
Introduction
Balloon aortic valvuloplasty (BAV) was originally devised as an alternative approach for treating aortic valve stenosis (AS) in patients who could not undergo aortic valve replacement (AVR)1. However, BAV only provided temporary clinical and haemodynamic improvement and its use was therefore limited to palliative therapy2. Transcatheter aortic valve implantation (TAVI) has revolutionised the treatment of symptomatic severe AS in elderly patients with an elevated operative risk3. Treating this increasingly frail population has renewed interest in a therapy which could temporarily alleviate symptoms in order to bridge patients to definitive valve replacement therapy or other urgent therapy.
INDICATIONS FOR A SINGLE-ACCESS BAV
Actual BAV indications include unmasking the futility for AVR, bridging a period of acute heart failure or bridging to non-cardiac therapy4,5,6. BAV is still used for palliative purposes, though less frequently. In the past, BAV proved to be challenging with regard to access-site management, optimal balloon sizing and efficient gradient monitoring7. Contemporary procedure simplification has tackled these issues and, additionally, converted BAV into a single-access intervention, without the need for general anaesthesia and minimising the burden for a high-risk population. Herein, we describe the step-by-step BAV technique incorporating contemporary access-site management, balloon size selection tailored to the procedure objectives, evaluation of BAV effect and guidewire-mediated pacing.
Methods
STEP 1: PREPARATION
All of the required materials are illustrated in Figure 1 and Figure 2. The procedure is typically conducted under local anaesthesia with the administration of subcutaneous lidocaine at the access site only.
