
Abstract
Aims: We aimed to demonstrate the feasibility and investigate the safety of a novel, self-expanding transcatheter heart valve in a selected patient population with severe aortic stenosis.
Methods and results: Between January and September 2013, a total of 21 patients with symptomatic severe aortic stenosis were eligible for transcatheter aortic valve implantation (TAVI) with the self-expanding NVT Allegra bioprosthesis (New Valve Technology, Hechingen, Germany) at two cardiovascular centres. Patients were elderly (age 83.8±4 years), predominantly female (95.2%), and all were considered to be at prohibitive risk for surgical aortic valve replacement (logistic EuroSCORE 30.4±11%). Procedural and device success was achieved in 95.2% and 85.7%, respectively. Echocardiographic assessment at discharge showed favourable haemodynamic results with a reduction of the mean transvalvular aortic gradient from 48.0±21 mmHg to 8.9±3 mmHg. In the majority of patients (90.5%), none or trace aortic regurgitation was recorded. Permanent pacemaker implantation was required in 23.8% of patients within the first 30 days of follow-up. Apart from one procedural death, no other serious adverse events were observed during the periprocedural period. TAVI with the NVT Allegra system was highly effective in alleviating symptoms and reducing NYHA functional class at 30-day follow-up.
Conclusions: The first-in-human experience with the NVT Allegra transcatheter heart valve prosthesis was associated with a high rate of procedural success. Furthermore, the NVT Allegra bioprosthesis was able to achieve favourable haemodynamic results and effectively alleviate symptoms at 30-day follow-up. The larger, multicentre NAUTILUS study will provide further information on the safety and efficacy of this novel, second-generation transcatheter aortic bioprosthesis.
Introduction
Transcatheter aortic valve implantation (TAVI) is the treatment of choice for inoperable and high-risk patients with symptomatic, severe aortic stenosis1. Since the first TAVI procedure in 20022, the technology has been constantly improved and refined3. Today, TAVI represents a standardised treatment with low rates of periprocedural complications. Rates of device success are high across various multicentre studies4-6; however, in contemporary clinical practice, malpositioning or embolisation of TAVI devices is still reported in up to 4% of cases6. Novel TAVI devices aim to overcome the shortcomings of earlier-generation devices such as prosthesis malpositioning or embolisation and paravalvular aortic regurgitation, as well as new onset of conduction disturbances. Herein, we describe the technical features of the NVT Allegra transcatheter heart valve (New Valve Technology, Hechingen, Germany) and report the first-in-human clinical experience during 30 days of follow-up.
Methods
TAVI with the NVT Allegra bioprosthesis was performed in 21 selected patients with symptomatic, severe aortic stenosis at two cardiovascular centres (Department of Cardiology, Swiss Cardiovascular Center Bern, Bern University Hospital, Bern, Switzerland, and the Department of Cardiology, Heart Center Oldenburg, Oldenburg, Germany) (Figure 1).
Patient population
The inclusion criteria of the study are provided in the Online Appendix. Patients were considered eligible for participation in this first-in-human study when deemed at high or prohibitive risk for conventional surgical aortic valve replacement (SAVR) after the assessment of a multidisciplinary Heart Team. Patient screening and selection was performed using a detailed pre-procedural work-up, including coronary angiography, multislice computed tomography scan and transoesophageal echocardiography, to comprehend fully the periprocedural risk and the anatomical setting of the patient. All patients provided written informed consent for treatment with an investigational device and for prospective data acquisition, which was approved by the local ethics committees and the German/Swiss healthcare authorities. Short-term clinical outcomes were reported according to the standardised endpoint definitions recommended by the Valve Academic Research Consortium7.
