor outcomes in patients with AS and CS4069. Interestingly, in the IREMMI Registry, the time between shock onset and TEER for acute MR was around 30 days70. Prior to TEER, 66% of patients were stabilised with IABP or Impella and 12% with VA-ECMO. However, the authors advocated for early MR correction irrespective of the LVEF and development of CS70.
The Heart Team must decide the indication, timing and mode of intervention (surgical versus transcatheter) after taking into account the patient’s clinical status and risk profile, anatomical considerations (i.e., type of VHD, presence of combined VHD, aortic disease or CAD), the role of VHD in the CS, as well as institutional expertise and the patient’s values and preferences. Contraindications for intervention in patients with CS, include the following:
1. Severe frailty, limited life expectancy (<12 months), or refusal of life-saving treatment;
2. Non-severe VHD;
3. End-stage CS with severe end organ failure (the “point of no return” was crossed);
4. CS complicated by resuscitated cardiac arrest with unfortunate neurological outcome;
5. Possibility and indication for urgent heart transplantation with or without previous MCS as a bridge therapy (i.e., end-stage HF patients with functional MR).
Some of these contraindications are relative and dynamic; hence, patients should be closely monitored and the decision adjusted according to the patient’s clinical status. Regarding the mode of intervention (surgical vs transcatheter), very few data are available to support either choice. Urgent/emergent cardiac surgery in VHD and CS is associated with high morbidity and mortality risks717273, and a less invasive approach with at least equal results might be preferable. This can be more intuitive with TAVI but is less evident with other transcatheter valve interventions. RCTs are needed to confirm this hypothesis, but they are hard to carry out in this setting. Regardless of the strategy, acute correction of VHD in CS patients can potentially reverse the fatal process, allowing for recovery and improving long-term patient outcomes. Emergent or urgent surgical treatment of VHD complicated by CS is advisable as a first-line therapy (particularly in young patients and those with low comorbidity) or as the only therapeutic option in certain settings (i.e., active endocarditis or acute AR associated to type A aortic dissection) if surgical risk allows it. A benefit of early surgery in infective endocarditis is uncertain because of a high recurrence rate, and its timing must be carefully selected. Therefore, surgery in the acute setting is restricted to specific clinical situations (HF, uncontrolled infection and prevention of embolic events) and eligible patients74. In other cases, and in the absence of haemodynamic refractory instability, surgery is postponed to allow 1 or 2 weeks of antibiotic treatment under careful clinical and echocardiographic observation7475.
Few surgical reports indicate that immediate surgical aortic valve replacement is feasible in critically ill and decompensated patients with AS, with an in-hospital mortality of 25-30%767778. In MR patients with CS, primary and secondary MR should be distinguished. In MR caused by AMI, the standard of care is acute surgical revascularisation with simultaneous mitral valve repair or replacement, despite the high risk in patients with CS (early mortality up to 20-30%)79. Perioperative short-term MCS may be beneficial. In acute ischaemic MR, only papillary muscle and chordal ruptures usually need immediate intervention. Accordingly, of ~8% of patients with CS due to severe MR complicating AMI in the SHOCK Trial Registry, only 46% underwent mitral valve surgery80. Surgery of papillary muscle rupture carries a higher mortality rate compared to regular mitral surgery owing to the acute setting80. As rapid deterioration after papillary muscle rupture is unpredictable, early intervention is mandatory, even though intravenous diuretic and vasodilator/inotropic support may initially stabilise patients81.
Emergency transcatheter valve treatments across different structural VHD complicated by CS are described below, in dedicated sections.
In general, specific contraindications for transcatheter intervention include the following:
1. Unfavourable valve or vascular anatomy;
2. Percutaneous intervention not achievable (i.e., intrachamber thrombus, valvular thrombosis, mitral valve anatomy not suitable for TEER − same contraindications as in stable patients);
3. Active endocarditis (for transcatheter implantation of devices and valvular replacement);
4. Feasible and potentially more beneficial valvular surgery despite increased surgical risk according to Heart Team consensus.
