Abstract
Background: The next-generation ACURATE neo2 transcatheter aortic valve was designed for simplified implantation and to mitigate the risk of paravalvular leak (PVL) compared to the earlier device.
Aims: We sought to collect clinical outcomes and device performance data, including echocardiography and 4-dimensional computed tomography (4D-CT) data, with the ACURATE neo2 transcatheter heart valve in patients with severe aortic stenosis (AS).
Methods: The ACURATE neo2 Post-Market Clinical Follow-up (PMCF) Study is a single-arm, multicentre study of patients with severe AS treated in routine clinical practice. The primary safety endpoint was all-cause mortality at 30 days. The primary imaging endpoint was hypoattenuated leaflet thickening (HALT), measured by core laboratory-adjudicated 4D-CT at 30 days. Secondary endpoints included Valve Academic Research Consortium safety endpoints, procedural success, and evaluation of valve performance via core laboratory-adjudicated echocardiography.
Results: The study enrolled 250 patients at 18 European centres (mean age: 80.8 years; 63.6% female; mean Society of Thoracic Surgeons score: 2.9±2.0%); 246 patients (98.4%) were successfully treated with the ACURATE neo2. The 30-day rates for mortality and disabling stroke were 0.8% and 0%, respectively. The new permanent pacemaker implantation rate was 6.5%. HALT >50% was present in 9.3% of patients at 30 days. Valve haemodynamics improved from baseline to 30 days (mean aortic valve gradient: from 47.6±14.5 mmHg to 8.6±3.9 mmHg; mean aortic valve area: from 0.7±0.2 cm2 to 1.6±0.4 cm2). At 30 days, PVL was evaluated as none/trace in 79.2% of patients, mild in 18.9%, moderate in 1.9%, and severe in 0%.
Conclusions: The study results support the safety and efficacy of transcatheter aortic valve implantation with the ACURATE neo2 in patients in routine clinical practice.
Introduction
Studies have demonstrated that transcatheter aortic valve implantation (TAVI) with the first-generation ACURATE neo transcatheter heart valve (THV; Boston Scientific) is a safe and effective treatment for patients with aortic stenosis (AS)123. However, recent comparative studies have noted higher rates of paravalvular leakage (PVL) with the ACURATE neo compared with other TAVI devices45.
The next-generation ACURATE neo2 THV (Boston Scientific) features an improved sealing skirt with active sealing technology designed to mitigate PVL and a radiopaque marker for the facilitation of accurate THV positioning during implantation6. Early results from the prospective, multicentre ACURATE Neo AS clinical study demonstrated a favourable safety profile and improvement in valve haemodynamics in a selected patient population treated with the ACURATE neo26. The goal of the ACURATE neo2 Post Market Clinical Follow-up (PMCF) Study was to collect real-world safety and imaging data with the ACURATE neo2 THV when used to treat patients with severe AS in routine clinical practice. The study employed 4-dimensional computed tomography (4D-CT) imaging to assess the prevalence of hypoattenuated leaflet thickening (HALT) and reduced leaflet mobility and its relationship, if any, to clinical events.
This report presents the 30-day clinical outcomes and the computed tomography and echocardiographic results from the study, including the primary endpoints for safety and subclinical leaflet thrombosis.
Methods
The ACURATE neo2 aortic valve system consists of a self-expanding nitinol stent frame with three porcine pericardial tissue leaflets in a supra-annular position, and inner and outer porcine pericardium skirts6. The valve features a self-aligning design and a novel radiopaque marker to facilitate accurate positioning in the native aortic valve. The upper crown on the valve reduces the risk of coronary occlusion, while the lower crown reduces contact with the conduction system tissue, lowering the risk of conduction disturbances. Finally, the double-pericardium skirt is intended to reduce paravalvular leak. The device is currently available in 3 sizes (small [S], medium [M], and large [L]), intended for a native aortic annulus diameter range between 21 mm and 27 mm.
Study design
ACURATE neo2 PMCF is a prospective, open-label, single-arm, multicentre, observational post-market surveillance study. All patients signed an informed consent form prior to enrolment. A patient was considered enrolled once an attempt was made to insert the ACURATE neo2 transfemoral delivery system. This study adhered to the principles set forth in the Declaration of Helsinki and all applicable local and country regulations. The study is registered at ClinicalTrials.gov: NCT04655248.
The study sponsor (Boston Scientific) had a role in the study design and conduct, including data collection and analysis, and in the preparation of the manuscript. The principal investigators (L. Sondergaard and W-K. Kim) had unrestricted access to the data and wrote the manuscript first draft; all authors provided a critical review of the manuscript content.
Patients
Patients with severe aortic stenosis and who were deemed treatable with the ACURATE neo2 THV were approached to participate in the study. No specific inclusion criteria were set for this post-market surveillance study. In order to enrol patients who would be able to participate in the CT-imaging portion of the study, exclusion criteria were 1) prior aortic bioprosthesis valve, 2) chronic kidney disease stage IV or V (defined as eGFR <30 mL/min/1.73 m2), 3) atrial fibrillation that could not be controlled to a ventricular response rate <60 beats per minute to allow for high-quality 4D-CT, or 4) expected need for chronic anticoagulation therapy post-procedure (short-term anticoagulation post-procedure was permissible, but all imaging assessments were performed 30 days after discontinuation of anticoagulation).
