Abstract
Aims: The introduction of transcatheter aortic valve implantation (TAVI) has generated a renewed interest in the treatment of high-risk patients with severe aortic stenosis. This study describes the indications and long-term outcome of balloon aortic valvuloplasty (BAV) in recent years.
Methods and results: Between 2000 and 2010, 415 consecutive patients at our institution underwent BAV. The number of BAV per year increased sharply after the introduction of TAVI. Patients were 77.5±10.9 years old and showed important comorbidities (average logistic EuroSCORE=23.9±15.3%). We identified four cohorts according to the indications: 1) bridge for TAVI (B-TAVI; n=162); 2) bridge for aortic valve replacement (B-AVR, n=97); 3) cardiogenic shock (n=23); 4) palliation (n=133). Baseline characteristics were significantly different among groups. In-hospital mortality was 5.1%, and occurred predominantly in patients who underwent BAV in the setting of cardiogenic shock (56.5% vs. around 2% in the other subgroups). Other major events were stroke (0.5%), major vascular complications (2.2%), and life-threatening bleedings (1.5%). The cumulative one-year and two-year mortality rates were 33.2% and 57.4%, respectively, with the highest incidence in the shock group (70.7% and 80.4%) and the lowest in the B-AVR group (21.7% and 38.4%). Rehospitalisation for heart failure was 26.3% at one-year and 47.2% at two-year follow-up.
Conclusions: The number of BAV is increasing, mainly due to increased referral of high-risk patients and to the emerging indication of bridge for TAVI. In this complex population, BAV is relatively safe but two-year survival remains poor, and more effective and definitive treatments should be pursued in a timely fashion.
Introduction
Until recently, percutaneous balloon aortic valvuloplasty (BAV) had been almost abandoned in most catheterisation laboratories because of the limited prognostic impact and the perceived high procedural risk. Indeed, large registries reported an incidence of serious complications (including death, stroke, myocardial infarction, prolonged hypotension and shock, cardiopulmonary resuscitation, pulmonary oedema, cardiac tamponade, severe aortic regurgitation, need for pacemaker implantation, systemic embolisation and vascular surgery) around 25% within the first 24 hours and in-hospital mortality between 3.5% and 10%1-3. Current guidelines recommend BAV with very restrictive indications: bridge to aortic valve replacement (AVR) in haemodynamically unstable patients at high risk for surgery or in patients with symptomatic severe aortic stenosis (AS) who require urgent major non-cardiac surgery, and palliation in occasional individual cases4. The recent advent of transcatheter aortic valve implantation (TAVI) has led to a renewal of interest in BAV, opening the way to new possible indications5,6.
With populations ageing, the prevalence of degenerative AS is increasing progressively7. Data from many studies indicate that a considerable proportion of patients referred for TAVI are eventually not eligible for the procedure8-11. The use of BAV for palliation of symptoms has probably been undervalued in this group. Given these premises, it is assumed that the number of BAV might increase in the next few years and a reconsideration of the technique and its clinical results seems advisable.
The aim of this study was to assess the clinical outcome of a large cohort of patients undergoing BAV with different indications, and to ascertain the factors associated with mortality during follow-up.
Methods
PATIENT POPULATION
At our institution data on all patients with severe symptomatic AS (aortic valve area, AVA<1 cm2 and AVA indexed <0.6 cm2/m2) undergoing BAV between 2000 and July 2010 were prospectively collected in a dedicated database. Patients were retrospectively subdivided into four cohorts according to the indications: 1) bridge for TAVI (B-TAVI); 2) bridge for AVR (B-AVR); 3) cardiogenic shock; 4) palliation of symptoms in patients without other therapeutic options (palliation). In accordance with guidelines the B-AVR group included patients potentially eligible for AVR who required urgent major non-cardiac surgery (for example because of cancer) or haemodynamically unstable patients at high risk for cardiac surgery. Patients with cancer were included in this group only if they had a reasonable chance of healing and of subsequently undergoing AVR. The B-TAVI group included patients deemed ineligible for surgical AVR with a temporary contraindication to the TAVI procedure defined by one or more of the following factors10,12: very low left ventricular ejection fraction (LVEF <30%), frailty or enfeebled status, symptoms of uncertain origin (i.e., dyspnoea in patients with concomitant severe chronic obstructive pulmonary disease, COPD), critical conditions (acute pulmonary oedema, acute coronary syndrome), ≥grade 3 mitral valve regurgitation.