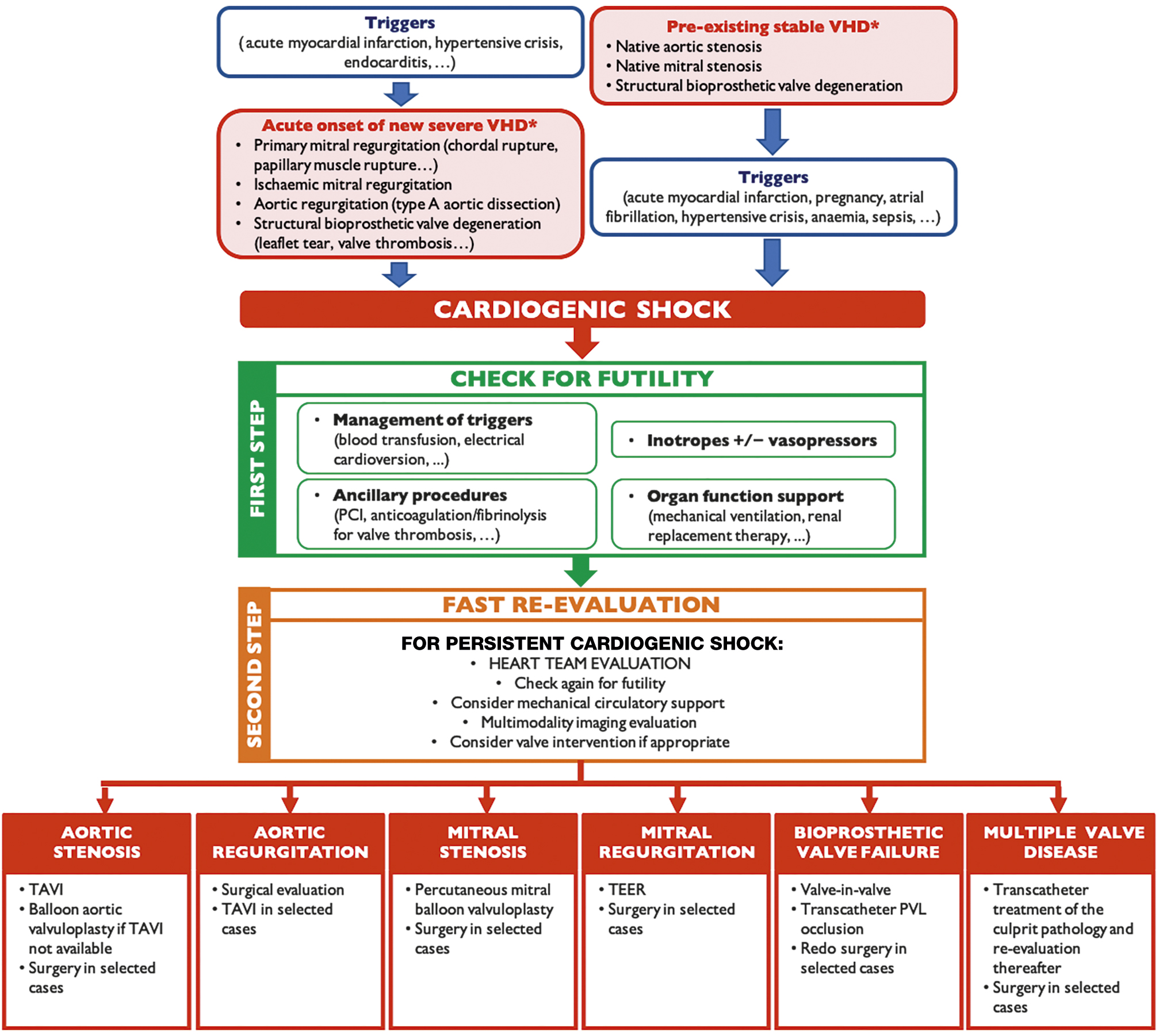
Central illustration. Diagnostic and therapeutic algorithm in cardiogenic shock and valvular heart disease. Diagnostic and therapeutic algorithm leading to valve intervention when valvular heart disease is either the primary cause or an aggravating factor in cardiogenic shock. *The mentioned valve disorders are the most common examples. PCI: percutaneous coronary intervention; PVL: paravalvular leak; TAVI: transcatheter aortic valve implantation; TEER: transcatheter edge-to-edge repair; VHD: valvular heart disease
Urgent/emergent transcatheter valve treatments across different VHD
Native AS and CS
TAVI has a class I indication in symptomatic severe AS patients at high or prohibitive surgical risk9. CS represents a high-risk surgical condition, but RCTs of TAVI in this setting are not available, as CS was an exclusion criterion in most RCTs evaluating therapies targeting both AS and HF. Therefore, current guidelines still recommend balloon aortic valvuloplasty (BAV) in AS with decompensated HF and/or CS for stabilisation as a bridge to TAVI or surgical aortic valve replacement (Class IIb, Level of Evidence C)9. However, despite the initial success of urgent BAV, early mortality of these patients remains high (up to 71%)82838485868788899091. Recently, urgent/emergent TAVI has been suggested as an alternative when available404969889293 (Table 2). Theoretical advantages of TAVI over BAV in this setting may be better and could offer sustained haemodynamic improvement with complete relief of afterload mismatch and low residual AR risk, potentially translating into better outcomes and lower rates of early readmission94. Notwithstanding, TAVI may be more challenging because of larger vascular access, higher risk of vascular complications and the inconstant availability and feasibility of preprocedural MSCT88. Moreover, urgent TAVI is not feasible in all hospitals, and a transfer might be needed. Finally, even in hospitals with TAVI availability, urgent TAVI may not be rapidly feasible for logistical reasons (Table 3).