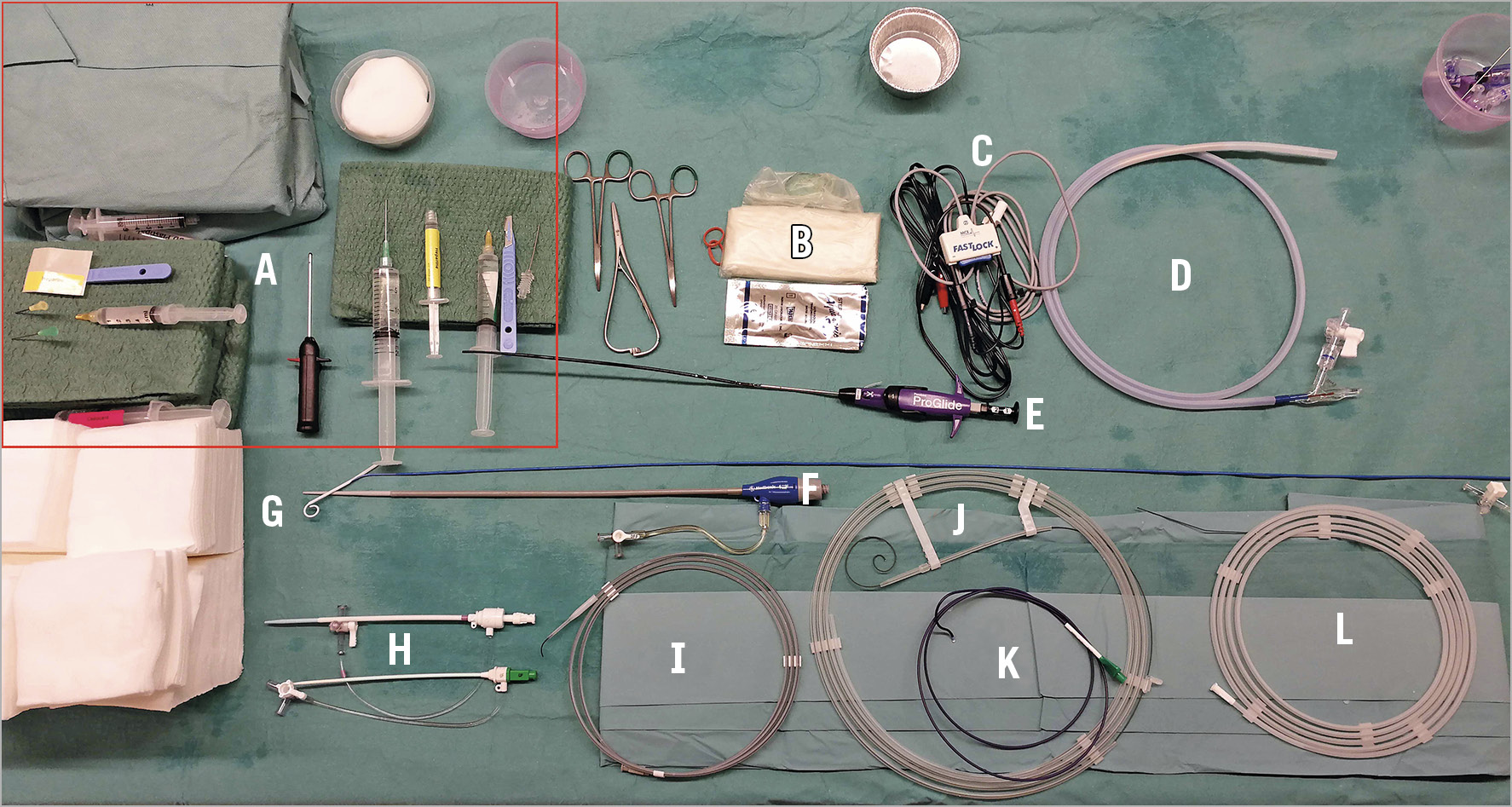
Figure 1. Pre-procedure set-up of a single-access balloon aortic valvuloplasty. A) Needles and local anaesthetic. B) Sterile ultrasound probe cover. C) Alligator clamps to be connected to external pacemaker wires. D) Valvuloplasty balloon, in this example a True Dilatation Balloon. E) Suture-based vascular closure device, in this example a ProGlide device. F) 12 Fr introducer sheath, in this example a Sentrant™ (Medtronic, Minneapolis, MN, USA). G) 6 Fr Langston Dual Lumen Catheter, with its white pigtail tip. H) Vascular access sheaths. Top: 10 Fr Fast-Cath™ Hemostasis Introducer (St. Jude Medical, St. Paul, MN, USA). Bottom: 6 Fr Input® Introducer Sheath (Medtronic). I) Peripheral J-shaped guidewire 0.038”, Radifocus® (Terumo Corp., Tokyo, Japan). J) Guidewire, Safari 0.035”. K) Amplatz Left 2, 6 Fr diagnostic coronary wire (Medtronic). L) Diagnostic guidewire 0.035” Straight, AngioFLEX™ (Kimal plc, Worcester, UK).
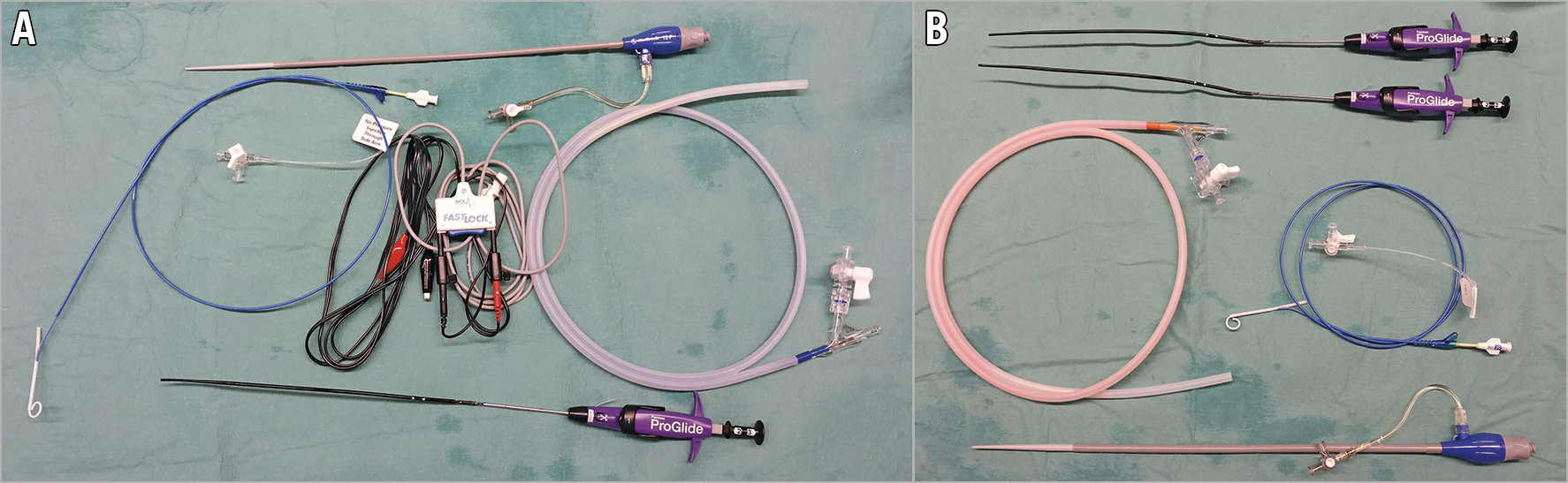
Figure 2. Balloon aortic valvuloplasty: occluding vs non-occluding balloon. A) Essential requirements for BAV with occluding aortic balloon: introducer sheath 12 Fr (top), Langston Dual Lumen Catheter (centre left), alligator clamps connected to external pacemaker wires to facilitate pacing over the wire (centre), aortic valvuloplasty balloon (centre right), one suture-based vascular closure device (bottom). B) Essential requirements for BAV with non-occluding balloon: two suture-based vascular closure devices (top), non-occlusive aortic valvuloplasty balloon (centre left), Langston Dual Lumen Catheter (centre right), introducer sheath 16 Fr.