BALLOON AORTIC VALVULOPLASTY
All procedures were performed with the Cristal Balloon™ (Balt, Montmorency, France) using the standard retrograde technique. A low-dose heparin bolus was administered after sheath insertion in all patients (40-50 IU/kg). As per local protocol, in the vast majority of cases BAV was performed with a 20 mm diameter balloon unless annular diameter measured by transthoracic echocardiography was >24 mm or <18 mm. In fact, the semi-compliant balloon material enables some variation of diameters above or below the nominal level. Thus, balloon diameter was modulated with manual inflation in order to achieve a balloon-to-annulus ratio 1:1, confirmed by the complete sealing of the valvular orifice and aortic pulse abrogation. The objective was to perform three manual inflations with complete abrogation of aortic pulse for a few seconds. Rapid ventricular pacing was used only occasionally. The procedure was terminated based on one of the following criteria: three dilatations with complete pulse abrogation independent from haemodynamic results; balloon rupture or exchange and evidence of average gradient reduction of ≥50%; acute severe aortic regurgitation. Access site haemostasis was accomplished using the 8 Fr Angio-Seal™ device (St. Jude Medical, St. Paul, MN, USA), application of a compressive bandage, and bed rest for 24 hours. The Cristal Balloon is, in fact, compatible with 9 Fr (18 mm and 20 mm) and 10 Fr (23 mm and 25 mm) introducer sheaths, which can be safely sealed with the 8 Fr Angio-Seal™ device13.
DEFINITIONS AND OUTCOMES
Peripheral artery disease included a history of claudication, previous vascular surgery, or documented peripheral arterial stenosis >50%. Chronic kidney disease (CKD) was considered to be a glomerular filtration rate <60 ml/min calculated by the Cockcroft-Gault formula. The diagnosis of anaemia was made at a level of serum haemoglobin <13 g/dL for men and <12 g/dL for women. Severe COPD was identified by a forced expiratory volume in one second <1 litre or long-term use of bronchodilators, steroids or oxygen. Cardiogenic shock was characterised by systolic blood pressure <90 mmHg with signs of low peripheral perfusion or the necessity to administer inotropes for circulatory support. The logistic European System for Cardiac Operative Risk Evaluation score (EuroSCORE) was calculated for all patients. The occurrence of the following in-hospital events was recorded: death, myocardial infarction (MI), stroke, vascular complications, bleedings, acute kidney injury (AKI), surgical AVR, severe acute aortic regurgitation. Myocardial infarction was defined by a rise in the creatine kinase level to more than twice the upper normal limit with an increased creatine kinase-MB or newly developed Q-waves. Stroke was diagnosed by a neurologist on the basis of neurological signs or symptoms consistent with stroke and a positive neuroimaging study. Vascular complications, bleedings and AKI were classified retrospectively using the Valve Academic Research Consortium (VARC) criteria14. Severe acute aortic regurgitation was defined as a quick and deep drop of aortic diastolic pressure and the rapid equilibration of LV and aortic pressures which was not present at baseline, associated with fast circulatory collapse. We evaluated the incidence of the following events during follow-up: death, MI, stroke, hospitalisation for heart failure (HF), AVR, TAVI, repeat BAV. Follow-up was complete for all patients and used different sources: hospital files, outpatient clinic, hospital discharge records, and municipal civil registries of mortality.
STATISTICAL ANALYSIS
Continuous variables were expressed as mean±standard deviation and comparisons between groups were performed with the one-way analysis of variance (ANOVA). Categorical variables were compared using Pearson’s chi-square test. All tests were two-sided and statistical significance was defined as p<0.05. Cumulative event rates were estimated using the Kaplan–Meier method and compared by the log-rank test. Patients were censored at the time of the last contact. In order to avoid the bias of an intention-to-treat analysis especially in the bridge groups (risk of overestimating the benefit of BAV in these groups by prolonging the follow-up beyond the TAVI or the AVR procedure), the last follow-up for all patients undergoing TAVI or AVR following BAV was considered the day of these procedures. The Cox proportional hazard regression method (and a logistic regression analysis at 30 days) was used to examine the association of clinical, echocardiographic and procedural variables with mortality during follow-up. Multivariate analyses with all variables with p≤0.10 at univariate analysis were performed to identify independent predictors of mortality at 30 days and one year. In order to avoid overadjustment, the EuroSCORE was excluded from the analyses, whilst all the single variables used to calculate it were included. Similarly, variables showing a significant correlation with other variables were not included in the multivariate analysis. All analyses were performed with the SAS v9.2 (SAS Institute, Cary, NC, USA).
The study was conducted according to the Declaration of Helsinki. The local medical ethics committee approved the protocol and written informed consent was obtained from every patient.
Results
The study included 415 consecutive patients; the number of BAV per year is shown in Figure 1. Patients undergoing BAV were 77.5±10.9 years old, with a slight predominance of women (55.7%) and a high prevalence of concomitant diseases (Table 1). The average logistic EuroSCORE was 23.9±15.3%. When comparing the four subgroups, the compassionate (palliation) cohort was significantly older, with a greater prevalence of neurological dysfunction and dyspnoea. Patients in the shock cohort were younger, with a greater prevalence of previous MI and CKD, lower LVEF, and more often presenting with acute coronary syndrome (ACS). The B-AVR patients more frequently had cancer but a lower logistic EuroSCORE, whilst in the B-TAVI cohort there were more cases of porcelain aorta and CKD, and a mild trend towards older age, more frequent COPD, PAD, and prior cardiac revascularisation (Table 1).