Valve implantation was performed per the ACURATE neo2 instructions for use and local standard of care. As per the instructions for use, implantation of the ACURATE neo2 should be preceded by balloon dilation of the stenotic native aortic valve. Aortic annulus sizing was determined per site-based CT measures; the final THV size was at the operators’ discretion. Patients were recommended to be treated with aspirin and/or a P2Y12 inhibitor for at least 1 month following valve implantation, with subsequent antiplatelet therapy per the treating investigator’s discretion. Combination treatment with oral anticoagulation and dual antiplatelet therapy was not recommended.
Follow-up occurred per local standard of care at predischarge and 30 days and will continue annually from 1 up to 5 years post-index procedure. Additionally, all implanted patients were to undergo transthoracic echocardiography (TTE) predischarge and at 30 days post-TAVI, as well as 4D-CT imaging at 30 days. Echocardiography and 4D-CT imaging will be repeated at 1-year follow-up for eligible patients. Enrolled patients who were not treated with an ACURATE neo2 THV in the aortic position were followed for safety up to 30 days after the initial attempted index procedure but did not undergo imaging, and patients who required a second transcatheter valve or conversion to surgery during the index procedure will continue to be followed for safety for 1 year but are not required to undergo protocol-mandated imaging assessment.
Endpoints
The primary safety endpoint was all-cause mortality at 30 days. The primary imaging endpoint was HALT, as measured by 4D-CT at 30 days. Secondary endpoints were based on Valve Academic Research Consortium (VARC)-2 guidelines7. Per the study protocol, prosthetic aortic valve performance was evaluated via TTE predischarge and at 30 days post-TAVI, including an assessment of the effective orifice area (EOA), the mean and peak aortic gradients, and aortic regurgitation. Additionally, functional status per the New York Heart Association (NYHA) classification was evaluated at baseline and 30 days.
Adverse events (death, stroke, bleeding, major vascular complications, and hospitalisation for valve-related symptoms or worsening congestive heart failure) were adjudicated by an independent clinical events committee (CEC). Echocardiographic imaging evaluation at discharge and 30 days was performed by an independent echocardiographic core laboratory (MedStar, USA) according to the American Society of Echocardiography guidelines (available at www.asecho.org); 4D-CT data were evaluated by an independent CT core laboratory (Department of Radiology, The University of British Columbia, Vancouver, BC, Canada) according to published guidelines8.
Statistical analyses
Baseline, procedural, and follow-up data were expressed as mean±standard deviation (n) for continuous variables and as percentage (n/N) for categorical variables. The primary safety endpoint and safety outcomes were analysed in the intention-to-treat population, which consisted of all enrolled patients regardless of whether the ACURATE neo2 THV was implanted; outcomes at 30 days were evaluated in patients who experienced a CEC-adjudicated event up to 30 days post-procedure or who were event-free, with their last follow-up at least 23 days post-procedure. Imaging outcomes were analysed in the implanted population, which consisted of all enrolled patients who were successfully implanted with an ACURATE neo2 THV. Event rates are shown as proportions for follow-up up to 30 days, and no formal statistical testing was prespecified for this single-arm study. All statistical analyses were performed using the SAS System software, version 9.4 or later (SAS Institute).
Results
Study flow and patients
The study enrolled 250 patients at 18 centres in 8 European countries between December 2020 and January 2022 (Supplementary Table 1). For all patients, the local Heart Team agreed that the patient had an approved indication for TAVI and that TAVI with the ACURATE neo2 THV was appropriate. Among these, 246 patients (98.4%) had successful implantation of the THV (defined as successful vascular access, delivery, and deployment of the valve with successful retrieval of the delivery system), comprising the implanted population (Figure 1). Per protocol, only patients with a device implanted in the correct position underwent imaging; among patients with successful implantation of the ACURATE neo2, 91% (224/246) had 30-day TTE and 83% (204/246) had 30-day 4D-CT performed. Clinical follow-up data were available at 30 days for 94% (236/250) of patients.
Baseline demographics and patient characteristics are shown in Table 1. The mean age of patients was 81 years, with 64% female, a baseline Society of Thoracic Surgeons (STS) risk score of 2.9±2.0%, and 52.0% were considered NYHA Class III or IV. Based on operator assessment, approximately two-thirds of patients were considered to be at either high/extreme (31.2% [7/250]) or intermediate (31.6% [79/250]) surgical risk. Based on CT core laboratory assessment, the native valve morphology was tricuspid in 98.4% of patients and bicuspid in 1.6% of patients. The mean baseline aortic valve area was 0.7±0.2 cm2 and the mean baseline aortic valve gradient was 47.6±14.5 mmHg. Additional baseline echocardiographic and CT measurements are shown in Table 1.