STEP 2: ACCESS-SITE MANAGEMENT
Ultrasound (US) guidance facilitates the identification of the common femoral artery and helps to avoid calcified segments and to prevent side wall or posterior wall puncture8. US-guided access precludes additional radiation exposure to the patient and operator, increases first pass success and reduces time to successful insertion9. The US system requires a linear 3-9 MHz probe (probe L9-3) with colour Doppler.
Access to the common femoral artery is obtained under US guidance for a 12 to 16 Fr sheath. First, the femoral artery bifurcation is identified in a long-axis view (Figure 3A, Moving image 1). A 90-degree counter-clockwise probe rotation pictures the superficial and common femoral artery in a short-axis view. By advancing the probe in a cranial direction, the exact location of the femoral artery bifurcation and common femoral artery is obtained. Colour Doppler helps to appreciate pulsatile flow in the artery and compressible continuous flow in the surrounding venous structures. With the US probe steady in one hand, the operator can advance a hollow needle with the other hand to puncture the common femoral artery under direct vision. After successful needle entry in the common femoral artery, a 6 Fr sheath is inserted per conventional Seldinger technique. The sheath is ultimately upscaled for a larger sheath to accommodate the valvuloplasty balloon.
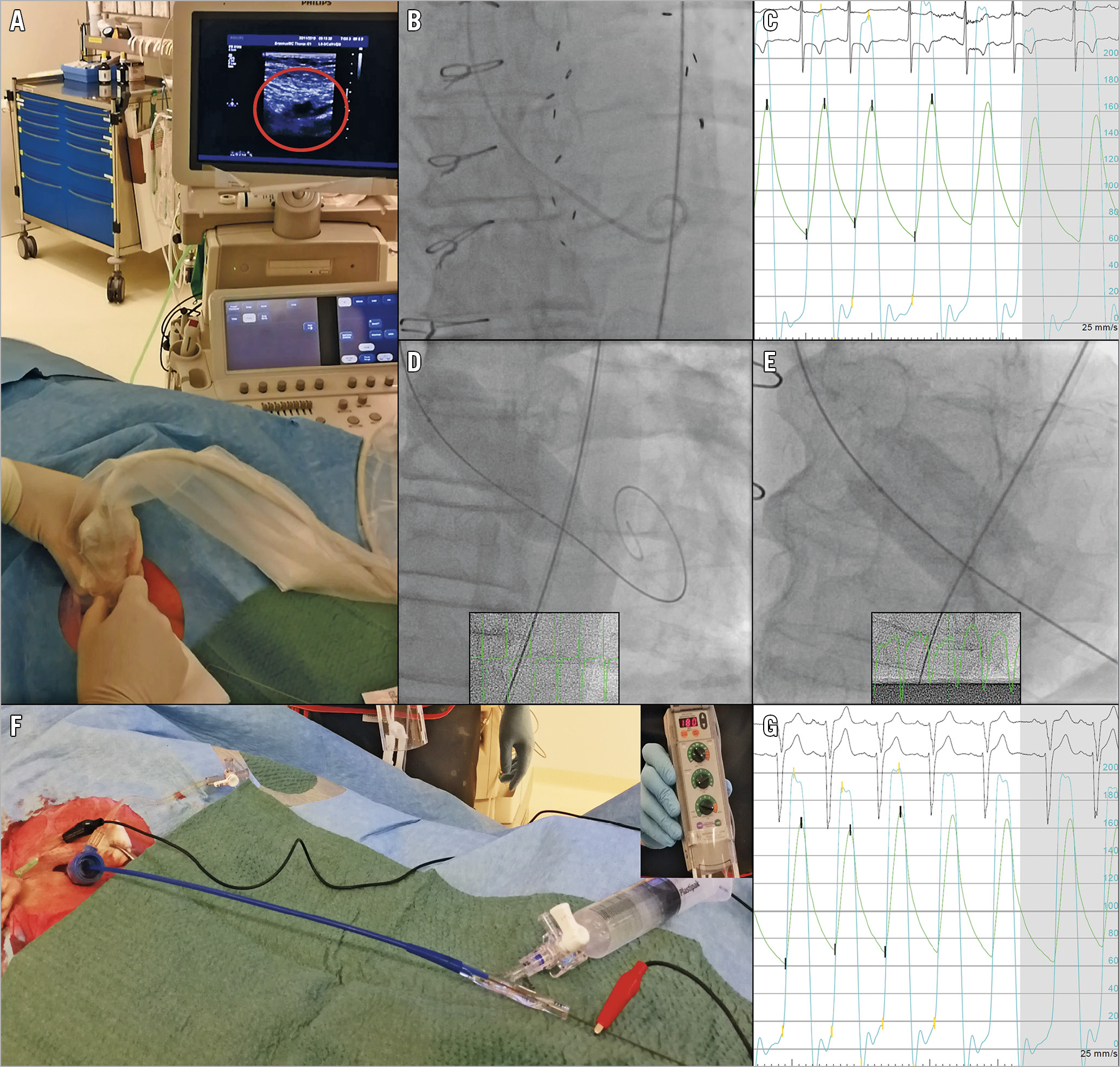
Figure 3. Balloon aortic valvuloplasty: procedural details. A) Ultrasound-guided puncture of the right common femoral artery, with tenting of the vessel wall shown in the red circle. B) Fluoroscopic image of the Langston Dual Lumen Catheter in situ. C) Simultaneous aortic (green) and ventricular (light