Masha et al reported the largest TAVI series in CS (4.1% of the US TAVI population)40, comparing 2,220 emergent TAVI for CS to 12,851 high-risk patients without CS (median Society of Thoracic Surgeons [STS] score 10.2) included in the STS/American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) registry between 2014 and 2017. Despite similar optimal gradient relief, the CS population had higher complication rates and 30-day mortality (19.1% in patients with CS vs 4.9% in those without CS), primarily driven by preprocedural shock severity rather than procedural complications. The impact of CS duration prior to treatment is well known, and available evidence suggests that AS should be promptly corrected (BAV or TAVI) − ideally within 48 hours from CS onset, as >48 hours delay was linked to worse prognosis858889. However, the ideal time window and accurate criteria for intervention remain unknown. Given the procedural risks, it should be undertaken after Heart Team discussions in experienced centres. Decision-making should consider feasibility, efficacy and utility of emergent TAVI over other treatments, including medical management, BAV, durable LVADs, and palliative care (Figure 3). There is no uniform definition of futility; however, in TAVI, it can be described as death and/or an absence of functional improvement 6 to 12 months post-procedure95.
Considerable knowledge gaps also exist regarding specific technical considerations, such as 1) timing of the coronary revascularisation of concomitant CAD; 2) valve choice; and 3) usefulness of intraprocedural MCS (i.e., “protected TAVI”)9697. Regarding valve choice, there is no RCT to support this and interventionalists should rather use the device they are most familiar with. Furthermore, devices anticipating the best outcomes with least haemodynamic compromise during deployment should be preferred.
Table 2. Summary of registries on BAV and TAVI in CS.
