Abstract
Aims: The aim of this study was to demonstrate the safety and efficacy of the next-generation balloon-expandable Myval transcatheter heart valve (THV) in an intermediate- or high-risk patient population with severe symptomatic native aortic stenosis.
Methods and results: MyVal-1 was a first-in-human, prospective, multicentre, single-arm, open-label study. Between June 2017 and February 2018, a total of 30 patients were enrolled at 14 sites across India. Mean age was 75.5±6.7 years; 43.3% had coronary artery disease. The mean Society of Thoracic Surgeons score was 6.4±1.8% and 100% of the patients were in New York Heart Association (NYHA) functional Class II/III/IV pre-procedure. The six-minute walk test and Kansas City Cardiomyopathy Questionnaire (KCCQ) scores were recorded. After successful implantation of the Myval THV, 96.6% and 100% were in NYHA functional Class I/II at 30-day and 12-month follow-up, respectively. Outcomes of the six-minute walk test (148.0±87.4 vs 336.0±202.9 m) and KCCQ score (36.6±11.0 vs 65.9±11.4) improved from baseline to 12-month follow-up. The effective orifice area (0.6±0.2 vs 1.8±0.3 cm2, p<0.0001), mean aortic valve gradient (47.4±8.8 vs 12.0±3.3 mmHg, p<0.0001), peak aortic valve gradient (71.7±13.0 vs 20.3±5.9 mmHg, p<0.0001) and transaortic velocity (4.5±0.4 vs 2.2±0.4 m/s, p<0.0001) improved substantially from baseline to 12 months post procedure. Four all-cause mortality cases were reported up to 12 months. Moreover, there was no other moderate/severe paravalvular leak, aortic regurgitation or need for new permanent pacemaker (PPM) up to 12-month follow-up.
Conclusions: The MyVal-1 study demonstrated the primary safety and efficacy of the Myval THV with no new PPM requirement up to 12-month follow-up. However, future trials with a larger number of patients and long-term follow-up are warranted to establish the safety and efficacy of the device.
Introduction
Aortic stenosis (AS) is a common type of valve disorder in the elderly population, and its prevalence is increasing in ageing societies1. Two decades ago, surgical aortic valve replacement (SAVR) was the only available treatment for AS2. In 2002, a “proof-of-concept” case of transcatheter aortic valve replacement (TAVR) was performed by Cribier and his colleagues3. TAVR was introduced as an alternative treatment for selected patients with severe AS who were not eligible for surgery4. Moreover, TAVR was found to be non-inferior to SAVR with a lower rate of mortality and reduced cardiac arrest in intermediate- and high-risk patient populations5,6. TAVR has been successfully carried out in more than 200,000 patients across 65 countries, and is currently considered to be the best strategy for treatment of calcific, severe AS in patients with intermediate- to high-risk surgical scores7.
Some of the approved TAVR systems (SAPIEN 3, SAPIEN XT [Edwards Lifesciences, Irvine, CA, USA], Lotus™ [Boston Scientific, Marlborough, MA, USA], and CoreValve®, Evolut™ PRO [Medtronic, Minneapolis, MN, USA]) are well established. However, some reports demonstrated certain challenges during implantation or post procedure in low as well as intermediate and high operative risk patients, which included a requirement for new permanent pacemaker (PPM), paravalvular leak (PVL), increased risk for valve dislocation, annular rupture, aortic regurgitation (AR) and need for a second TAVR implantation8,9,10,11,12.
The Conformité Européenne (CE) approved Myval™ transcatheter heart valve (THV) (Meril Life Sciences Pvt. Ltd., Vapi, India) is a next-generation balloon-expandable TAVR system with features that facilitate accurate positioning and favourable clinical outcomes compared to current-generation TAVR systems. The present study aimed to demonstrate its safety and efficacy in an intermediate- or high-risk patient population with severe symptomatic native AS.
Methods
STUDY DESIGN
MyVal-1 was a first-in-human, prospective, multicentre, single-arm, open-label study (Clinical Trials Registry-India: CTRI/2016/11/007512) performed at 14 sites across India to evaluate the safety and efficacy of the next-generation balloon-expandable Myval THV in symptomatic patients with severe AS. The study protocol was approved by the institutional ethics committees at the participating sites. All patients received and signed informed consent. There was an independent data safety monitoring board that adjudicated all the adverse events.
PATIENT POPULATION
A total of 36 patients were screened for the study. Out of these, six patients were excluded due to reasons shown in Figure 1. All the inclusion and exclusion criteria for the implantation of the study device are shown in Supplementary Appendix 1.