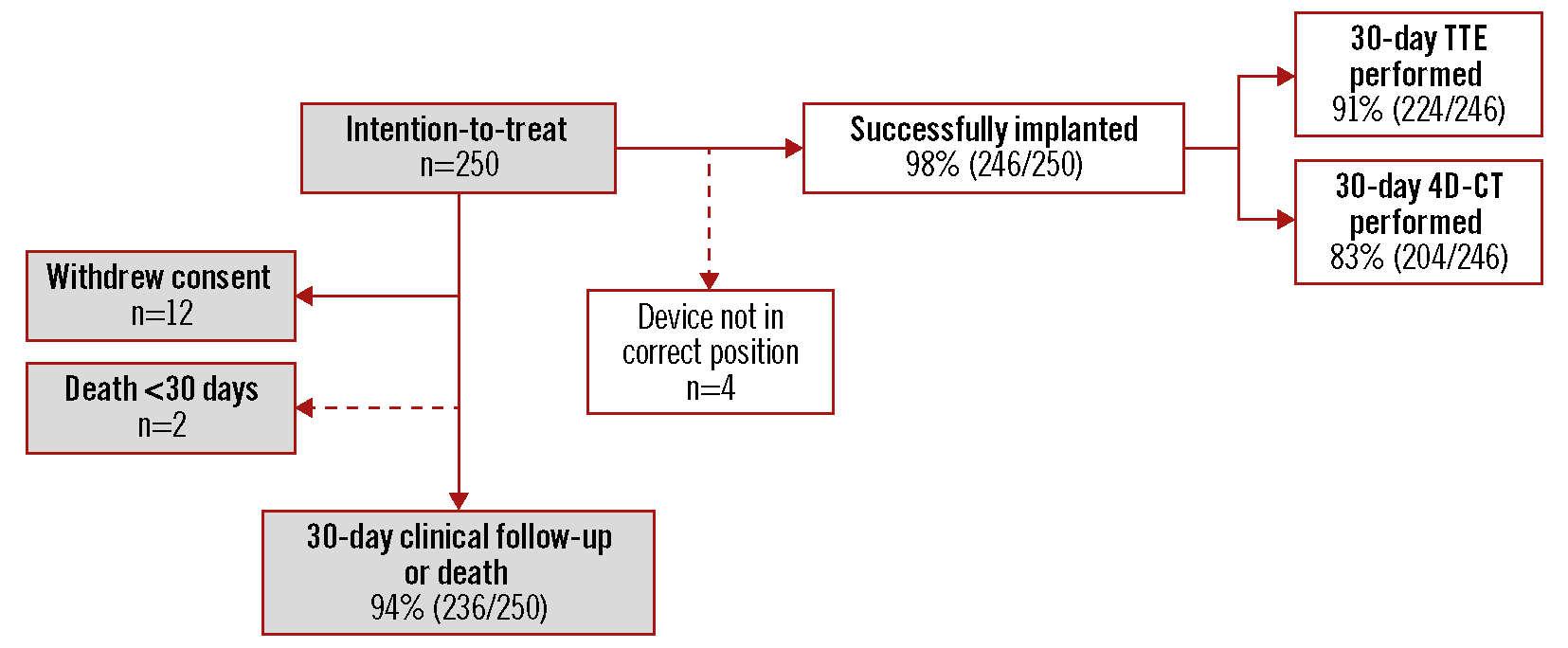
Figure 1. Patient disposition up to 30 days. Four patients did not have a study valve implanted in the correct position and, per protocol, did not undergo 30-day imaging but were followed for clinical safety. 4D-CT: 4-dimensional computed tomography; TTE: transthoracic echocardiography
Table 1. Baseline demographics and patient characteristics.
| ITT population (N=250) | |||
|---|---|---|---|
| Patient characteristics | Age, mean (years) | 80.8±6.2 | |
| Sex, female | 63.6% (159/250) | ||
| Diabetes, medically treated | 24.0% (60/250) | ||
| Hypertension | 80.8% (202/250) | ||
| Coronary artery disease | 40.8% (102/250) | ||
| History of atrial fibrillation | 4.4% (11/250) | ||
| History of stroke | 5.2% (13/250) | ||
| Prior pacemaker at baseline | 6.0% (15/250) | ||
| Pre-existing conduction abnormality | 13.3% (33/248) | ||
| Atrioventricular block I | 42.4% (14/33) | ||
| Atrioventricular block II – Type 2 | 3.0% (1/33) | ||
| Left bundle branch block | 33.3% (11/33) | ||
| Right bundle branch block | 36.4% (12/33) | ||
| Left anterior fascicular block | 15.2% (5/33) | ||
| NYHA Functional Class | I | 8.4% (21/250) | |
| II | 38.8% (97/250) | ||
| III | 51.2% (128/250) | ||
| IV | 0.8% (2/250) | ||
| Unknown | 0.8% (2/250) | ||
| Risk assessments | Mean STS score (%) | 2.9±2.0 (143) | |
| STS score (category) | STS score <3% | 68.5% (98/143) | |
| STS score ≥3% to <8% | 28.7% (41/143) | ||
| STS score ≥8% | 2.8% (4/143) | ||
| EuroSCORE II (%) | 3.3±2.8 (201) | ||
| Operative risk group (as per Heart Team assessment) | High operative risk | 31.2% (78/250) | |
| Intermediate operative risk | 31.6% (79/250) | ||
| Low operative risk | 37.2% (93/250) | ||
| Baseline echocardiography (site reported) | Aortic valve area, cm2 | 0.7±0.2 (237) | |
| Aortic valve gradient, mmHg | 47.6±14.5 (240) | ||
| Left ventricular ejection fraction, % | 56.5±8.6 (244) | ||
| Screening CT (core laboratory) |
Bicuspid native valve morphology | 1.6% (4/250) | |
| Systolic annular measurements | Perimeter (mm) | 73.5±5.1 (216) | |
| Perimeter-derived diameter (mm) | 23.4±1.6 (216) | ||
| Area (mm2) | 416.6±58.9 (216) | ||
| Right coronary artery height (mm) | 16.0±3.1 (243) | ||
| Valve leaflet calcification | None | 0.4% (1/244) | |
| Mild | 6.6% (16/244) | ||
| Moderate | 75.0% (183/244) | ||
| Severe | 18.0% (44/244) | ||
| Annulus calcification | None | 67.6% (165/244) | |
| Mild | 12.3% (30/244) | ||
| Moderate | 14.8% (36/244) | ||
| Severe | 5.3% (13/244) | ||
| LVOT calcification | None | 70.5% (170/241) | |
| Mild | 14.1% (34/241) | ||
| Moderate | 11.2% (27/241) | ||
| Severe | 4.1% (10/241) | ||
| Values are means±standard deviation (n) or percentages (n/N). CT: computed tomography; ITT: intention-to-treat population; LVOT: left ventricular outflow tract; NYHA: New York Heart Association Functional Class; STS: Society of Thoracic Surgeons | |||
Procedural results
Procedural details are presented in Table 2. As noted above, 4 patients did not have an ACURATE neo2 THV implanted in the correct position; in all of these cases, this was due to valve embolisation in the aortic direction (pursuant to use of an oversized THV in a small anatomy [n=1], and positioning the THV too high in the annulus [n=3]). In 3 of the 4 patients a non-study balloon-expandable THV was successfully implanted in the aortic position. In the remaining patient a non-study balloon-expandable THV was implanted in the aortic position in a TAV-in-TAV procedure; however, this patient experienced cardiac tamponade and left main coronary occlusion and died during the procedure.