blue) pressure measurement prior to BAV, peak-to-peak gradient of 70 mmHg. D) Fully inflated occlusive balloon under rapid pacing. E) Fully inflated non-occlusive balloon on angiography without rapid pacing. F) Single-access pacing over the wire. The cathode (red) is connected to the stiff wire (right); the anode (black) is connected to a subcutaneous needle adjacent to the femoral access site of the guidewire in the patient’s groin (left). In the upper right corner of the image the external pacemaker is shown, set to a pacing rate of 180 bpm. G) Simultaneous aortic and ventricular pressure measurement post BAV. Note a significant reduction of transvalvular gradient, peak-to-peak gradient of 35 mmHg.
STEP 3: PRE-BAV TRANSVALVULAR PRESSURE GRADIENT MEASUREMENT
The aortic valve is crossed with a 0.035” straight-tipped guidewire through a 6 Fr AL-1 or AL-2 diagnostic catheter. Once crossed, the diagnostic catheter is exchanged for a 7 Fr Langston® Dual Lumen Pigtail Catheter (Vascular Solutions, Inc. [now Teleflex], Minneapolis, MN, USA) (Figure 3B). This catheter has separate exits at the catheter’s end and 7.9 cm more proximally, allowing simultaneous pressure recordings in the left ventricle (LV) and the ascending aorta. Alternatively, a standard pigtail and introducer sheath can be used for simultaneous pressure measurement. The invasive haemodynamic assessment includes determination of the transvalvular pressure gradient and the aortic regurgitation index (ARI) (Figure 3C). With a cut-off <25, the ARI has 86.3% sensitivity and 75.1% specificity for identifying at least moderate AR. The specificity increases to 93.2% if one relies on ARI before and after intervention10. An ARI >25% makes more than moderate AR unlikely11.