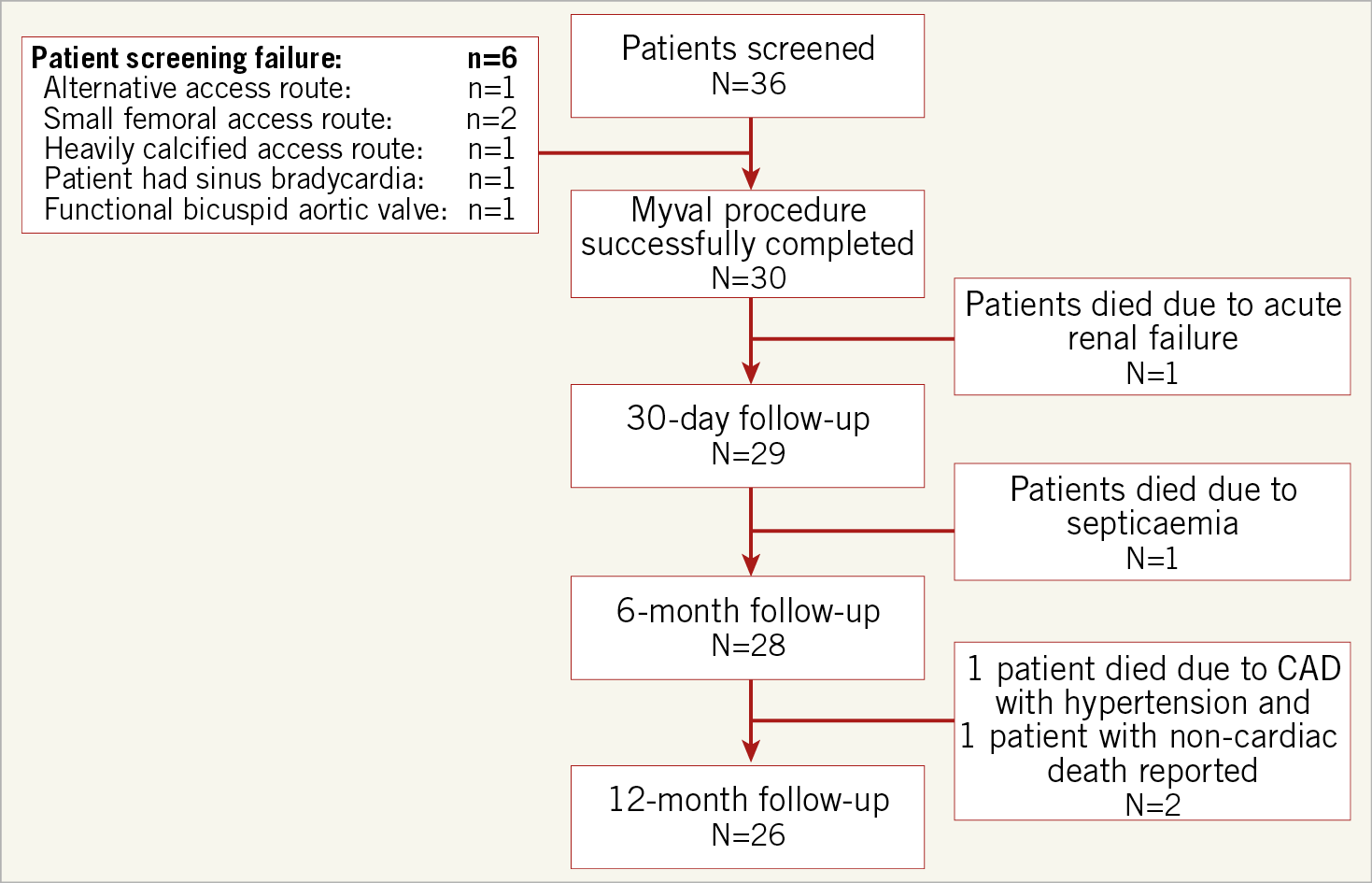
Figure 1. Patient enrolment and disposition.
DEVICE DESCRIPTION AND PROCEDURE
The Myval THV is a balloon-expandable TAVR system (Figure 2). The device is characterised by a nickel-cobalt alloy frame which is composed of a single design element – hexagons. These are arranged in a hybrid honeycomb fashion which allows 53% of the frame to have large open cells towards the aortic end and 47% to have closed cells with higher annular radial force towards the ventricular end. This novel design geometry on crimping gives rise to a unique alternative dark-light band-like pattern which allows precise positioning, placement, and deployment of the THV across the native annulus (Figure 3). The valve construction material is decellularised bovine pericardium tissue, which receives an anti-calcification treatment and is crafted into a trileaflet valve, fixed at three equipoise vertical commissural posts (separated by 120°) on the metal frame. The lower closed cell part of the valve frame is covered externally with a protective sealing cuff of polyethylene terephthalate (PET) to form an external buffing. This feature has a significant benefit in terms of minimising or eliminating PVL. The Myval THV is manufactured in diameters of 20 mm, 21.5 mm, 23 mm, 24.5 mm, 26 mm, 27.5 mm, 29 mm, and 32 mm.

Figure 2. Design of the Myval THV.