The NVT Allegra TAVI system
The NVT Allegra transcatheter heart valve (Figure 2) is a self-expanding TAVI prosthesis which was designed to avoid haemodynamic compromise and facilitate correct positioning during the stepwise implantation phase of the prosthesis. The prosthesis incorporates a trileaflet, bovine pericardial bioprosthetic aortic heart valve attached to a nitinol stent frame. The nitinol stent frame has a closed cell, diamond-shaped configuration with a variable cell size distribution allowing improved coronary perfusion and easy access for possible percutaneous coronary intervention at a later stage. Different levels of radial force enhance a safe anchoring of the prosthesis within the native aortic annulus. Six radiopaque gold markers are placed at the level of the valve plane to indicate the distal part of the semilunar valve and to assist correct valve positioning. The ventricular inflow section of the prosthesis is covered by a bovine pericardial sealing skirt in order to mitigate paravalvular prosthetic regurgitation. The NVT Allegra transcatheter heart valve is available in three sizes (23, 27 and 31 mm) to match aortic annulus dimensions ranging from 19 to 29 mm. For this first-in-human clinical trial only the 23 mm and the 27 mm bioprostheses were available, offering treatment for aortic annulus diameter dimensions from 19 to 25 mm. Detailed information on the prosthesis specifications is provided in Table 1.
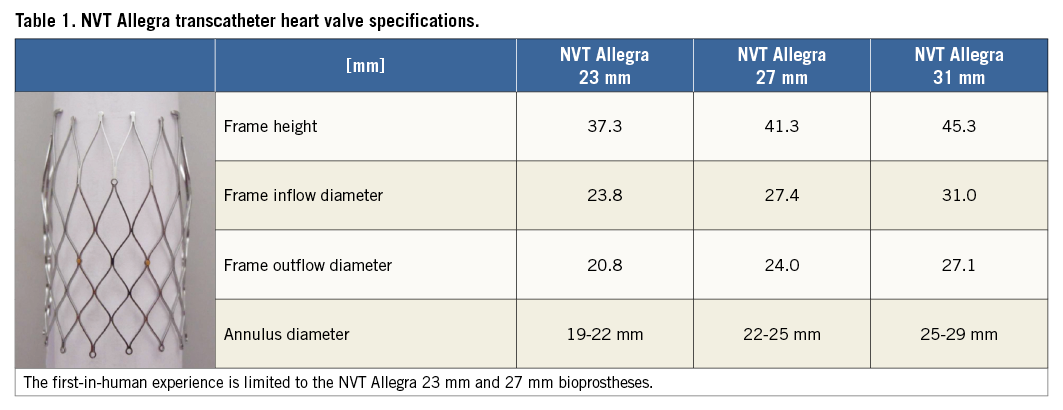
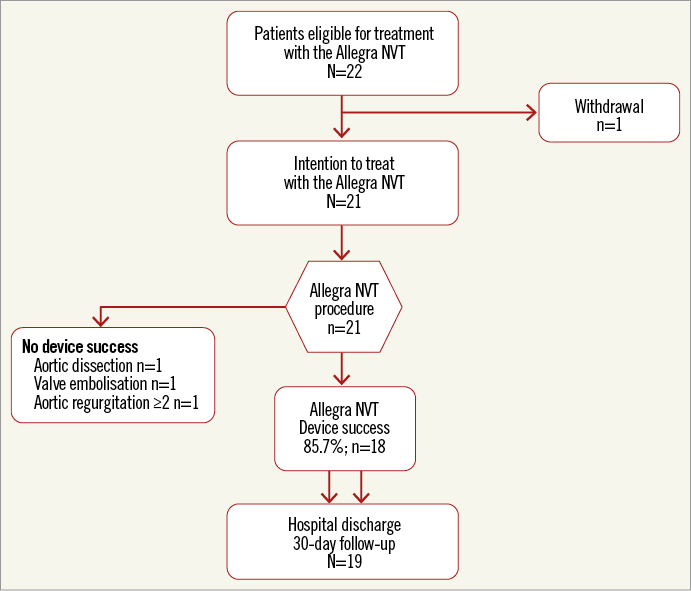
Figure 1. Patient recruitment and study flow.