| Author | Setting | Population | Age, years | LVEF, % | 30-day mortality, % |
|---|---|---|---|---|---|
| BAV in patients with CS | |||||
| NHLBI, 199182 | Multicentre | 39 BAV | - | - | 51.0 |
| Cribier et al, 199283 | Single centre | 10 BAV | 64±9 (54-79) | 25±6 | 20.0 |
| Moreno et al, 199484 | Single centre | 21 BAV | 74±3 (35-90) | 29±3 | 43.0 (in-hospital) |
| Buchwald et al, 200185 | Single centre | 14 BAV | 74±11 (50-91) | - | 71.0 |
| Saia et al, 201386 | Single centre | 23 BAV | 70±13 | 40±15 | 56.5 |
| Theiss et al, 201487 | Single centre | 13 BAV | 79±2 | 33±3 | 38.5 |
| Bongiovanni et al, 201788 | Multicentre | 118 BAV | 81±8 | - | 33.0 |
| Debry et al, 201889 | Multicentre | 44 BAV | 77±8 | 30±14 | 47.0 |
| Eugène et al, 201890 | Single centre | 17 BAV | 79±9 | 27±11 | 48.0 |
| Varela et al, 201991 | Single centre | 14 BAV | 76±7 | - | 21.4 |
| TAVI in patients with CS | |||||
| D’Ancona et al, 201292 | Single centre | 21 TAVI TA | 75±11 | 26±13 | 19.0 |
| Frerker et al, 201669 | Single centre | 27 TAVI | 78±9 | 40±15 | 33.3 |
| Bongiovanni et al, 201788 | Multicentre | 23 TAVI | 76±11 | - | 23.8 |
| Fraccaro et al, 201951 | Multicentre | 51 TAVI | 76±13 (31-93) | 43±15 | 11.8 |
| Huang et al, 201993 | Single centre | 31 emergent TAVI (26/31 in CS) | 73±14 | 32±15 | 19.4 |
| Masha et al, 202040 | Multicentre | 2,220 TAVI | 83 (median) | 53 (median) | 19.1 |
| Steffen et al, 2022150 | Single centre | 47 TAVI | - | - | 42.6 (at 90 days) |
| Data are n, mean±standard deviation, mean±standard deviation (range) or %, unless indicated otherwise. BAV: balloon aortic valvuloplasty; CS: cardiogenic shock; LVEF: left ventricular ejection fraction; TA: transapical; TAVI: transcatheter aortic valve implantation | |||||
Table 3. Pros and cons of BAV and TAVI in CS.
| BAV | TAVI | |
|---|---|---|
| Residual transvalvular gradient | ↑↑ | ↓↓ |
| Risk of significant postprocedural AR | ↑↑ | ↓ |
| Insertion profile | ↓ | ↑ |
| Availability | ↑↑ | ↑↓* |
| Feasibility | ↑↑ | ↑↓** |
| Costs | ↓↓ | ↑↑ |
| *potential need for transfer; **need for emergent CT scan AR: aortic regurgitation; BAV: balloon aortic valvuloplasty; CS: cardiogenic shock; CT: computed tomography; TAVI: transcatheter aortic valve implantation | ||
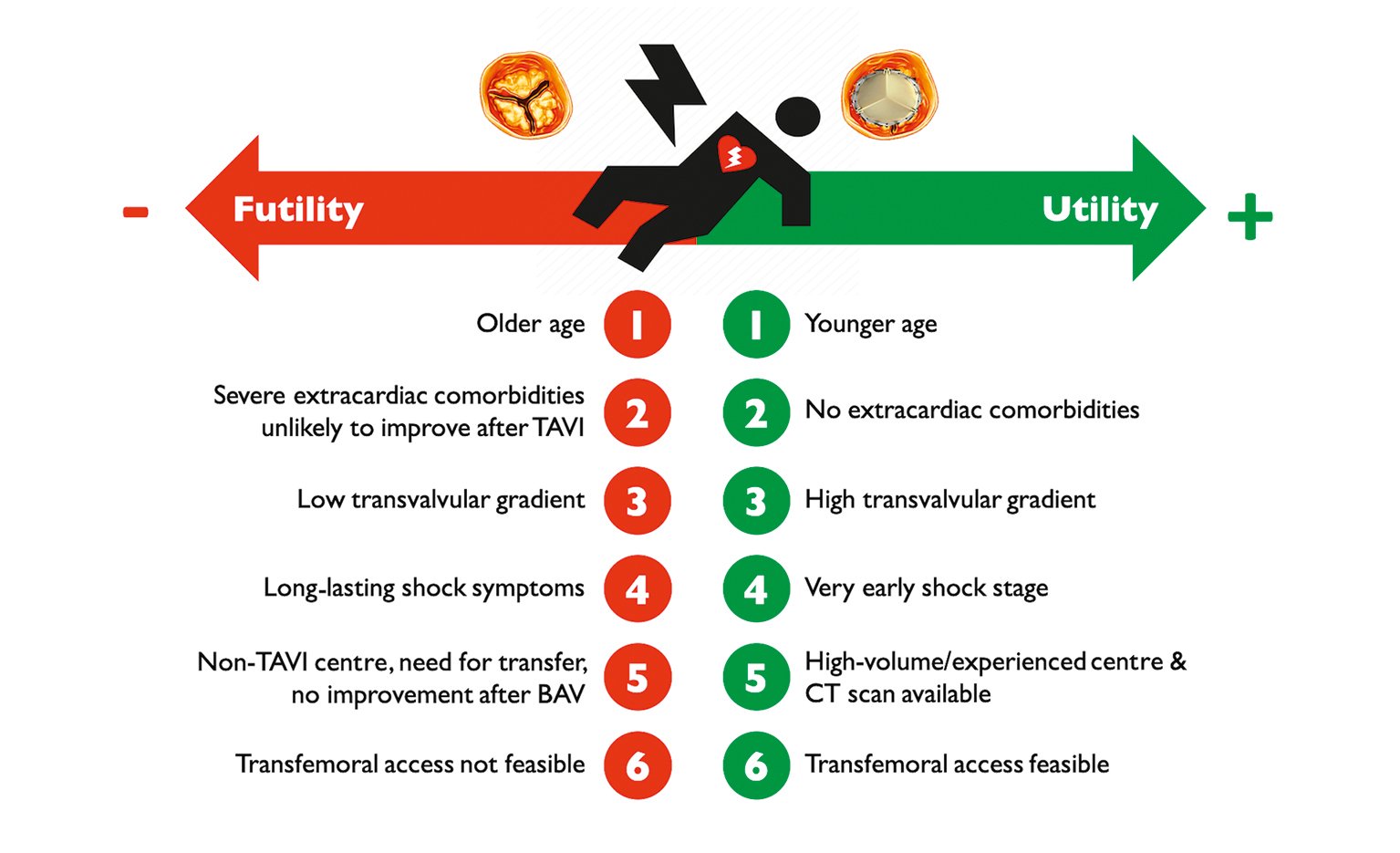
Figure 3. Factors influencing utility versus futility of emergent TAVI in case of patients with AS and CS. AS: aortic stenosis; BAV: balloon aortic valvuloplasty; CS: cardiogenic shock; CT: computed tomography; TAVI: transcatheter aortic valve implantation