After obtaining the pre-BAV pressure gradient, a 0.035” stiff guidewire is inserted through the Dual Lumen Catheter, which is exchanged for a valvuloplasty balloon. Current-generation pre-shaped stiff wires limit ventricular perforations (e.g., Safari™ guidewire [Boston Scientific, Marlborough, MA, USA], Confida™ [Medtronic, Minneapolis, MN, USA], and the INNOWI® [Symedrix GmbH, Deisenhofen, Germany]).
STEP 4: BALLOON SELECTION
The balloon size can be based on the minimum diameter of the aortic annulus by multiplanar reconstruction if multislice computed tomography (MSCT) evaluation of the aortic root is available or on the left ventricular (LV) outflow tract diameter as measured by transthoracic echocardiography (TTE). Deliberate balloon undersizing (relying on the smallest annulus diameter by MSCT) may reduce the risk of BAV-induced aortic regurgitation, aortic rupture, trauma to the cardiac conduction system and, arguably, debris dislodgement. Furthermore, published data suggest clinical efficacy with limited increases in post-BAV aortic valve area (AVA)6,7. Conventional BAV techniques require rapid ventricular pacing for balloon stability across the aortic valve during inflation (Figure 3D, Moving image 2). Novel, non-occlusive balloon technology consists of a ring of multiple cylinder-shaped balloons with a central orifice, allowing blood to pass during inflation (True™ Flow balloon; Bard Peripheral Vascular Inc., Tempe, AZ, USA) and tends to be more stable during inflation, potentially precluding rapid pacing and minimising haemodynamic instability during BAV12 (Figure 2B, Figure 3E, Figure 4). Of note, the non-occlusive balloon requires a larger access sheath (i.e., 16 Fr) than a standard valvuloplasty balloon.
Figure 4. Non-occlusive aortic valvuloplasty balloon. True Flow balloon inflated. Note the palisade-shaped balloons creating a lumen in the centre allowing fluid to traverse while inflated.
STEP 5: GUIDEWIRE-MEDIATED PACING
Rapid pacing is generally required to stabilise the aortic balloon during inflation13. With the single-access technique there is no deep venous access, hence no temporary pacing wire. Instead, the stiff wire in the LV over which the balloon is advanced across the aortic valve is connected to an external pacemaker source with alligator clamps (Figure 3F),14. The cathode is connected to the wire, and the anode is connected to a subcutaneous needle adjacent to the femoral access site of the guidewire in the patient’s groin. Previous studies have reported successful procedural guidewire-mediated pacing in all performed BAVs without the need for additional venous access in any of the patients15,16. Novel balloon technology may preclude the need for ventricular pacing (e.g., the True Flow balloon) (Figure 4). However, due to its relatively short length, stable positioning of the non-occlusive balloon may be challenging. A certain degree of pacing (i.e., 120-140 bpm) may still be needed to stabilise the balloon.
Rapid pacing is performed over the LV guidewire at a rate of between 180 and 220 bpm to allow stable balloon inflation. Total ventricular pacing time is approximately 10 seconds (Figure 3C).
STEP 6: POST-BAV TRANSVALVULAR PRESSURE GRADIENT MEASUREMENT - ASSESSING BAV EFFICACY
After balloon inflation, haemodynamics are reassessed with the dual-lumen catheter (Figure 3G). Multimodality assessment of aortic regurgitation is performed by contrast aortography, TTE and calculation of the ARI.
STEP 7: ARTERIOTOMY CLOSURE
Percutaneous arteriotomy closure, using either suture- or plug-based closure devices, concludes the procedure. Arteriotomy closure relies on either suture-based closure with one or two ProGlide® devices or one Prostar® (both Abbott Vascular, Santa Clara, CA, USA) through a pre-closure technique or using dedicated large-bore closure devices17. Suture-based closure allows immediate re-access to the puncture site. Collagen-based closure needs approximately six months of resorption time.
Conclusion
BAV has become a simplified and safe single-access technique and still represents a valuable asset in the treatment toolbox for patients with severe AS. This step-by-step description of the contemporary BAV procedure may aid the interventionalist in managing the procedure efficiently.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Ultrasound-guided puncture of the right common femoral artery.
Moving image 2. Balloon aortic valvuloplasty: inflation under rapid pacing.