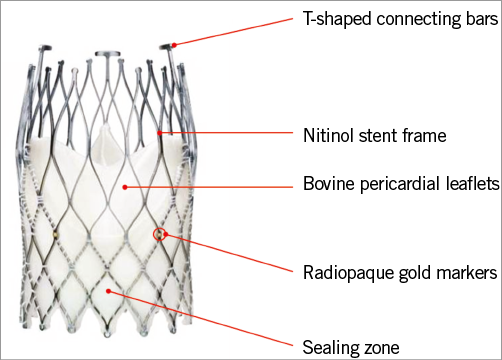
Figure 2. Device-specific characteristics of the NVT Allegra bioprosthesis.
The NVT Allegra transfemoral delivery system consists of an 18 Fr catheter and an attached handle, which integrates distinct functional components for controlled prosthesis release and deployment (Figure 3). Using a three-step release mechanism, the novel implantation technology (PermaFlow®; New Valve Technology) allows a controlled positioning of the NVT Allegra prosthesis without interfering with the left ventricular outflow. As long as the prosthesis is attached to the catheter system and until 70% of complete deployment, the transcatheter heart valve can easily be recaptured and fully retrieved from the circulation (Figure 4).
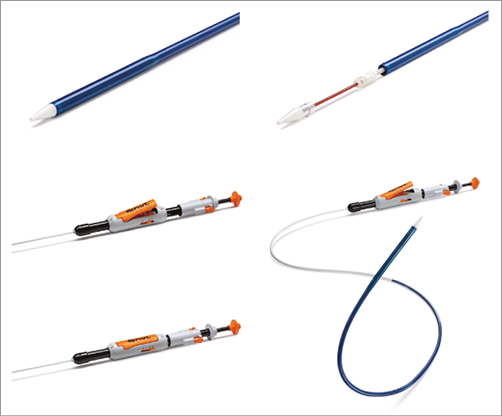
Figure 3. NVT catheter delivery system.
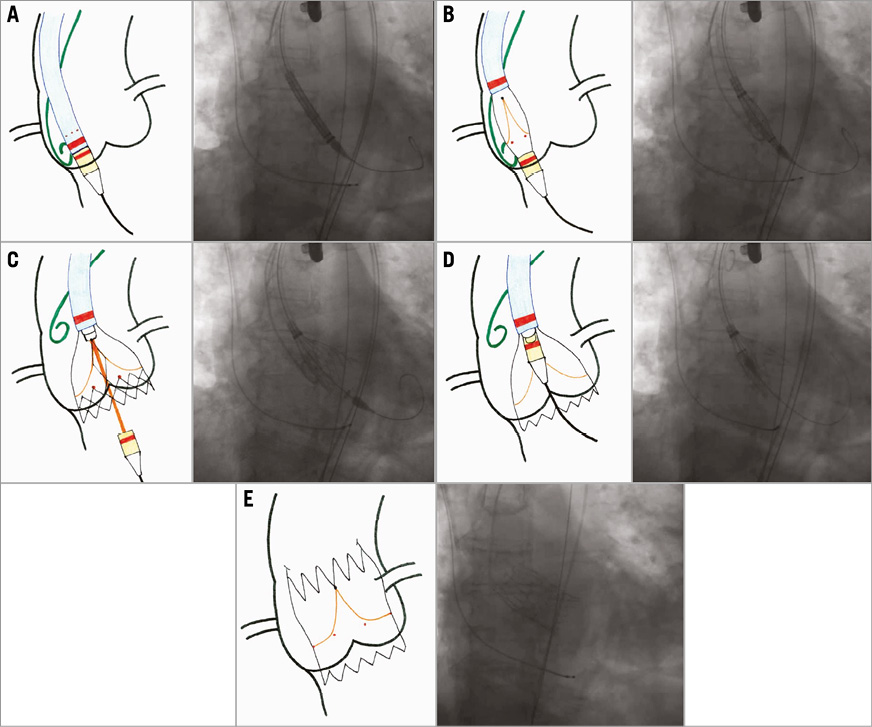
Figure 4. Implantation sequence of the new-generation NVT Allegra transcatheter heart valve. A) Introduction of the delivery catheter into the LV. The pigtail catheter is placed in the non-coronary cusp and marks the aortic annulus. B) Starting the deployment of the NVT Allegra prosthesis in a stable position. C) After angiographic confirmation of the optimal height of the prosthesis, controlled release of the inflow part of the Allegra NVT. During this step the valve is already fully functioning and can be easily recaptured and fully retrieved from the circulation. D) Final angiographic check and retraction of the nose cone to enable the valve release mechanism. E) Allegra NVT fully deployed in appropriate annular position.