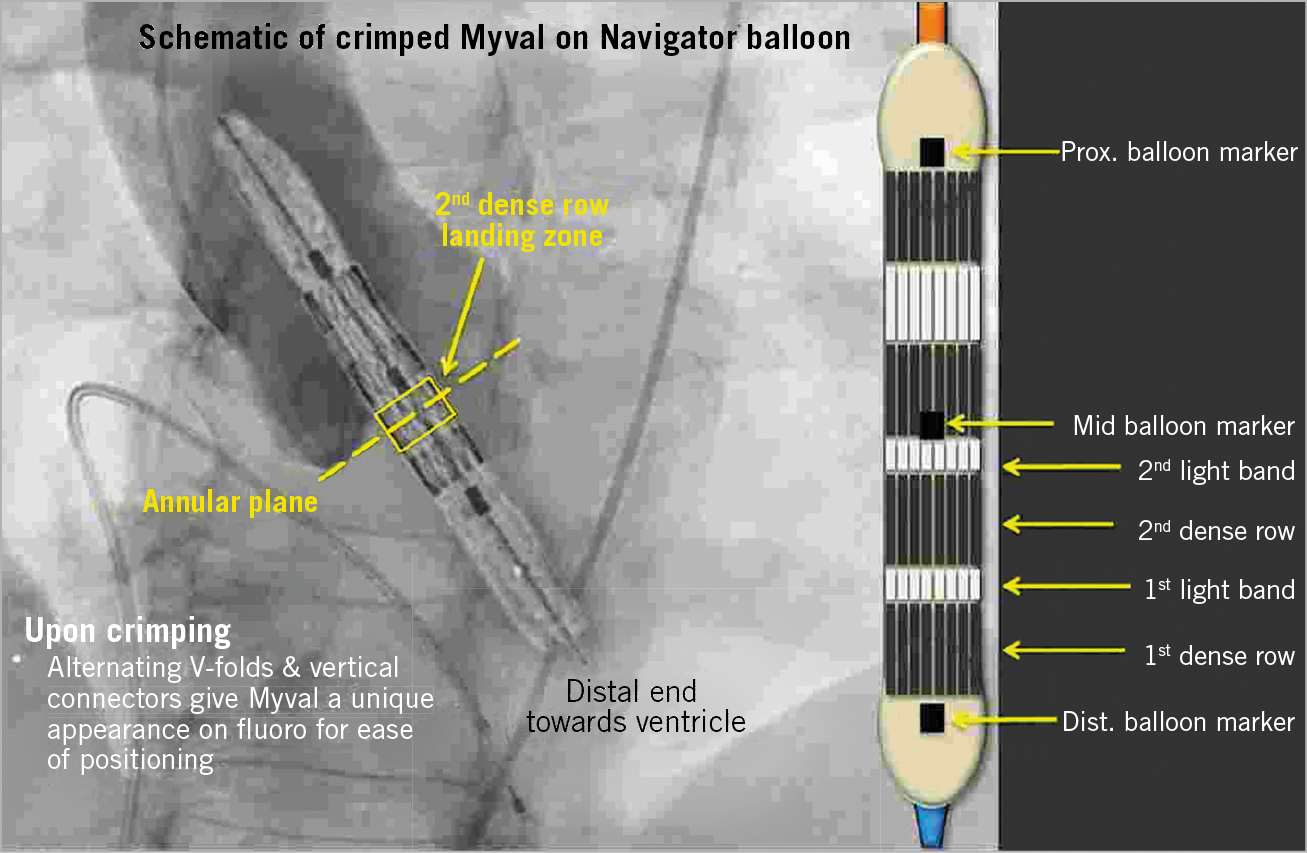
Figure 3. Positioning of the Myval THV.
The Myval THV is recommended to be crimped on its novel specially designed hi-flex, over-the-wire Navigator™ balloon catheter system (Meril Life Sciences Pvt. Ltd.) prior to insertion within the vessel (Figure 4). The Navigator has a unique construction characterised by a proximal deep flexion handle and a distal balloon with two counter-opposing soft stoppers within that create a shallow, low-profile crimping zone and thus a snug fit that prevents any inadvertent dislocation of the Myval THV during negotiation through the sheath or thereafter. Additionally, the delivery system allows flexion of the distal catheter system which ensures trauma-free negotiation across the aortic arch and minimises or eliminates any threat of a periprocedural stroke during arch navigation. Furthermore, the balloon has two internal expansion ports, which facilitate simultaneous expansion distally and proximally (similar to a dog bone), stabilising the valve during deployment and ensuring precision placement. The crimped THV is inserted via a specially designed 22 Fr (THV sizes: 20, 23 and 26 mm) or 24 Fr (THV size: 29 mm) expandable sheath. In situations where the operator is not able to deploy the valve in the desired orthotopic position, the unique sheath design allows valve retraction within the sheath.