The most common valve size implanted was medium (42% of patients). In most patients (77.6%), the ACURATE neo2 was implanted using a 3-cusp view; 22.4% of cases used a cusp-overlap view. There was an attempt at commissural alignment in 174 patients. There were no cases with unplanned use of cardiopulmonary bypass or conversion to surgery during the procedure. In a post hoc analysis, 93% of patients met the criteria for VARC-3 device success9 (Supplementary Table 2). Ten percent of procedures used a concomitant cerebral embolic protection device. At hospital discharge, 89% (218/246) of patients were treated with aspirin, 33% (81/246) with a P2Y12 inhibitor, 25% (60/246) with dual antiplatelet therapy, and 14% (35/246) with anticoagulants. At 30 days, 90% of patients (209/233) were treated with aspirin, 31% (73/233) with a P2Y12 inhibitor, 24% (55/233) with dual antiplatelet therapy, and 5% (11/233) with anticoagulants.
Table 2. Procedural characteristics.
| ITT population (N=250) | ||
|---|---|---|
| Successful vascular access, delivery, and deployment of valve with successful retrieval of delivery system* | 98.4% (246/250) | |
| Conversion to surgery | 0% (0/250) | |
| Unplanned cardiopulmonary bypass performed | 0% (0/250) | |
| TAV-in-TAV | 0.4% (1/250) | |
| Second valve deployed (non TAV-in-TAV) | 1.2% (3/250) | |
| Anaesthesia type | General anaesthesia | 7.6% (19/250) |
| Conscious sedation | 92.4% (231/250) | |
| Total procedure time, min | 63.2±32.3 (245) | |
| Total fluoroscopy time, min | 16.1±9.5 (248) | |
| Total contrast media used for procedure (mL) | 113.9±91.2 (248) | |
| Predilation balloon aortic valvuloplasty performed | 96.8% (242/250) | |
| Rapid pacing using during valve deployment | 46.0% (115/250) | |
| Embolic protection device used | 10.4% (26/250) | |
| Post-dilatation performed | 26.0% (65/250) | |
| Implanted ACURATE neo2 valve size | Small | 26.0% (64/246) |
| Medium | 42.3% (104/246) | |
| Large | 31.7% (78/246) | |
| Valve malpositioning | 1.6% (4/250) | |
| Valve embolisation | 1.6% (4/250) | |
| Major vascular complication | 3.2% (8/250) | |
| Cardiac tamponade (≤3 days after index procedure) | 0.4% (1/250) | |
| Acute kidney injury (Stage 2 or 3) | 0% (0/250) | |
| Values are site-reported means±standard deviations (n) or percentages (n/N). Binary rates.*4 patients were implanted with a non-study device following embolisation of the ACURATE neo2 valve during implantation. ITT: intention-to-treat; TAV-in-TAV: transcatheter aortic valve in valve implantation | ||
Primary endpoints
The primary safety endpoint of 30-day all-cause mortality was 0.8% (2/250) (Central illustration, Table 3). One patient experienced haemodynamic instability from bleeding of unknown aetiology, leading to circulatory arrest and resulting in death on day 4 post-procedure. The second patient died post-index procedure following the aforementioned THV embolisation and unsuccessful TAV-in-TAV. For the primary imaging endpoint, HALT was detected in 50 of 204 patients with imaging data available (24.5%). Among those, HALT severity was >75% in 8 patients (3.9%) and >50% to 75% in 11 patients (5.4%) (Central illustration). In the 50 patients exhibiting some degree of HALT, there was evidence of restricted leaflet mobility in one leaflet in 46.9% of patients, in two leaflets in 36.7% of patients, and in all three leaflets in 14.3% of patients. The presence of HALT was not detected in any patients who were on oral anticoagulant therapy. The mean aortic valve gradient was not increased in patients with HALT (HALT: 7.2±2.7 mmHg; no HALT: 8.8±3.7 mmHg).