Procedure
All procedures were performed using general anaesthesia and transoesophageal echocardiographic guidance. An 18 Fr delivery sheath was introduced into the femoral vasculature and an extra-stiff guidewire was placed in the left ventricle. Balloon aortic valvuloplasty was mandatory prior to valve implantation and was performed using rapid ventricular pacing. Stepwise and controlled deployment of the NVT Allegra transcatheter heart valve was performed under fluoroscopic guidance. After assessing the correct position, the valve was completely released without the use of fast ventricular pacing. Immediately after prosthesis deployment, haemodynamic function and paravalvular aortic regurgitation were assessed using transoesophageal echocardiography and aortography. Dual antiplatelet therapy with low-dose acetylsalicylic acid (100 mg) and a short-term duration of clopidogrel (75 mg) was recommended for all patients during the first six months after TAVI, followed by indefinite treatment with low-dose acetylsalicylic acid.
Statistical analysis
Continuous data are reported as mean (±standard deviation [SD]), and categorical variables are reported as number of patients (% of patients) where appropriate. Mean values of continuous data were compared by using the Student’s t-test/signed-rank Wilcoxon test. Two-sided p-values <0.05 were considered statistically significant. All analyses were performed with SAS version 9.4 (SAS, Cary, NC, USA).
Results
PATIENT POPULATION
A total of 21 patients with symptomatic, severe aortic stenosis were considered eligible for treatment with the NVT Allegra TAVI prosthesis and were enrolled in this first-in-human trial between January and September 2013. Mean age was 83.8±4 years and the majority of patients (95.2%) were female. Patients were highly symptomatic, with 85.7% presenting in NYHA functional Class III or IV. Mean aortic valve area was 0.61±0.2 cm2 and mean transvalvular gradient 48.0±21 mmHg. Patients were considered to be at high surgical risk with an estimated risk of mortality at 30 days of 30.4±11% according to the logistic EuroSCORE. Detailed information on baseline clinical characteristics is summarised in Table 2.
PROCEDURAL CHARACTERISTICS AND CLINICAL OUTCOMES
Procedural characteristics and results are presented in Table 3. Transfemoral TAVI was initiated in 21 patients and successfully completed in 20 patients (95.2%). One patient experienced a local type A aortic dissection during the advancement of the delivery catheter through the aortic arch and expired soon after the procedure. In another patient, embolisation of the NVT Allegra prosthesis was observed directly after successful placement of the prosthesis in correct intra-annular position due to the withdrawal of the delivery catheter, followed by bail-out implantation of a commercial balloon-expandable prosthesis. VARC device success with the NVT Allegra transcatheter heart valve was achieved in 18 out of 21 patients (85.7%). Post-dilatation of the prosthesis was performed in 11 patients (55%) due to mild or moderate paravalvular aortic regurgitation leading to an improvement of the immediate post-procedural result. Echocardiographic assessment at discharge showed favourable haemodynamic results with a reduction of mean transvalvular aortic gradient from 48.0±21 mmHg to 8.9±3 mmHg and an increase in aortic valve area from 0.61±0.2 cm2 to 1.68±0.5 cm2 (Figure 5). Moderate aortic regurgitation (grade 2) was observed in only one patient, while all others showed no relevant aortic regurgitation (grade 0 and 1). Permanent pacemaker implantation was required in five patients (23.8%) during the periprocedural observation period. In three patients a complete AV block was the indication for the implantation.