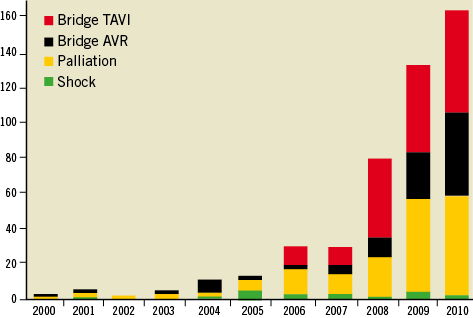
Figure 1. Number of balloon aortic valvuloplasty procedures per year.
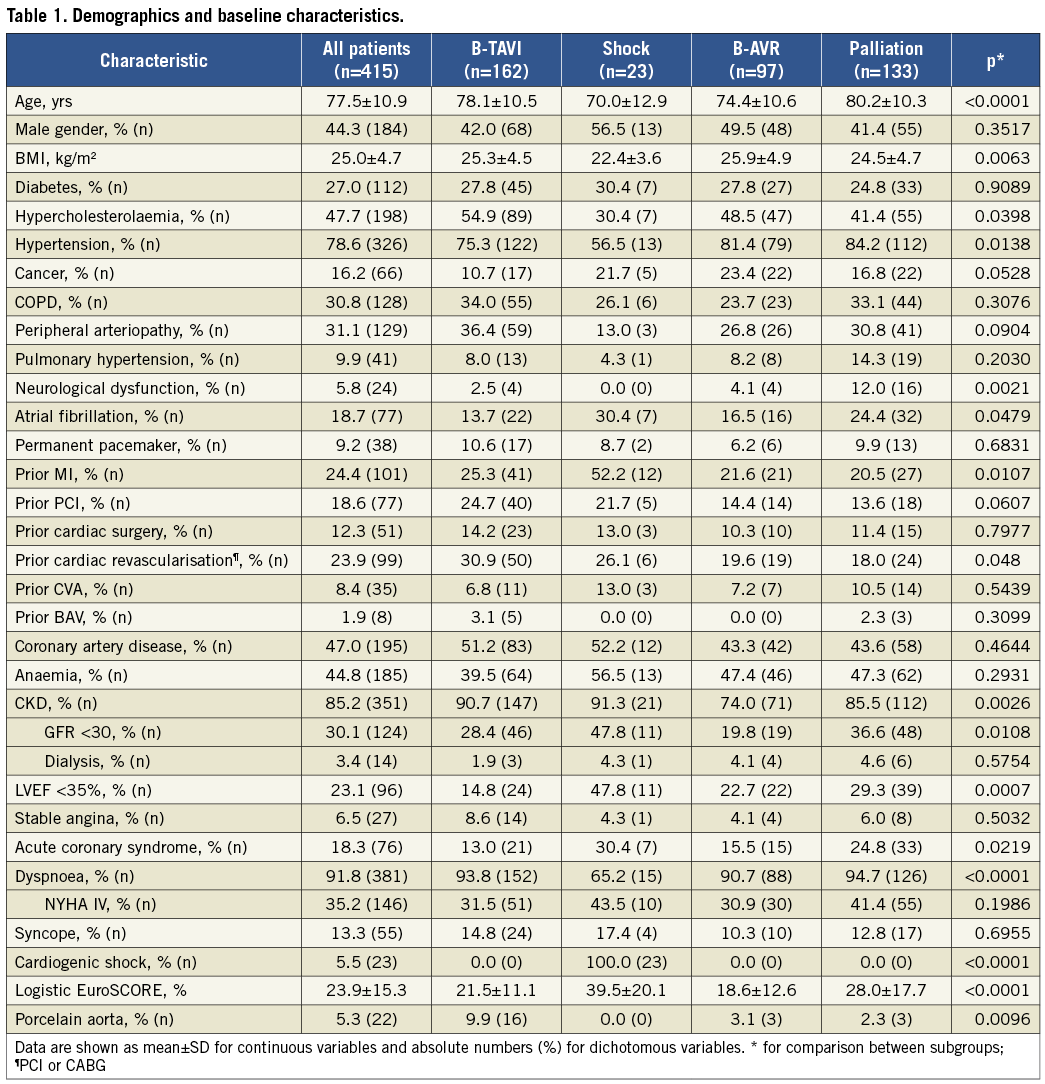
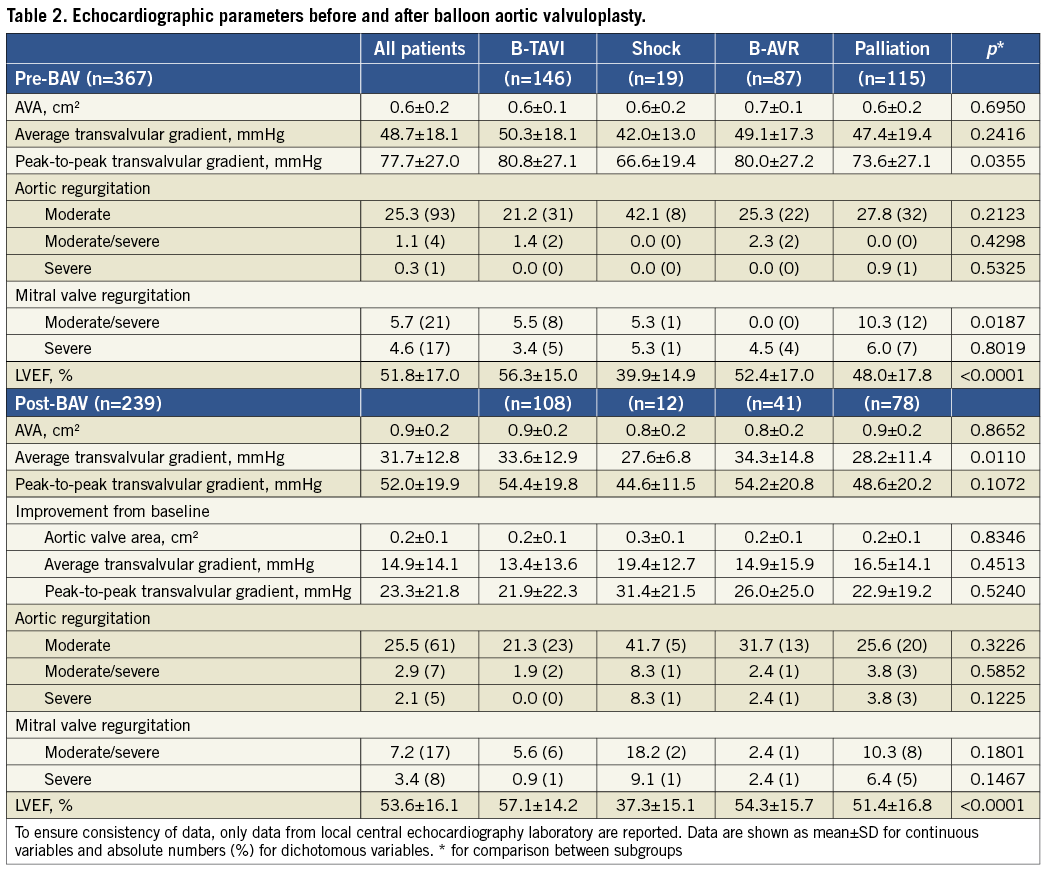
Table 2 summarises echocardiographic parameters before and immediately after BAV, whilst haemodynamic and procedural data are shown in Table 3. In 331 patients (79.8%) we were able to perform at least three dilatations with complete pulse abrogation; in 40 patients (9.6%) the procedure was terminated because of a reduction ≥50% of the mean transvalvular gradient after balloon rupture or exchange; in the remaining 44 patients (10.6%) the procedure was terminated prematurely for other reasons (acute aortic regurgitation, patient intolerance, other clinical problems). Acute aortic regurgitation occurred in 11 patients (2.6%), but it was successfully managed in the catheterisation laboratory in eight (final grade ≤2). In fact, in the majority of cases massive aortic regurgitation is caused by the immobilisation of a cusp in the open position. The cusp can be remobilised through the manipulation of a pigtail catheter, reinforced with a stiff guidewire, inserted into the sinus of Valsalva, between the aortic wall and the blocked cusp15. Among the three patients who left the catheterisation laboratory with acute aortic regurgitation, one died shortly after BAV (presentation was cardiogenic shock), the second was discharged with severe AR and died two months later of terminal heart failure, and the third one survived and showed a reduction to moderate AR at three-month echocardiography. Nine months after BAV he underwent successful AVR. The incidence of post-procedural severe aortic regurgitation appeared somewhat higher at echocardiography, being 0.3% at baseline and 2.1% after BAV. Overall, ≥50% gradient reduction was achieved in 215 patients (51.8%), 30-49% reduction in 105 (25.3%), and <30% in 95 (22.9%). After three dilatations with complete pulse abrogation, 175 patients (53.2%) achieved ≥50% gradient reduction and 75 (22.8%) a reduction between 30% and 49%.