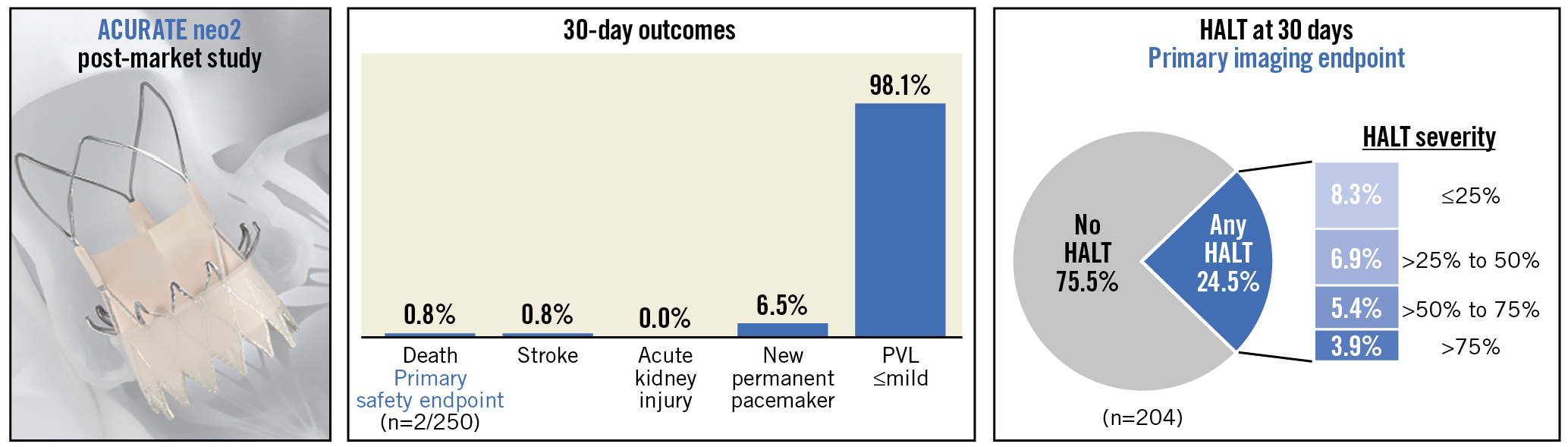
Central illustration. The ACURATE neo2 PMCF observational post-market surveillance study collected clinical outcomes and device performance data in 250 patients with severe aortic stenosis and at high operative risk who were treated with the ACURATE neo2 aortic valve system in routine clinical practice. HALT: hypoattenuated leaflet thickening; PVL: paravalvular leak
Table 3. Safety outcomes up to 30 days (ITT population; N=250).
| Event | Discharge/7 days | 30 days* | |
|---|---|---|---|
| All-cause mortality | 0.4% (1) | 0.8% (2) | |
| Cardiovascular mortality | 0.4% (1) | 0.8% (2) | |
| All stroke | 0.4% (1) | 0.8% (2) | |
| Disabling stroke | 0.0% (0) | 0.0% (0) | |
| Non-disabling stroke | 0.4% (1) | 0.8% (2) | |
| Bleeding | Life-threatening or disabling | 2.0% (5) | 2.9% (7) |
| Major | 2.4% (6) | 2.4% (6) | |
| Myocardial infarction | 0.8% (2)† | 0.8% (2) | |
| Repeat procedure for valve-related dysfunction | 0.4% (1) | 0.4% (1) | |
| Hospitalisation for valve-related symptoms or worsening congestive heart failure | 0% (0) | 0% (0) | |
| Prosthetic aortic valve thrombosis‡ | 0.4% (1) | 0.4% (1) | |
| Prosthetic aortic valve endocarditis | 0.0% (0) | 0.4% (1) | |
| Newly implanted permanent pacemaker | Among all patients | 5.2% (13) | 6.1% (15) |
| Among pacemaker-naïve patients (n=235) | 5.5% (13) | 6.5% (15) | |
| Values are percentages (n). Binary rates. *245 patients were evaluable for safety at 30 days (defined as subjects who experience a CEC-adjudicated event up to 30 days post-procedure or who were event-free with last follow-up at least 23 days post-procedure). †Periprocedural (≤72 hours post-index procedure). ‡Occurred in a non-study valve that was implanted subsequent to ACURATE neo2 valve embolisation. CEC: clinical events committee; ITT: intention-to-treat | |||
Other clinical endpoints
Additional safety assessments at discharge and 30 days are shown in Table 3; 245/250 patients (98.0%) were evaluable for safety at 30 days. The overall rates of adverse events up to 30 days included 0.8% stroke (2/245, both non-disabling), 2.9% life-threating/disabling bleeding (7/245), and 0.4% repeat procedure for valve-related dysfunction (1/245). Major vascular complications were reported in 3.3% of patients; all were access site-related. Permanent pacemaker implantation was required in 15 of 231 (6.5%) pacemaker-naïve patients. Of the patients implanted with a new pacemaker, 6 patients (40%) had evidence of a conduction disturbance at baseline (right bundle branch block [n=5]; second-degree atrioventricular block [n=1]).
At 30 days, 51.0% (107/210) of patients were NYHA Class I, 41.0% (86/210) were Class II, and 8.1% (17/210) were Class III; no patients were Class IV. Overall, 71% of patients improved by at least 1 functional class from baseline to 30 days, and 20% improved by at least 2 classes.