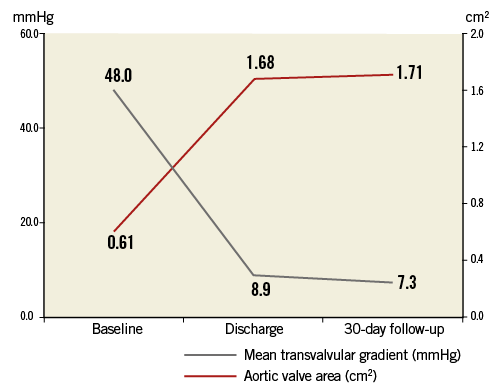
Figure 5. Mean transvalvular gradient and aortic valve area at baseline, discharge and during echocardiographic follow-up at 30 days.
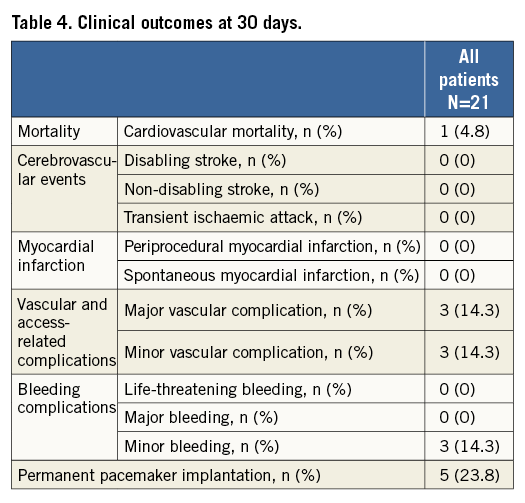
Short-term clinical outcome within the first 30 days of follow-up is presented in Table 4. Apart from one death during the periprocedural period, all patients were alive at 30 days of follow-up. None of the patients experienced cerebrovascular events (disabling, non-disabling stroke, transient ischaemic attack), myocardial infarction, kidney injury or life-threatening bleeding. Furthermore, there was no repeat unplanned valvular intervention during the periprocedural observational period.
TAVI was effective in alleviating symptoms with an improvement in NYHA functional Class III/IV in 85.7% down to 21.1% at 30 days of follow-up.
Discussion
This feasibility study and first-in-human experience with the NVT Allegra transcatheter heart valve provides favourable short-term clinical and haemodynamic results in a selected patient population with symptomatic, severe aortic stenosis. The NVT Allegra TAVI system is a novel transcatheter heart valve system with the following advantages and technical specifications:
– The NVT Allegra transcatheter heart valve system is a second-generation TAVI device allowing repositioning and complete removal of the valve after the deployment of the functional part (until 70% of complete deployment).
– Six radiopaque gold markers indicate the level of the leaflets of the bioprosthesis assisting correct valve positioning.
– The specific design of the NVT Allegra transcatheter heart valve incorporates different levels of radial force providing a stable fixation within the native aortic annulus. Furthermore, the low profile of the frame and the cell geometry of the nitinol stent facilitate the percutaneous access to the coronary arteries in case of staged coronary intervention.
– Prior to valve release from the catheter, the correct implantation level and paravalvular aortic regurgitation can be easily assessed with a functional valve prosthesis in place.