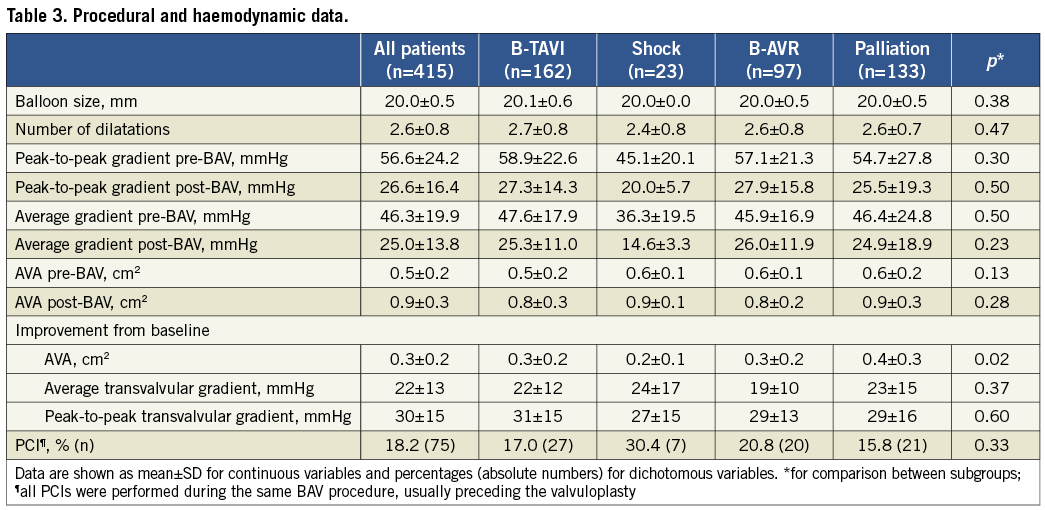
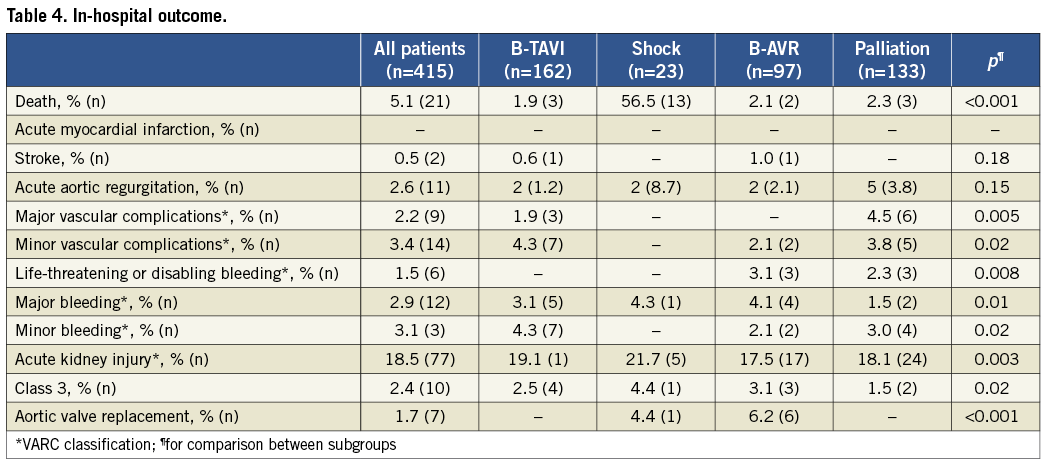
In-hospital mortality was 5.1%, and occurred predominantly in patients with cardiogenic shock (56.5% vs. around 2% in the other subgroups) (Table 4). There were eight deaths in the other subgroups, but only in four cases was death related to periprocedural complications (one cardiac tamponade, one mesenteric artery embolisation, one severe acute aortic regurgitation with multiorgan failure, and one infective endocarditis); all these patients were severely compromised before the procedure. The other four patients who died were in a critical condition before the procedure and did not show any improvement afterwards. The incidence of stroke was 0.5% (two patients, one with complete functional recovery). Life-threatening bleedings occurred in 1.5%, major vascular complications in 2.2%, and severe AKI in 2.4% of the patients.