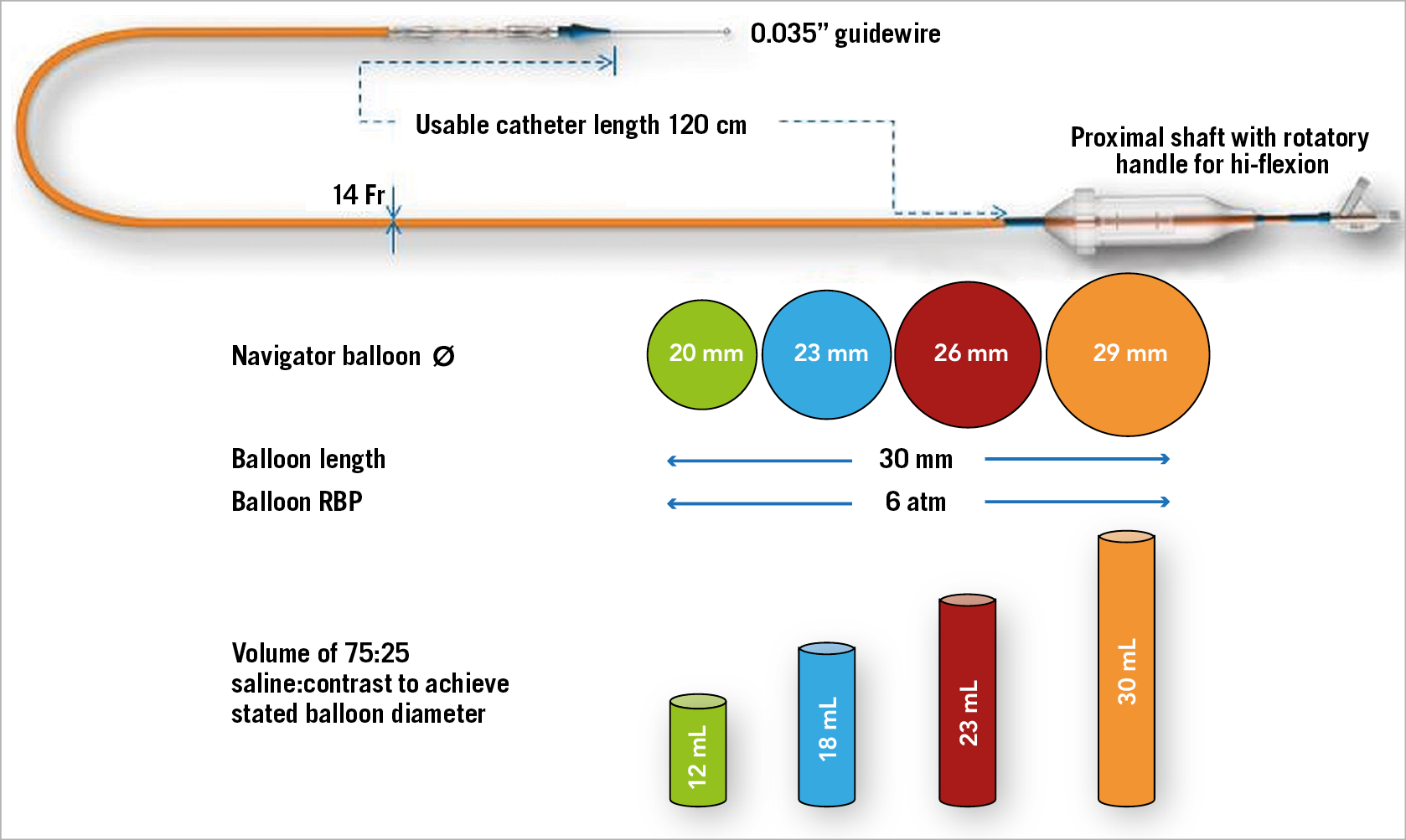
Figure 4. Navigator delivery system for the Myval THV.
All the patients received a loading dose of aspirin (325 mg/day) and clopidogrel (300 mg/day) before the procedure. In most of the cases, using standard percutaneous techniques, a predilatation was performed using an over-the-wire balloon (Mammoth™; Meril Life Sciences Pvt. Ltd.) compatible with a 0.035” guidewire. Once the THV was positioned accurately across the annulus, a dry pacing run at 180-200 bpm was conducted to ensure the valve positioning. The Myval THV was deployed under fluoroscopic guidance by connecting an inflation device pre-filled with a mixture of saline and contrast (75:25) using controlled emptying of the syringe under rapid pacing. Once the THV was fully deployed, the Navigator THV delivery system balloon was fully deflated, and the temporary pacing was stopped. The delivery system was withdrawn from the implantation site, and the post-procedural echo was recorded to check the accuracy of valve deployment, to confirm the absence of any trauma to adjoining zones, to measure the gradients and to check for the presence of AR or PVL. Figure 5 depicts positioning of the Myval THV in a case example from the MyVal-1 study.

Figure 5. Case example. A) Baseline aortogram. B) Predilatation. C) Navigator flexion avoids scraping against the contralateral arch wall. D) Valve positioning. E) Precise placement and deployment. F) Final orthotopic deployment.