Bicuspid aortic valve disease
In the specific and not uncommon setting of bicuspid severe AS, especially in youth, cardiac surgery should be considered. BAV can be undertaken in non-calcified valves with minimal AR (for example, during pregnancy), as a bridge to surgery. In older patients with calcified valves, TAVI remains an option, provided accurate valve evaluation, sizing and preprocedural planning by MSCT have been carried out.
Native AR and CS
Given the high surgical risk, TAVI may be an alternative for pure (non-calcified) AR9899100101102103104 also in CS, as reported in case reports or small series (Table 4)105106107108. It is generally contraindicated in endocarditis and aortic dissection. The Endo-Bentall procedure for transcatheter treatment of acute aortic dissection complicated by acute AR is promising109. Concerning AR in LVAD patients, casuistics and meta-analyses demonstrate the challenges and potentials of transcatheter treatment107110.
There is currently only one European conformity (CE)-marked device for pure AR98. The procedural challenges with TAVI in pure AR include 1) lack of calcification for annulus visualisation and valve anchoring; and 2) large annular size exceeding the manufacturer’s recommendations for available transcatheter heart valve sizes. Future device iterations may overcome these limitations.
Table 4. Summary of studies (single case reports) on emergent TAVI for the treatment of acute AR during CS.
| Author | Setting | Treatment |
|---|---|---|
| Spina et al, 2019105 | Acute AR after iatrogenic surgical injury during complicated mitral valve surgery | Transfemoral TAVI (Medtronic Evolut R) |
| Herrmann et al, 2017106 | Acute AR after iatrogenic injury from Impella implantation | Transfemoral TAVI (Edwards Lifesciences SAPIEN 3) |
| Van der Werf et al, 2017107 | Acute AR in an LVAD patient | Transfemoral TAVI (Medtronic CoreValve) |
| Abdelaziz et al, 2018108 | Acute AR and aortic root dissection after previous supracoronary aortic replacement | Transapical TAVI (Edwards Lifesciences SAPIEN S3) |
| AR: aortic regurgitation; CS: cardiogenic shock; TAVI: transcatheter aortic valve implantation | ||
Native MS and CS
Percutaneous mitral balloon valvuloplasty (PMBV) in rheumatic MS has revolutionised the treatment of rheumatic MS since its introduction in 1984111112. It is recommended for severe symptomatic MS without unfavourable anatomical characteristics for mitral commissurotomy, according to the 2021 ESC/EACTS Guidelines for the management of VHD9.