Haemodynamic performance
The mean aortic valve area improved from 0.7±0.2 cm2 at baseline to 1.6±0.4 cm2 at both discharge/7 days and at 30 days (Figure 2). The mean aortic valve gradient decreased from 47.6±14.5 mmHg at baseline to 9.7±5.4 mmHg at discharge/7 days and then to 8.6±3.9 mmHg at 30 days (Figure 2). Results were similar in a paired analysis of patients with evaluable echocardiographic data available at all timepoints (Supplementary Figure 1). Among all patients with core laboratory-evaluable echocardiograms, PVL was evaluated as none/trace in 79.2% of patients, mild in 18.9%, moderate in 1.9%, and severe in 0% (Figure 3A). Results were similar in a paired analysis of 121 patients who had evaluable echocardiograms at both discharge/7 days and 30 days (Figure 3B). In the paired analysis, most patients (77%) exhibited stability in PVL (i.e., no interindividual change) between discharge and 30 days.
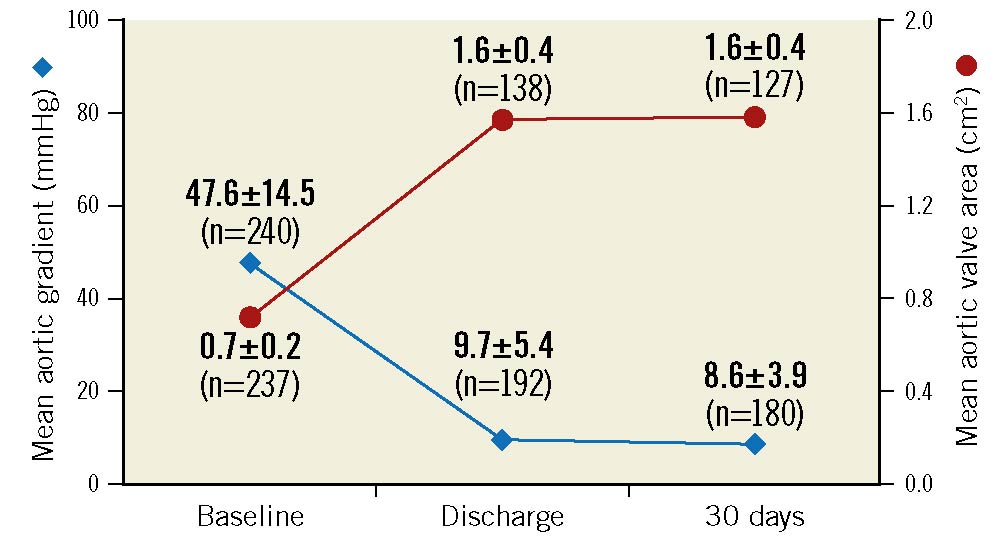
Figure 2. Valve haemodynamics in the implanted population. Mean aortic gradient and aortic valve area were evaluated by an echocardiography core laboratory at baseline, discharge, and 30 days in patients successfully implanted with the ACURATE neo2 (n=246).
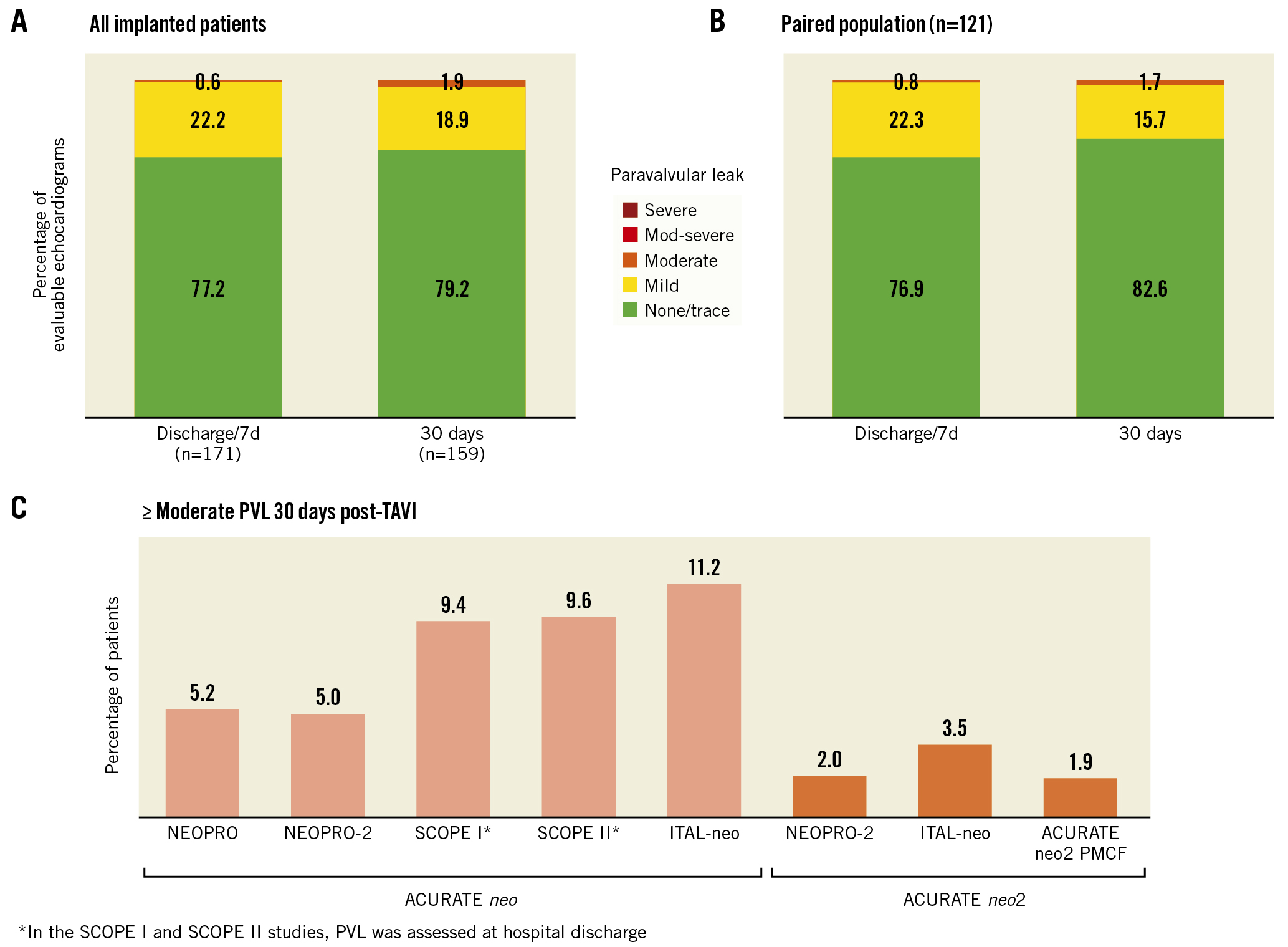
Figure 3. Paravalvular leak. Paravalvular leak was assessed at hospital discharge or 7 days (whichever came first) and 30 days in (A) all patients successfully implanted with the ACURATE neo2 (n=246) and (B) the paired population of patients with evaluable echocardiograms at both discharge/7 days and 30 days (n=121). C) The rate of moderate or greater PVL at 30 days was higher in patients treated with the prior-generation ACURATE neo device compared with the next-generation ACURATE neo2 THV. PMCF: post-market clinical follow-up; PVL: paravalvular leak; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve.
Discussion
In this post-market registry of patients with aortic stenosis treated in routine clinical practice with the ACURATE neo2 THV, a clinical events committee adjudicated VARC-2 adverse events, and core laboratories were employed for echocardiographic analyses related to haemodynamic performance, as well as for 4D-CT imaging to provide insight into the prevalence of HALT and restricted leaflet motion in a real-world setting.
Patients in the study demonstrated a high rate of procedural success (98.4%) and a favourable safety profile. The primary safety endpoint of 30-day all-cause mortality was 0.8%. This rate was similar to or better than previously observed in studies that evaluated the ACURATE alongside other THVs451011, or with contemporary devices in other large post-approval THV registries (2.2% with SAPIEN 3 [Edwards Lifesciences] in the SOURCE 3 registry12; 0.0% with SAPIEN 3 Ultra in the S3U registry13; 4.8% with Evolut PRO [Medtronic] in the FORWARD PRO registry14; 2.6% with Portico [Abbott] in the CONFIDENCE Registry15). Likewise, the 