This analysis investigating the first-in-human safety and feasibility of the NVT Allegra transcatheter heart valve demonstrates high procedural success, favourable haemodynamic results and a low rate of serious adverse events during the first 30 days of follow-up. The technical features of the NVT Allegra valve may improve procedural and functional TAVI outcomes as reflected in the high rate of VARC device success, which is comparable to other second-generation CE-approved TAVI devices – Lotus™ transcatheter heart valve (90.1% in the Reprise I study) (Boston Scientific, Marlborough, MA, USA)8, the Portico™ prosthesis (90% in the first-in-man experience) (St. Jude Medical, St. Paul, MN, USA)9, and the Direct Flow Medical® transcatheter heart valve (93% in a multicentre evaluation) (Direct Flow Medical, Santa Rosa, CA, USA)10.
Furthermore, haemodynamic results of the NVT Allegra transcatheter heart valve as well as paravalvular aortic regurgitation are comparable to other CE-marked second-generation devices. Of note, more than 50% of patients (57.9%) required balloon post-dilation in order to reduce and minimise paravalvular aortic regurgitation. While the available literature points towards an increased risk of cerebral embolism after additional prosthetic post-dilation to improve paravalvular sealing11,12, this additional procedural step did not translate into clinically apparent stroke in this selected patient population.
Almost one out of four patients (23.8%) treated with the NVT Allegra prosthesis had atrioventricular (AV) conduction disturbances necessitating permanent pacemaker implantation, which was complete AV block in three patients. A permanent pacemaker rate beyond 20% of patients is common after the implantation of a self-expanding transcatheter heart valve prosthesis4,13. During the early experience of other second-generation, self-expanding TAVI devices, remarkable differences have been observed in rates of permanent pacemaker implantation. While the implantation of the Portico valve did not result in the additional need for permanent pacing9, one out of three patients was found to be pacemaker-dependent after the implantation of a Lotus valve8. The reasons for this might be explained by inherent differences in stent frame design and radial force that might provoke additional conduction disturbances leading to permanent pacemaker implantation. However, it must be pointed out that permanent pacemaker implantation does not have any impact on clinical outcomes up to one year after TAVI14, and is only associated with an economic burden.
Limitations
The results of the present pilot study and first-in-human experience with this novel investigational device need to be interpreted with caution as only selected high-risk patients were considered for inclusion in this study. Furthermore, as the vast majority of patients were female, the results of this study might not be representative of an unselected all-comer patient population with symptomatic, severe aortic stenosis. Additional understanding of the relevance and impact of this novel valve technology on clinical outcomes, haemodynamic results, paravalvular aortic regurgitation and the need for permanent pacemaker implantation can only be gained in a larger study of unselected patients.
Conclusion
The first-in-human experience with the NVT Allegra transcatheter heart valve prosthesis was associated with a high rate of procedural success. Furthermore, the NVT Allegra bioprosthesis was able to achieve favourable haemodynamic results and effectively alleviate symptoms at 30-day follow-up. The larger, multicentre NAUTILUS study will provide further information on the safety and efficacy of this novel, second-generation transcatheter aortic bioprosthesis.
| Impact on daily practice The NVT Allegra transcatheter heart valve prosthesis represents a novel self-expanding system with the possibility of repositioning and retrieving the device due to the step-wise deployment system. Therefore, this first-in-human experience summarises the feasibility of a novel transcatheter system already incorporating features of a second-generation device for the treatment of valvular aortic stenosis. The short-term clinical outcome after successful implantation shows favourable results with a haemodynamic performance comparable to CE-approved TAVI devices. Further trials are needed to prove the benefits of this novel implantation technology. |
Conflict of interest statement
P. Wenaweser received proctoring and lecture fees from Medtronic, Edwards and Boston Scientific, and a research grant from Medtronic to the institution. The other authors have no conflicts of interest to declare.
Online Appendix. Patient inclusion and exclusion criteria for the Allegra NVT FIM study
INCLUSION CRITERIA
Patients must meet the following inclusion criteria for trial inclusion:
1. Patients of both gender ≥75 years old.
2. Patient has symptomatic severe, degenerative aortic stenosis with the following echocardiographic parameters (baseline echocardiographic assessment has to be performed within 60 days of enrolment):
a. Mean transvalvular pressure gradient >40 mmHg and/or
b. Jet velocity >4.0 m/s and/or
c. Aortic valve area of <1.0 cm² (or aortic valve area index ≤0.6 cm²/m²).