This is particularly appealing in CS, as the procedure is less invasive than surgery and can be performed quickly, on an emergency basis, and under local anaesthesia. Several case reports have described its use in this setting113114115116. In patients who are not good candidates for PMBV, transcatheter mitral valve implantation (TMVI) could offer a minimally invasive alternative, even though most techniques are much more challenging, require general anaesthesia and thorough preprocedural planning. Besides, widespread availability and longer-term follow-up is lacking.
In the specific case of pregnant women with severe HF, use of PMBV has been described with substantial improvement in clinical outcomes and acceptable safety117. Its yield in CS has been described in a case report118. Radiation exposure during PMBV carries a foetal risk, especially during organogenesis. Every effort should be made to postpone the procedure to the second trimester, after the fourth month, when organogenesis is complete and the thyroid is still inactive119. However, when CS occurs, postponing the procedure may not be possible. In this case, radiation doses should be kept as low as reasonably achievable, and dedicated protocols are warranted to minimise foetal radiation and iodine-based contrast medium because of the risk of neonatal hypothyroidism (Supplementary Table 2).
Native MR and CS
Postoperative outcome of emergency surgery (repair or replacement) for acute severe MR, regardless of aetiology, is poor with an overall 30-day mortality of 22.5%, even higher in AMI-related MR complicated by CS (up to 26.9%)120. The role of transcatheter interventions in patients with MR and CS has not been fully demonstrated. There are no specific RCTs completed to date − the “Transcatheter Mitral Valve Repair for Inotrope Dependent Cardiogenic Shock (MINOS)” trial is ongoing (ClinicalTrials.gov: NCT05298124) − and patients in CS were excluded from landmark trials of transcatheter mitral valve repair121122123. However, several case reports and recent observational studies described good results (Table 5)586170124125126127128129130.
Available evidence concerns almost exclusively one TEER device; use of TMVI in this setting has not been reported. Of note, most data pertain to secondary, especially ischaemic, MR.
Comparison of studies is limited by differences in population, methods and outcome assessment. Still, available evidence suggests that the MitraClip (Abbott) is associated with high procedural success (72.7-100%), and acceptable short- and midterm outcomes (30-day or in-hospital mortality 0-27.3%, with a single-centre study reporting 30-day mortality of 60%; 6-month or follow-up mortality 16.7-63.0%)586170124125126127128129130. In the largest study published to date, Jung et al pooled data from several observational studies and performed a patient-level analysis of 141 patients with CS and moderate to severe acute ischaemic MR; 78.7% of patients required inotropes and about half were on MCS. Most had secondary MR (75.2%). Procedural success was high (88.7%), with a relatively low overall mortality (in-hospital mortality 15.6%, 90-day mortality 29.5%, and 1-year mortality 42.6%). Successful TEER was associated with a 74% relative reduction in both in-hospital and 90-day mortality124. Tang et al compared the outcome of patients with CS and MR receiving the MitraClip during the index hospitalisation to those who did not, using propensity-matched data from the Centers for Medicare and Medicaid Services in the US. They showed increasing device use throughout the study and significantly lower in-hospital (24.8% vs 35.4%; odds ratio 0.6, 95% confidence interval [CI] 0.47-0.77; p<0.001) and 1-year mortality (hazard ratio 0.76, 95% CI: 0.65-0.88; p<0.001) in patients undergoing TEER. This benefit was consistent in all subgroups, except for patients requiring acute MCS or haemodialysis at the time of intervention131.
These preliminary results, even if encouraging, should be considered with caution. More robust data should be obtained, and the role of other techniques including TMVI must be assessed. In the meantime, TEER in patients with CS should be considered only in experienced hands and after careful feasibility evaluation.