After the procedure, anaesthesia/sedation was reversed, and patients were transferred to the intensive care unit. All the patients were prescribed 75 mg/day aspirin and clopidogrel for at least six to 12 months post procedure.
ENDPOINTS AND FOLLOW-UP
Clinical follow-up and echocardiography were performed post procedure and up to 12 months. Thereafter, clinical and echocardiographic follow-up will be performed annually up to five years post procedure. The safety endpoint was Kaplan-Meier survival up to 12-month follow-up. Additional safety endpoints were all-cause death and stroke up to 12-month follow-up. The efficacy endpoints were improvement in NYHA functional classification, effective orifice area (EOA), and six-minute walk test from baseline up to 12-month follow-up. Additionally, quality of life (QoL) as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), and freedom from major adverse cardiac cerebrovascular and renal events (MACCRE) were assessed at follow-up. MACCRE was defined as the composite of cardiovascular death, evidence of prosthetic valve dysfunction (haemolysis, infection, thrombosis, or valve migration), stroke, procedure-associated and/or device-associated adverse cardiac events, or kidney dysfunction. Device success, early safety at 30 days, clinical efficacy after 30 days, myocardial infarction, all-cause death and stroke were defined in accordance with the Valve Academic Research Consortium-2 (VARC-2) definitions13. All VARC-2 and the MACCRE definitions are shown in Supplementary Appendix 2 and Supplementary Appendix 3.
STATISTICAL ANALYSIS
Patient demographics, device performance, risk factors, and clinical outcomes were summarised using descriptive statistics for continuous variables and frequency tables for categorical variables. Continuous variables were reported as mean±standard deviation. Categorical variables were expressed as numbers and percentages. All calculations were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Change from baseline for NYHA functional class was assessed using the paired Wilcoxon signed-rank test; the six-minute walk test was assessed using a paired t-test. Time-to-event analysis for survival was evaluated by Kaplan-Meier curve.
Results
BASELINE CHARACTERISTICS OF THE STUDY POPULATION
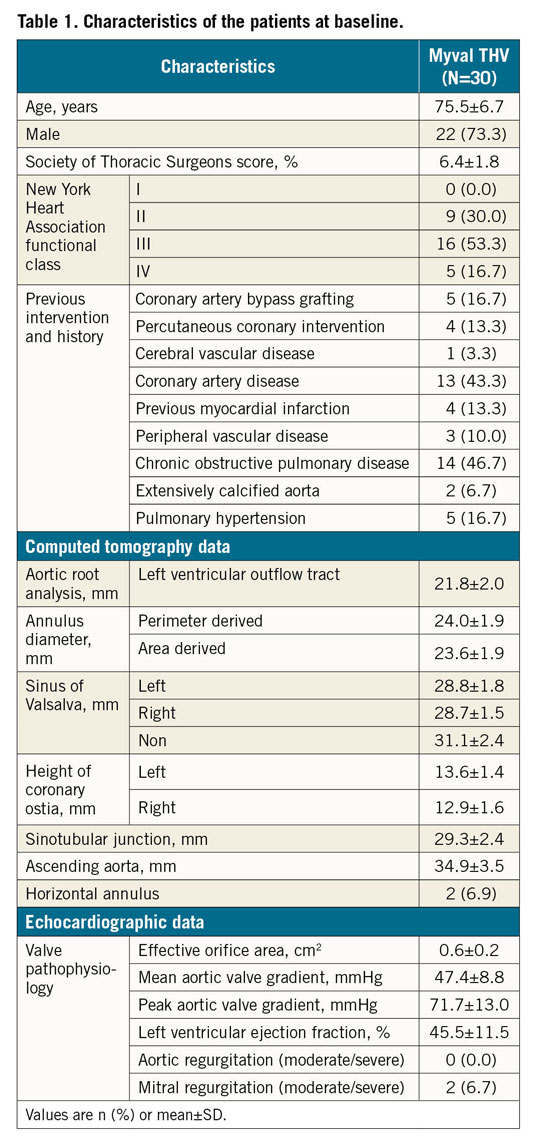
Between June 2017 and February 2018, a total of 30 symptomatic patients (73.3% male) with a mean age of 75.5±6.7 years were enrolled in the study. The number of patients enrolled at each participating site is shown in Supplementary Table 1. Baseline and demographic characteristics are listed in Table 1.
PROCEDURAL OUTCOMES
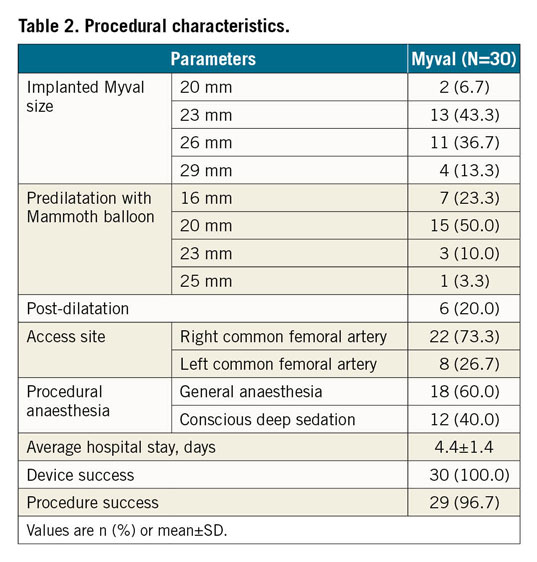
The Myval THV was implanted in all 30 patients enrolled in the MyVal-1 study. Procedural details are shown in Table 2. There were no cases of coronary obstruction, valve dislocation, annular rupture, or structural damage to the aortic valve apparatus. The placement of a second valve was not required in any of the cases.