30-day rates of disabling stroke (0.0%) and acute kidney injury (0.0%) in patients treated with the ACURATE neo2 in this study were lower than those reported for the ACURATE neo or its contemporary competitors in the aforementioned THV studies and registries. Four patients (1.6%) experienced embolisation of the THV; this incidence of embolisation is in line with the findings from the TRAVEL Registry, a multicentre TAVI cohort of over 29,000 patients, wherein valve embolisation and migration occurred in approximately 1% of cases overall, and in 1.4% with non-balloon-expandable THV in particular16. The 6.5% rate of new pacemaker implantation in this study is lower than the range previously reported for the first-generation ACURATE neo (8.3% to 12%)1451011 and comparable to the rates for the ACURATE neo2 in the recent NEOPRO-2 and ITAL-neo registries (8.0% and 7.6%, respectively)1718.
Patients in the ACURATE neo2 PMCF Study exhibited early haemodynamic improvements at discharge, which were maintained up to 30-day follow-up. At 30 days, paravalvular leak was mild or less in 98% of patients and moderate in 1.9% of patients, with no patients exhibiting greater than moderate PVL. In contrast, the 30-day rate of moderate or greater PVL observed with the prior-generation ACURATE neo in the recent SCOPE I and SCOPE II studies was 9.4% and 9.6%, respectively45 (Figure 3C). The results of the current study are comparable to the rates of moderate or greater PVL 30 days after ACURATE neo2 implantation in the ACURATE Neo AS study (3.0%)6, the NEOPRO/NEOPRO-2 registry (2.0%)17, and the ITAL-neo registry (3.5%)18. The substantial improvement in PVL can be attributed, in large part, to the active sealing technology of the improved sealing skirt on the ACURATE neo2. This type of improvement in PVL, coincident with device design progression, has also been noted for other THVs192021. As has been observed with the ACURATE neo, factors related to improvements in patient selection, sizing, and implant technique may also have played a role in the reduction of PVL rates2223.
In recent years, there has been increased interest in the use of 4D-CT to characterise HALT, which manifests as hypoattenuating thickening typically localised to the valve periphery and base of the leaflet, with a subjective grading scale used to describe the extent of leaflet involvement8. Data from studies to date have been variable with regard to the impact of HALT or restricted leaflet mobility on clinical outcomes and the utility of treatment for HALT in improving outcomes2425. Recent meta-analyses have suggested there is an association between imaging findings and major adverse cardiac and cerebrovascular events2627. While prospective studies have not confirmed that HALT affects patients’ clinical outcomes, there is a concern that it may potentially impact valve durability and increase thrombotic complication rates82829.