3. Patient is considered at high risk for surgical aortic valve replacement with a logistic EuroSCORE ≥20%, or documented Heart Team agreement that the patient is at high risk for surgery due to frailty and/or other coexisting comorbidities. The following conditions, which are not captured by conventional risk scores will be taken into account by the Heart Team and considered for trial inclusion:
a. Porcelain aorta or other preconditions which preclude cannulation for cardiopulmonary bypass, cross-clamping or surgical access to the mediastinum.
b. Previous radiation of the mediastinum.
c. Chest deformities or remote mediastinitis.
d. Chronic obstructive pulmonary disease with a FEV1 <70%.
e. Other conditions that could be associated with significant perioperative risk, e.g., prior thoracic or other heart surgery.
4. Aortic annulus dimension suitable for treatment with the NVT transcatheter heart valve (aortic annulus diameter between 19 mm and 29 mm).
5. Width of the sinus of Valsalva >26 mm for aortic annulus dimensions between 19 and 22 mm; width of the sinus of Valsalva >29 mm for aortic annulus dimensions between 22 and 29 mm.
6. Patient complies with the study protocol and agrees to return to the study hospital for all required scheduled follow-up visits.
7. Patient has provided written informed consent for trial participation.
EXCLUSION CRITERIA
Patient will be excluded from participation in the study in case one of the following criteria is met:
Anatomical exclusion criteria:
1. Unicuspid or bicuspid aortic valve anatomy.
2. Non-calcified aortic valve stenosis as assessed by echocardiography.
3. Predominant aortic regurgitation (AR greater than 3+).
4. Severe mitral regurgitation (MR greater than 3+).
5. Distance between the aortic annulus level and the take-off of one of the coronary arteries <10 mm.
6. Pre-existing prosthetic heart valve in mitral position.
7. Echocardiographic evidence of intracardiac mass, thrombus or vegetation.
8. Hypertrophic obstructive cardiomyopathy.
9. Significant aortic disease including:
a. abdominal or thoracic aneurysm of the aorta defined as maximal luminal diameter of 5 cm or greater;
b. significant vascular tortuosity;
c. aortic arch atheroma or stenosis of the abdominal or thoracic aorta.
10. Femoral artery lumen diameter <7.0 mm, or iliofemoral vascular conditions precluding the safe introduction of an 18 Fr delivery sheath or endovascular closure (severe calcification and tortuosity of the vasculature).
CLINICAL EXCLUSION CRITERIA
1. Left ventricular ejection fraction less than 20%.
2. Evidence of acute myocardial infarction within 30 days prior to the index procedure.
3. Any other invasive/surgical cardiac procedure within 30 days prior to the index procedure.
4. Untreated clinically significant coronary artery disease requiring revascularisation.
5. Cerebral vascular accident (CVA) or transient ischaemic attack (TIA) within six months (≤180 days) of the index procedure.
6. Symptomatic carotid artery disease requiring intervention.
7. History of endocarditis within 12 months of the index procedure.
8. Haemodynamic instability (e.g., cardiogenic shock) requiring inotropic support or mechanical heart assistance (e.g., VAD, IABP).
9. Uncontrolled atrial fibrillation.
10. Need for emergency surgery for any reason.
11. End-stage kidney disease with a creatinine clearance <20 ml/min, a serum creatinine >3.0 mg/dl (264 μmol/l) or requiring chronic renal replacement therapy.
12. Active peptic ulcer or upper gastrointestinal bleeding within the past 90 days prior to index procedure.
13. History of bleeding diathesis or coagulopathy or other haematological disorders as defined:
a. leucopaenia (white blood cell count <3×109/L).
b. anaemia (haemoglobin <10 g/dl).
c. thrombocytopaenia (platelet count <80 G/L).