ECHOCARDIOGRAPHIC FINDINGS
As shown in Figure 6, EOA (1.7±0.3 vs 0.6±0.2 cm2, p<0.0001) and mean aortic valve gradient (8.0±2.7 vs 47.4±8.8 mmHg, p<0.0001) improved significantly post procedure as compared to baseline. These results were sustained at 12 months with an EOA of 1.8±0.3 cm2 (p<0.0001) and a mean aortic valve gradient of 12.0±3.3 mmHg (p<0.0001), showing significant improvement from baseline to 12-month follow-up. Moreover, peak aortic valve gradient (20.3±5.9 vs 71.7±13.0 mmHg, p<0.0001) and transaortic velocity (2.2±0.4 vs 4.5±0.4 m/s, p<0.0001) remained significantly improved haemodynamically at 12-month follow-up as compared to baseline. Two patients had a mild PVL after Myval THV implantation which was treated by post-dilatation during the procedure itself. This did not result in any patient complication or change in haemodynamic performance.
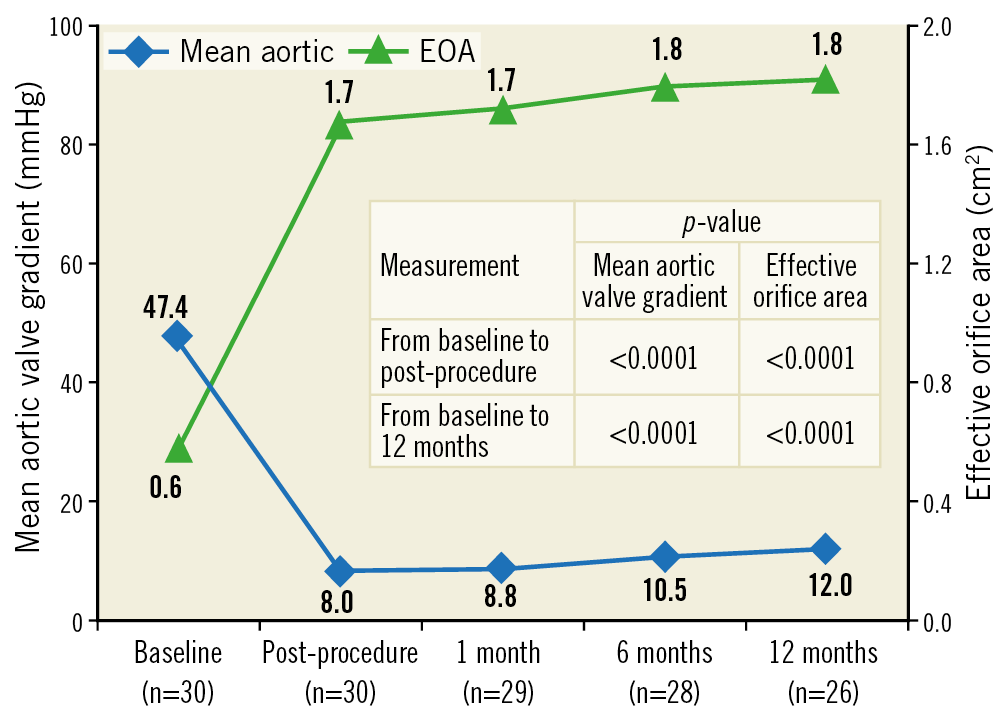
Figure 6. Mean aortic valve gradient and effective orifice area by echocardiography.
NYHA FUNCTIONAL CLASS AND QoL STATUS
NYHA functional class improved significantly (p<0.0001) from baseline to 12-month follow-up (Figure 7). The QoL improved from baseline to 12-month follow-up, according to the KCCQ score (36.6±11.0 vs 65.9±11.4). Outcomes of the six-minute walk test (148.0±87.4 vs 336.0±202.9 m) improved from baseline to 12-month follow-up.

Figure 7. Improvement in NYHA functional class.