Post-discharge follow-up was available for 394 patients (94.9%); one-year follow-up was available for 383 patients (92.3%) and two-year follow-up for 303 patients (73%). Median follow-up was 595 days (IQR 455-896). The cumulative one-year and two-year mortality rates were 33.2% and 57.4% (Figure 2, Table 5), respectively, with the highest incidence in the shock group (70.7% and 80.4%) and the lowest in the B-AVR group (21.7% and 38.4%). Rehospitalisation for HF was 26.3% at one-year and 47.2% at two-year follow-up. Among these patients, during the first year 51.6% were readmitted only once and 23.7% twice. When taking into account only those undergoing BAV for symptomatic relief (palliative group), the corresponding rates were 47.7% and 29.5%, respectively. At two-year follow-up, 42.2% had been rehospitalised only once and 28.8% twice. Information about NYHA functional Class was available for 365 patients (88%) at 13±12 months: 57.3% were in Class I-II, and 42.7% Class III-IV, which is consistent with rates of hospital readmission. Overall, 30.8% of the patients underwent a repeated BAV procedure within two years from the index procedure. As expected, AVR was performed mainly in the B-AVR group (33.2% of the patients after two years), whereas TAVI was performed in 49.8% at one-year and 58.1% at two-year follow-up of the B-TAVI group. The average delay between BAV and TAVI was 6.0±5.3 months, and between BAV and AVR it was 6.6±5.1 months. Patients who underwent TAVI showed a very good clinical outcome with a cumulative incidence of death of 13.1% at one year and 19.4% at two years. Patients undergoing AVR showed a mortality of 0 and 33.7% at one year and two years, respectively (p=ns for all), despite a numerically higher incidence of stroke at both time intervals (one year: 4.2% AVR vs. 1.1% TAVI, p=NS; two years: 8.5% AVR vs. 1.1% TAVI, p=0.08).
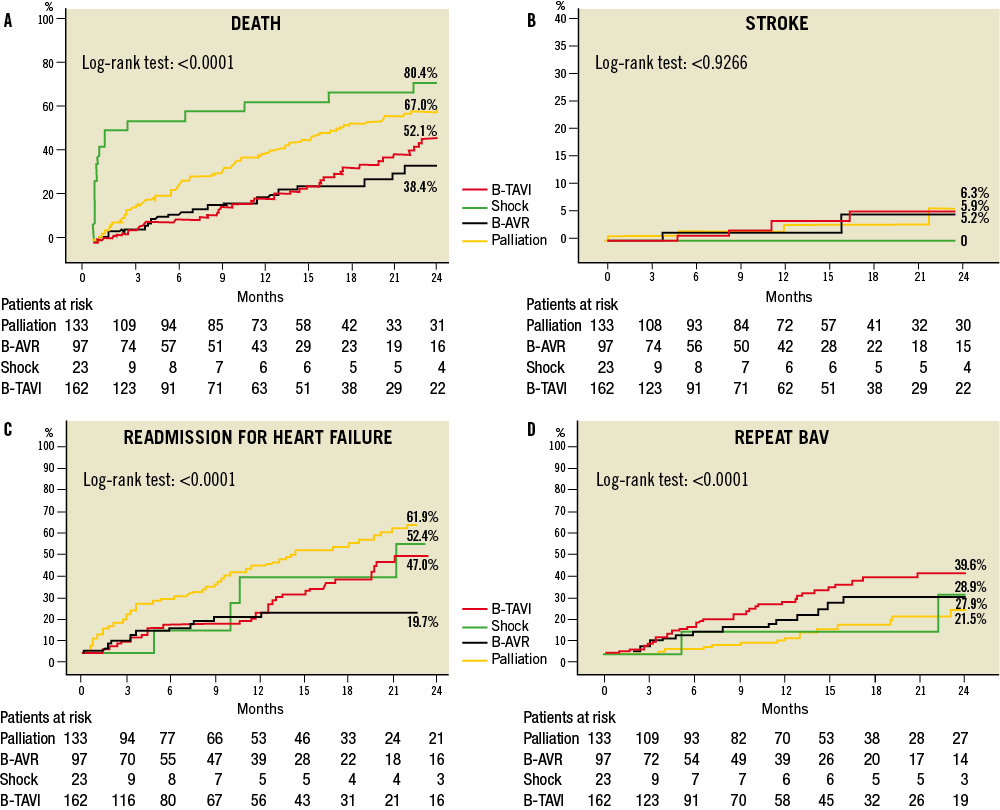
Figure 2. Two-year clinical outcome after BAV for different indications. A) Death. B) Stroke. C) Rehospitalisation for heart failure. D) Repeat BAV. BAV: balloon aortic valvuloplasty; B-TAVI: bridge for TAVI; B-AVR: bridge for AVR
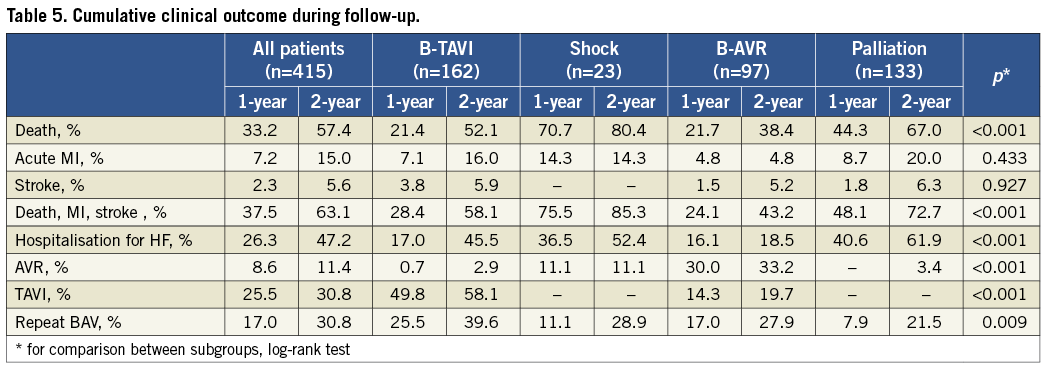
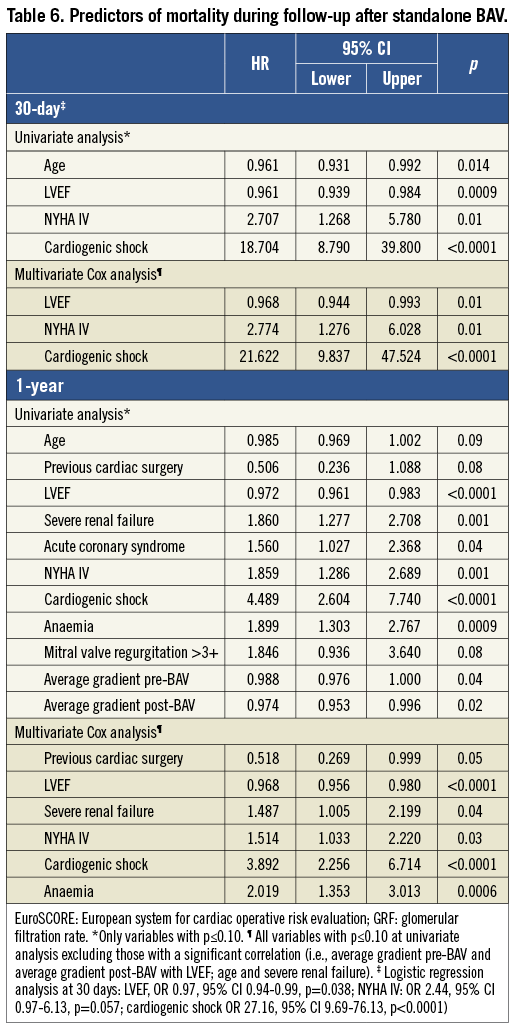
Table 6 lists the variables independently associated with mortality during follow-up after standalone BAV.
Discussion
This study reports the largest series of BAV after data from the historical National Heart, Lung, and Blood Institute Balloon Valvuloplasty Registry1 and the Mansfield Scientific Aortic Valvuloplasty Registry2 became available around 20 years ago. It provides a broad perspective on BAV indications and outcomes in recent years, through the description of a tertiary care centre experience. The main findings of the study are the following: 1) the number of BAV procedures increased sharply after the introduction of TAVI and will probably continue to increase in the near future; 2) new clinical indications for BAV have emerged in the TAVI era; 3) BAV is a relatively safe procedure in elective conditions but the mid-term clinical outcome remains poor.