Table 5. Observational studies of transcatheter mitral valve repair in patients with MR and CS*.
| Author | Setting | n | Clinical scenario | Device | Mechanical circulatory support | Procedural success | Outcomes |
|---|---|---|---|---|---|---|---|
| Adamo, 2017125 | Single centre | 4 | Secondary MR: 100% (acute MR post-AMI 100%) | MitraClip | IABP: 100% | 100% | 30-day mortality: 0% |
| Seizer, 2017126 | Single centre | 10 | N/A | MitraClip | IABP: 30.0% ECMO: 70.0% Impella: 30.0% |
90.0% | 30-day mortality: 60.0% |
| Flint, 2019127 | Single centre | 12 | Primary MR: 33.3% Secondary MR: 16.7% Mixed MR: 50.0% |
MitraClip | IABP: 33.3% ECMO: 8.3% |
75.0% | 30-day mortality: 16.7% Follow-up mortality (median 198 days): 41.7% |
| Chan, 2019128 | Single centre | 27 | Primary MR: 7.4% Secondary MR: 92.6% (ischaemic 92.0%) |
MitraClip | IABP: 18.5% | 92.6% | 30-day mortality: 25.9% Follow-up mortality (mean 202 days): 63.0% |
| Cheng, 201958 | Single centre | 29 | Secondary MR: 100% (non-ischaemic 65.5%, ischaemic 34.5%) | MitraClip | Impella: 17.2% IABP: 10.3% |
96.6% | In-hospital mortality: 17.2% Survival to 6 months: 75.6±8.0% |
| Garcia, 2020129 | Single centre | 11 | Primary MR: 63.6% Secondary MR: 36.4% |
MitraClip | IABP: 45.5% | 72.7% | 30-day mortality: 27.3% 1-year mortality: 66.7% |
| Jung, 2021124 | Multicentre | 141 | Primary MR: 23.4% Secondary MR: 75.2% Mixed MR: 1.4% |
MitraClip | 50.4% | 88.7% | In-hospital mortality: 15.6% One-year mortality: 42.6% |
| Estévez-Loureiro, 202170 | Multicentre | 50 | Secondary MR: 100% (acute MR post-AMI 100%) | MitraClip | IABP/Impella: 66.0% VA-ECMO: 12.0% |
90.0% | 30-day mortality: 10.0% Follow-up mortality (median 7 months): 28% |
| Vandenbriele, 202161 | 2 centres | 6 | Primary MR: 50.0% Secondary MR: 50.0% |
MitraClip | Impella: 100% | 100% | In-hospital mortality: 16.7% 6-month mortality: 16.7% |
| Falasconi, 2021130 | Multicentre | 31 | Secondary MR: 100% (papillary muscle rupture 12.9%) | MitraClip | IABP: 58.1% Impella: 22.6%ECMO: 6.5% |
87.1% | 30-day mortality: 22.6% 6-month mortality: 38.7% |
| *Studies including exclusively patients with cardiogenic shock or studies also including patients without cardiogenic shock but in which data on patients with cardiogenic shock could be extracted from the manuscript. AMI: acute myocardial infarction; CS: cardiogenic shock; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; MR: mitral regurgitation; N/A: not available; VA: venoarterial | |||||||
BVF and CS
According to the EAPCI consensus132 and Valve Academic Research Consortium-3 definition20, BVF is defined by 1) clinically expressive bioprosthetic valve dysfunction or irreversible severe HVD, 2) need for valve reintervention, and 3) valve-related death132133134.
Transcatheter treatment for Stage 3 HVD and CS depends on the underlying pathology and time from index procedure.
Short interval (<12 months)
Valve thrombosis is the most common cause of dysfunction5253. Its treatment was described earlier in this document. New valve regurgitation is related to valve migration, PVL, or endocarditis135. Plug implantation is the gold standard for non-surgical patients with PVL136137138. A valve-in-valve procedure can restore valve function and haemodynamics in unstable patients with a migrated transcatheter or sutureless valves and in inoperable patients with subacute endocarditis139140.
Long interval (>12 months)
The most common causes of BVF are degeneration and endocarditis, even if endocarditis decreases 1 year after valve implantation (approximately 1% per person-year vs 0.5% per person-year after 1 year)141. In case of valve-in-valve, characteristics of the bioprosthesis should be taken into account (Table 6). In the context of CS, patients would require a fast and effective transcatheter procedure. However, some anatomical conditions, like a small internal diameter of a degenerated bioprosthesis and a high coronary obstruction risk, or outflow tract obstruction risks, will require sophisticated techniques to achieve an optimal procedural outcome142143 (Table 7). Those situations will require general anaesthesia to allow TOE guidance and potentially MCS to stabilise haemodynamics during longer procedures.