CLINICAL FOLLOW-UP
Clinical outcomes along with MACCRE at different follow-up periods are shown in Table 3. Cumulative all-cause mortality at 1-month, 6-month, and 12-month follow-up was 1 (3.3%), 2 (6.7%), and 4 (13.3%), respectively. The Kaplan-Meier survival curve is shown in Figure 8. Of the four all-cause mortality cases, one patient died due to acute renal failure post procedure, one patient had died due to septicaemia at six-month follow-up, one patient died due to coronary artery disease with hypertension, and death related to a non-cardiac event was reported in another patient at 12-month follow-up. Major vascular complications were observed in two patients post procedure; no stroke, life-threatening bleeding or myocardial infarction, haemolysis, thrombosis, or valve migration was reported in any of the patients. Three patients were re-hospitalised at 30-day follow-up. All the re-hospitalised patients were successfully treated and discharged. None of the patients required a new PPM up to 12-month follow-up.
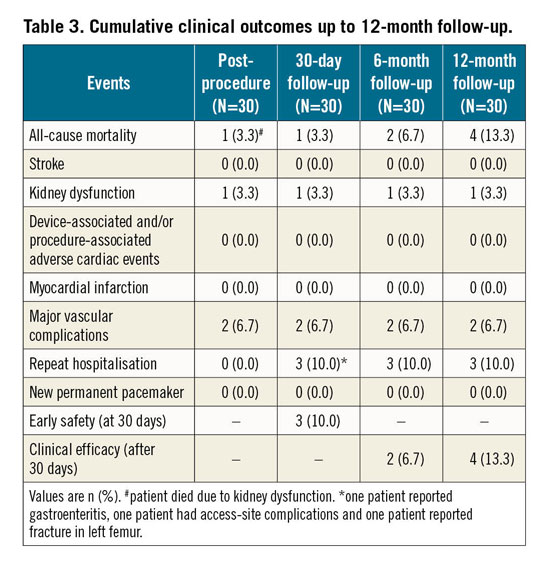
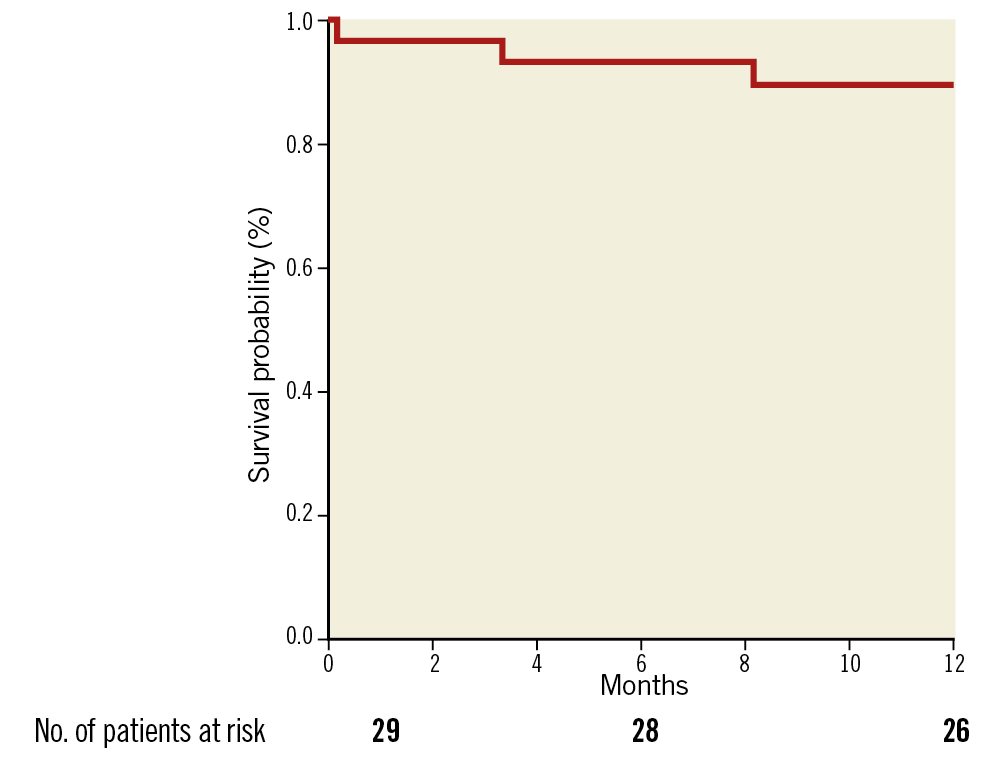
Figure 8. Kaplan-Meier survival curve.
Discussion
The first-in-human MyVal-1 study demonstrated the safety and efficacy of the next-generation balloon-expandable Myval THV implanted using a percutaneous transfemoral approach. The Myval THV was successfully implanted in all 30 patients with a low incidence of 30-day as well as 12-month all-cause mortality (4 out of 30 patients). The results of this study support the use of the Myval THV in intermediate- and high-risk patients with severe symptomatic native AS. No new PPM was required during or after the implantation of the Myval THV. Also, no moderate/severe PVL, haemolysis, thrombosis or valve migration was reported at 12-month follow-up.