The increase of BAV procedures after the start of our TAVI programme in 2007 is very evident. Other authors have observed a surge in referral for treatment of AS after the advent of TAVI, mainly due to increased referral of elderly patients with multiple risk factors who were previously untreated16,17. Remarkably, a significant proportion of patients remain without any option of definitive treatment despite the availability of TAVI8-10. The performance of a large number of balloon aortic valvuloplasties as a palliative therapy could be debated in the era of TAVI and selection criteria must necessarily be restrictive. The PARTNER trial and a wealth of other clinical studies have clearly demonstrated that TAVI is superior to medical therapy and BAV for inoperable patients with aortic stenosis18. As a consequence, all inoperable patients who are eligible for this procedure should receive it as soon as possible. However, with some exceptions, we can offer to most of the remaining no-option patients a low-cost and relatively safe procedure in experienced hands, associated with significant immediate haemodynamic and clinical improvement, and with an improved quality of life (and probably of death)19. Pain control and palliative service are an important part of medical care, and BAV might well fit within this area even in the era of TAVI. Some investigators suggested that repeat BAV might also improve three-year survival rates over a single dilatation20; a strategy of close outpatient clinic follow-up and eventual repeat BAV might merit further evaluation, but only in patients not eligible for TAVI. In our population, around one third of surviving patients had at least a second BAV procedure within two years: the second procedure was always performed for symptom recurrence and only if TAVI or surgical AVR were not indicated (palliative therapy or, for the bridge groups, because of the persistence of the temporary contraindication). Nevertheless, rehospitalisation for HF was relatively frequent (26.3% at one year and 47.2% at two years) confirming that BAV is often not durable, and, whenever possible, alternative treatments such as TAVI and AVR must be preferred in order to improve both symptoms and outcome18,20. Interestingly, however, among rehospitalised patients within the palliation group, around 80% were readmitted only once or twice during the first year (only falling to 70% after two years); this may further support a palliative success. Prospective studies addressing quality of life are mandatory before drawing strong conclusions.
