
For a decade, percutaneous balloon aortic valvuloplasty (BAV) was a treatment option for high surgical risk and inoperable patients with severe aortic stenosis (AS). It was first described by Cribier et al in 19861. Six years later, in 1992, Cribier et al reported the results of 10 patients treated by BAV as a life-saving procedure in an emergency setting due to cardiogenic shock (CS)2. In 2012, BAV was regarded as a bridge to surgery or transcatheter aortic valve implantation (TAVI) in haemodynamically unstable patients at high surgical risk, and as a palliative treatment alternative in patients for whom neither surgery nor TAVI was an option3.
In this issue of EuroIntervention, Debry and co-workers focus on the periprocedural and one-year outcomes of urgent BAV as a rescue therapy in patients with CS due to severe AS4.
The authors studied 44 patients with acutely decompensated severe AS who were treated by BAV at two centres. They divided the patients into two groups according to their blood pressure, hypotensive (n=31) and non-hypotensive CS (n=13). Hypotensive CS patients were classified as “classic” shock patients with a systolic blood pressure <90 mmHg and catecholamine dependency. Non-hypotensive CS patients had a systolic blood pressure >90 mmHg without vasopressor therapy but a combination of low cardiac index (<2.2 l/min/m²) and peripheral hypoperfusion. All patients were at extremely high surgical risk with a mean EuroSCORE II of 41.6±13.7%. The BAV procedure was performed 1.2±0.5 and 4.1±2.9 days after admission to the intensive care unit in patients with non-hypotensive CS and hypotensive CS, respectively. In 88.6% of the procedures, BAV was considered successful, defined as a reduction in transaortic pressure gradient of at least 50% and without moderate-to-severe aortic regurgitation (AR).
One-month and one-year mortality was 47% and 70%, respectively. Patients with a successful procedure had significantly lower in-hospital mortality (33% versus 80%; p=0.04). Furthermore, surviving patients had a lower post-procedural mean transaortic pressure gradient (22.5±9.7 vs. 30.5±12.6 mmHg; p=0.03). Univariate analysis identified a preoperative dose of dobutamine >5 µg/kg/min and a BAV delayed for >48 hrs as predictive of the primary endpoint of mortality or recurrent CS at one year.
The authors concluded that “despite initial success of urgent BAV, morbidity and mortality of CS related to severe AS remains dramatically high and is directly related to the duration of shock. Performing BAV before starting inotropic agents or within 48 hours of their initiation appears to be key to survival”.
So, should every patient in CS secondary to severe AS undergo a BAV procedure today? And what is the role of TAVI in this setting? The first TAVI procedure described by Cribier et al was performed in 2002 in a patient with CS due to severe AS5. This patient was declined cardiac surgery because of severe comorbidities and a haemodynamically unstable condition. He had undergone emergency BAV one week earlier, which was initially successful. However, the patient’s condition deteriorated with recurrent CS within one week. TAVI was then successfully performed5.
The key question here is: why not perform TAVI directly instead of BAV in patients with CS? We know from the literature that BAV has a high rate of recurrent AS. Patients in this study even had severe AS despite a successful procedure. The mean aortic valve area after BAV was 0.82±0.20 cm², corresponding to severe AS in the majority of patients. One could argue that particularly patients in CS should be treated optimally with respect to the correction of AS. The lower the transaortic pressure gradient in a patient with CS and severely reduced left ventricular ejection fraction the better the outcome. This was also shown by Debry et al in the lower post-procedural mean transaortic pressure gradients in surviving patients. When performing TAVI, the transaortic pressure gradient is going to be much lower than after BAV. One could argue that after TAVI patients will have a better outcome.
The second argument in favour of TAVI is the lower risk of significant post-procedural AR. A meta-analysis of more than 15,000 TAVI patients showed a 2.12-fold increase in overall (≥1 year) all-cause mortality in cases with ≥moderate AR6.
Third, transfemoral TAVI with the latest-generation devices can be performed via 14-16 Fr access. The mean French size for BAV in the current analysis was 11.6±0.9. This difference versus a TAVI procedure is rather low; thus, TAVI can be performed safely in most cases.
Despite these arguments favouring TAVI there are also arguments against TAVI. First, TAVI is not available at every hospital. Unstable patients in CS should not be transferred to another hospital because of the risk of further deterioration during transportation. Second, the size of the required transcatheter valve prosthesis can, in most cases, only be measured via echocardiography and angiography. A computed tomography scan of the aortic valve and the access vessels – which is the current state of the art – cannot be readily carried out in every patient without the risk of further clinical deterioration.
In summary, the paper by Debry et al underlines the need for a discussion on the best treatment option for patients with CS secondary to severe AS. In this author’s opinion, in experienced centres TAVI should be carried out as a first-line therapy provided this option is available and the aortic annulus can be measured safely and reliably, e.g., with 3D echocardiography. In all other centres and situations, BAV should be discussed as a treatment option, especially as a bridge to TAVI or surgical aortic valve replacement (Figure 1).
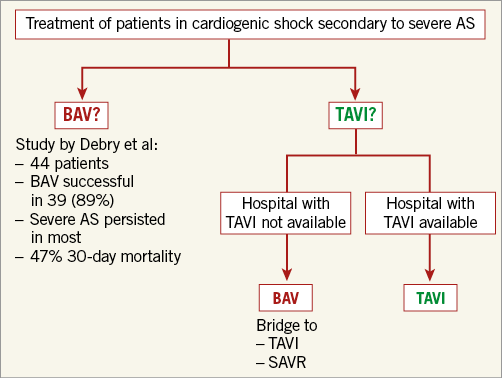
Figure 1. Decision tree for the emergency treatment of patients in cardiogenic shock secondary to severe aortic stenosis (AS). BAV: balloon aortic valvuloplasty; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation
Conflict of interest statement
C. Frerker received lecture honoraria and travel support from Medtronic, Edwards Lifesciences and Abbott Vascular.