Table 6. Transcatheter treatment options in case of BVF.
| No/mild calcifications | Severe calcification | |
|---|---|---|
| Stenosis | Valve-in-valve | Valve-in-valve (consider cerebral protection)151 |
| Regurgitation | Valve-in-valve Plug in case of severe PVL135136137 |
Valve-in-valve (consider cerebral protection)151 |
| BVF: bioprosthetic valve failure; PVL: paravalvular leak | ||
Table 7. Advanced techniques to overcome complex situations for valve-in-valve.
| Complex situations for valve-in-valve | Advanced techniques |
|---|---|
| Small aortic bioprosthesis (label size ≤21 mm) | Bioprosthetic valve ring fracture |
| Risk of coronary obstruction | Coronary chimney stenting, endovascular electrosurgery leaflet splitting144 |
| Risk of LV outflow tract obstruction in mitral valve-in-valve | Endovascular electrosurgery leaflet splitting145, alcohol septal ablation |
| LV: left ventricle | |
Conclusions
CS is a clinical condition with extremely high morbidity and mortality, and concomitant severe VHD is associated with increased mortality68. Acute onset of severe VHD may be the cause of CS, or triggering factors acting on pre-existing stable severe VHD can cause CS. In both situations, pharmacological support is the first-line therapy, including removal and treatment of triggering factors. However, if a patient’s haemodynamic status is not quickly reverted, rapid escalation to other non-pharmacological treatment, particularly correction of concomitant VHD, may be required. The treatment decision should consider procedural utility and futility. Given the extremely high mortality risk, less invasive transcatheter valve interventions can be used as an alternative to surgery in several situations. Heart Teams must guide decision-making regarding indications, timing and mode of intervention, according to patients’ clinical status and risk profile, anatomical considerations, VHD role, institutional expertise, and patients’ values and preferences. As outlined above, to date, most evidence stems from case series and registries. The very high mortality risk warrants dedicated, well-designed and adequately powered RCTs to further elucidate the role of transcatheter valvular interventions and could, if positive, have significant public health implications. While CS RCTs are complicated by time pressures and patients’ heterogeneity, clear inclusion criteria render such trials feasible and effective144. We believe that in the meantime, all CS patients, if not eligible for ongoing RCTs, should be included in registries embedded in a network of networks or a hub-and-spoke registry, that will allow high-quality retrospective analyses in a large, worldwide dataset, and may assist in future registry-based RCTs145.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement
N. Bonaros reports grants from Edwards Lifesciences and Corcym; and lecture fees from Edwards Lifesciences and Medtronic. P. Carrilho-Ferreira reports lecture fees from AstraZeneca, A. Menarini Diagnostics, Bayer, Biotronik, Medinfar, and Medtronic; and serves on an advisory board for Medtronic. M. Czerny is a consultant for Terumo Aortic, Medtronic, Endospan, and NEOS; and is a shareholder of TEVAR Ltd and Ascense Medical. C. Fraccaro reports support for attending meetings from Medtronic. C. Hassager reports research grants from the Novo Nordisk Foundation and the Lundbeck Foundation; and lecture honorarium from Abiomed. N. Karam reports consulting and lecture fees from Medtronic, Edwards Lifesciences, and Abbott Vascular. W-K. Kim reports lecture fees and honoraria from Abbott, Boston Scientific, Meril Life Sciences, Edwards Lifesciences, Medtronic, and Shockwave Medical. K.A. Krychtiuk reports lecture and/or consulting fees from Amgen, Novartis, and Sanofi. H. Möllmann received speaker honoraria/proctor fees from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. J. Pręgowski reports lecture fees from Abbott and Edwards Lifesciences; and contracts from Abbott. G. Tarantini reports lecture fees from Medtronic, Edwards Lifesciences, Abbott Vascular, Boston Scientific, GADA, and Abiomed. J. Ternacle reports consulting fees from Abbott, GE HealthCare, and Philips; and lecture fees from Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