Several randomised studies have demonstrated the effectiveness of TAVR over SAVR for symptomatic patients with AS (at low as well as intermediate to high operative risk) with a lower 30-day postoperative mortality rate4,5,12,14,15. The TAVR procedure is generally associated with a risk of acute kidney injury due to a variety of factors such as diabetes mellitus, chronic kidney disease, low glomerular filtration rate, peripheral vascular disease, haemodynamic instability during rapid pacing, previous stroke, and contrast agent volume16. In our study, one death due to acute renal failure was reported in a patient who was diabetic, hypertensive and had renal insufficiency. The analysis of the SWISS-TAVI registry, which comprised 3,491 consecutive patients, demonstrated a lower rate of in-hospital (2.9%) and 30-day mortality (3.8%) after a TAVR procedure17. The present study reported similar findings at 30 days with a mortality rate of 3.3%.
TAVR is frequently associated with moderate/severe paravalvular regurgitation (10-12%), which may require repeat intervention15,18,19. In the MyVal-1 study, prosthetic aortic regurgitation was not seen in any patient up to 12-month follow-up. Mild PVL was seen in 7.1% of the patients without any haemodynamic effects. Our data are in accordance with the recently published low-risk PARTNER 3 trial using the SAPIEN 3 valve where mild PVL was seen at a higher rate with TAVR than with surgery (29.4% vs 2.1%)20. Moreover, there was a substantial improvement in aortic valve haemodynamics from baseline in terms of EOA, mean aortic valve gradient, peak aortic valve gradient, and transaortic velocity up to 12-month follow-up.
Other major concerns after TAVR are neurological complications including stroke. Earlier studies have shown a greater risk of stroke within 30 days in TAVR than in SAVR18,21. In the PARTNER-I trial, the 30-day stroke rate was 3.6%21. Studies have suggested that in-hospital stroke events, both major and minor, were commonly seen in nonagenarian patients following TAVR19. In this study, no stroke during the 12-month follow-up was reported in any of the patients. Moreover, a recent study has favoured TAVR over SAVR with shorter hospital stay (3 days vs 7 days)20. Our procedural data are in accordance with that study concerning a shorter hospital stay.
The overall objective of TAVR in AS is to improve QoL, prolong life expectancy and improve NYHA functional class and six-minute walk test18,22. The QoL outcomes from recent studies have shown significant improvements after TAVR up to 1 month, 6 months, and 1 year23,24,25. Our results are in line with these observations, with significant improvements in QoL as well as NYHA functional class and six-minute walk test from baseline to 12-month follow-up.
New pacemaker implantation is a predictor of increased 30-day mortality following TAVR in low-flow AS and occurs due to atrioventricular block associated with deeper implantation of the valve26,27. The rates were shown to decrease with newer THV designs and advanced knowledge of predictors of pacemaker implantation28. The lowest pacemaker rate among the currently available valves is reported with the SAPIEN 3 (7.3%) in the PARTNER 3 trial20. Moreover, in the SURTAVI and Evolut Low Risk trials, PPM implantation was required in 25.9% and 17.4% of TAVR patients at 30-day follow-up, respectively12,15. Out of 30 patients evaluated in the MyVal-1 study, there was no new PPM implantation up to 12-month follow-up. This may be attributed to the fact that the design of the Myval THV allows positioning across the landing zone such that 70% of the THV lies in the aorta and 30% in the left ventricle, leading to marginal foreshortening of the frame from the ventricular end and thus resulting in a reduced depth of valve implantation within the left ventricular outflow tract.
Limitations
A limitation of this study is that it is a first-in-human experience, with a small sample size. The present study warrants future trials with larger populations and adequate power to validate the outcomes. Another limitation of the study is the assessment of safety and efficacy at short-term follow-up. Although pre-specified in the study protocol, our study was not statistically powered for clinical endpoints at 12 months, so the results should be considered hypothesis-generating only.
Conclusions
The results of this first-in-human study demonstrate the primary safety and efficacy of the Myval THV in patients with severe AS at intermediate or high risk for surgery. A high rate of device success was achieved without the need for a new PPM implant. The preliminary observations of the MyVal-1 study will serve as a basis for future trials with a larger number of patients and long-term follow-up to establish the safety and efficacy of the Myval THV further.
|
Impact on daily practice The MyVal-1 study showed acceptable safety and efficacy of the Myval THV with no need for a new PPM implant up to 12-month follow-up. Besides this, no stroke event was observed during the 12-month follow-up. The results are encouraging, with no device-related adverse events. The MyVal-1 study thus demonstrates that implantation of the Myval THV is safe in severe AS patients at intermediate or high risk for surgery. |
Appendix
STUDY COLLABORATOR
Anmol Sonawane, MD, DNB; Breach Candy Hospital, Maharashtra, India.
Funding
The MyVal-1 study was funded by Meril Life Sciences Pvt. Ltd., India.
Conflict of interest statement
S.K. Sharma and A. Seth are external scientific advisors to Meril Life Sciences Pvt. Ltd. R.S. Rao, P. Chandra and A. Sonawane are proctors for Myval THV technology and have received honoraria from Meril Life Sciences Pvt. Ltd. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.