Some of the patients referred for TAVI might be “temporarily” ineligible for the procedure. In a recent report we described how, in selected cases, BAV can be used as a bridge to TAVI12. In our series BAV allowed a careful reallocation of patients to their final therapeutic strategy (including surgical AVR in a considerable proportion of patients previously excluded). Perhaps, as also suggested by others, clinical improvement of critically ill patients after BAV might allow undertaking a safer TAVI procedure5. Nevertheless, a careful case-by-case evaluation by a multidisciplinary team is mandatory in order to estimate the risk/benefit ratio of a bridge BAV procedure in patients who are potential candidates for TAVI. A sizeable shift in the initial destination therapy was observed in both bridge groups, with some patients in the B-AVR group actually undergoing TAVI during follow-up and vice versa. This is consistent with the need for one or more subsequent clinical evaluations after BAV in the bridge groups. A change in clinical status can induce the “Heart Team” to reconsider the initial therapeutic strategy.
BAV is commonly perceived as a very high-risk procedure, based on the observations from the early registries1,2. Indeed, other early reports were most reassuring3, but at that time more emphasis was given to the lack of a positive prognostic impact of BAV22. In our large cohort, in line with other recent reports16, rates of periprocedural adverse events were certainly not trivial but, in our opinion, acceptable taking into account the patients’ characteristics. Short-term mortality, in line with previous reports1,16, was strongly linked to presentation with cardiogenic shock (HR 16.9, Table 6) and in the vast majority of cases causally linked to baseline critical status. In our series, the incidence of stroke was very low (0.5%) suggesting that major embolisation of debris from the aortic valve is a rare phenomenon with experienced operators, although silent microembolisation cannot be ruled out from our data. Acute aortic regurgitation deserves a special mention because it is a dramatic event leading to fast circulatory collapse and death. Over the years, we have learnt that the immobilisation of one of the cusps in the open position is a frequent underlying mechanism of this complication. Diagnosis must necessarily be immediate and can only be done with fluoroscopy. We described a coarse but quite effective method to manage acute aortic regurgitation, i.e., manipulation of a “reinforced” pigtail catheter to remobilise the “culprit” cusp15. The efficacy of the manoeuvre can be monitored in real time with aortic and left ventricular pressure curves. In eight out of 11 cases, we were able to improve the situation. Of course, alternative, more effective treatments must be considered (for example, TAVI or emergency AVR in potential candidates). In general, however, these options are not viable for patients with circulatory collapse. Remarkably, when compared to prior studies, we observed a significant reduction in vascular and bleeding complications1,2. Although our explanation can only be speculative, this might be the result of some pharmacologic and device-related advances over the last twenty years. Utilisation of a 20 mm balloon in the vast majority of patients allowed for the usage of smaller femoral arterial access sheaths and closure with the collagen-based closure device. Consistent with our data, a low rate of transfusions and vascular complications has been reported by the Washington group with this technique in quite a large number of patients, despite the use of 12 and 13 Fr sheaths in almost half of the cases13. Low heparin doses were probably helpful in the event of device failure and in reducing the incidence of occult bleedings.
A final observation is that in our series around 40% of the patients undergoing BAV as a bridge for TAVI or AVR did not undergo any of these procedures within two years. Whilst a good proportion of these patients were excluded for objective clinical reasons (terminal disease, persisting contraindication), some patients refused definitive treatment and others died while on the waiting list. Interestingly, due to our study design (i.e., termination of follow-up and censoring of the patients at the moment of TAVI or AVR), we assessed the outcome of these subgroups “as treated” only with BAV. Although the incidence of adverse outcomes was lower in the bridge groups as compared to other subgroups even when treated with BAV only, it clearly compares unfavourably with the results of TAVI and high-risk AVR described in the literature18,21,23. This finding indirectly confirms that these therapeutic options should be pursued whenever possible and excessive delays must be avoided. The average six-month delay between BAV and TAVI in our bridge patients cohort, although in some cases due to factors independent from our clinical judgement (including unavailability of the device), appears excessive and should definitely be reduced. These aspects deserve further investigation and application of preventive measures. Based on the available evidence, a more aggressive approach for earlier decision making towards a final destination therapy seems appropriate, and BAV as a bridge to TAVI should be limited to very selected patients.
Conclusion
The number of BAV has increased recently, due mainly to increased referral of high-risk patients and to the emerging indication of bridge for TAVI. In this complex population, BAV is relatively safe, but long-term survival remains poor indicating that, unless clearly contraindicated, more effective and definitive treatments such as AVR or TAVI must be performed without delay.
Conflict of interest statement
The authors have no conflicts of interest to declare.