The primary imaging endpoint of the ACURATE neo2 PMCF Study was evidence of any degree of HALT at 30 days post-TAVI. Published studies have found that post-TAVI HALT typically occurs in 10-15% of patients, although in some cases the rate is as high as 35-40%24. In the current study, 24.5% of patients exhibited some degree of HALT, with the severity of HALT >50% in 9.3% of patients. Of the patients with HALT, virtually all exhibited some degree of restricted leaflet mobility, but there were no evident clinical implications. In general, patients demonstrated improved valve haemodynamics, and the mean gradient was not increased in patients with HALT. The small sample size in this study (n=50 patients with HALT) makes it difficult to characterise the relationship between HALT and gradient; additional CT analysis in a larger population may provide additional insight. There were no unusual safety signals in patients with HALT during 30 days of follow-up – no patients experienced a stroke, and there was only one case of prosthetic valve thrombosis. In the short follow-up time frame for this study, it was not possible to evaluate whether HALT had an impact on valve durability or longer-term clinical outcomes. More in-depth analyses of the CT results are ongoing.
Limitations
As a single-arm study with only 250 patients, the results from this registry cannot be directly compared to clinical outcomes with other THV devices. Enrolment decisions were made per the discretion of the local Heart Team, thus the possibility of site-based differences in patient selection cannot be ruled out. It should be noted that this study was conducted during the COVID-19 pandemic, which may have affected site activation and enrolment, as well as limited clinical follow-up for some endpoints or at some sites, due to pandemic-related restrictions. The study did not require routine neurological assessment by an independent neurological professional; thus, it is possible that cerebrovascular complications may have been underreported. Although the study employed core laboratories for the evaluation of echocardiographic and CT data, in some cases the data collected were not analysable due to poor image quality or other technical issues, leading to a reduced sample size available for follow-up. To date, only short-term (30-day) follow-up data are available; however, patients will continue to be followed for 5 years, with echocardiographic and 4D-CT evaluations repeated at 1 year and analysed by the core laboratories.
Conclusions
The ACURATE neo2 PMCF Study results support the safety and efficacy of transfemoral TAVI with the ACURATE neo2 THV in routine clinical practice. Patients exhibited early improvements in valve haemodynamics that were maintained up to 30 days, with low rates of new pacemaker implantation and paravalvular leak.
Impact on daily practice
The first-generation ACURATE neo transcatheter heart valve (THV) is a safe and effective treatment for aortic stenosis; however, higher rates of paravalvular leakage (PVL) have been reported compared with other THVs. In this study of the next-generation ACURATE neo2 THV, which features improved sealing designed to mitigate PVL and a radiopaque marker for precise positioning, patients had a low pacemaker implantation rate, early improvements in valve haemodynamics that were maintained up to 30 days, and a low rate of moderate or greater PVL. The ACURATE neo2 PMCF Study results support the safety and efficacy of TAVI with the ACURATE neo2 in patients in routine clinical practice.
Acknowledgements
The authors wish to acknowledge Songtao Jiang, MS (Boston Scientific) for statistical support and MaryEllen Carlile Klusacek, PhD (Boston Scientific) for assistance with manuscript preparation.
Funding
The ACURATE neo2 PMCF Studywas sponsored and funded by Boston Scientific Corporation (Marlborough, MA, USA). The data and study protocol for this clinical trial may be made available to other researchers in accordance with Boston Scientific’s data sharing policy (http://www.bostonscientific.com/en-US/data-sharing-requests.html).
Conflict of interest statement
W-K. Kim reports personal fees from Boston Scientific, Edwards Lifesciences, Abbott, Medtronic, and Meril. C. Tamburino is a consultant for Medtronic. H. Möllmann reports speaker/proctor/advisor honoraria from Abbott, Boston Scientific, and Edwards Lifesciences. M. Montorfano reports consultant fees from Abbott, Boston Scientific, Kardia, and Medtronic. T.K. Rudolph has received speaker honoraria from Boston Scientific. N.M. Van Mieghem reports consulting fees/honoraria from Daiichi Sankyo, Abiomed, Anteris, Amgen, and Teleflex; and research grants from Medtronic, Boston Scientific, ACIST, PulseCath, Edwards Lifesciences, and Abbott Vascular. M. Hilker has participated on advisory boards for and received proctor fees from Boston Scientific. I. Amat-Santos is a proctor for Boston Scientific, Medtronic, and Meril Life. C.J. Terkelsen reports proctor/speaker fees from Edwards Lifesciences, Meril, Boston Scientific, and Terumo. A.S. Petronio reports research funds from Medtronic, Boston Scientific, and Abbott; and is a consultant for Medtronic, Boston Scientific, and Abbott. M. Götberg serves as a proctor for Boston Scientific and reports consulting honoraria from Boston Scientific. A. Rück reports advisory board participation for Edwards Lifesciences and Boston Scientific; lecture fees from Abbott, Edwards Lifesciences, and Boston Scientific; consulting for ANTERIS; and has received institutional research support from Boston Scientific. R. Trillo serves as a proctor for Medtronic and Boston Scientific. M. Barbanti is a consultant for Edwards Lifesciences, Medtronic, and Boston Scientific. P. Blanke is a consultant to Edwards Lifesciences; and has provided institutional CT Core Laboratory services for Boston Scientific, Medtronic, Edwards Lifesciences, and Abbott, for which no direct personal compensation was received. R. Modolo is an employee and shareholder of Boston Scientific. D.J. Allocco is an employee and shareholder of Boston Scientific. L. Sondergaard has received consultant fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic, and SMT. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

