Abstract
Background: Transcatheter aortic valve implantation is an effective treatment for patients with aortic stenosis; however, complications related to paravalvular leakage (PVL) persist, including increased risk of mortality, cardiovascular mortality, and rehospitalisation.
Aims: We sought to evaluate the clinical outcomes and valve performance at 1 year in patients with severe aortic stenosis treated with the ACURATE neo2 valve in a post-market clinical setting.
Methods: Valve Academic Research Consortium-2 safety events were assessed up to 1 year. Independent core laboratories evaluated echocardiographic measures of valve performance and hypoattenuated leaflet thickening (HALT; as measured by four-dimensional computed tomography).
Results: The study enrolled 250 patients (64% female; mean age: 81 years; baseline Society of Thoracic Surgeons risk score: 2.9±2.0%); 246 patients were implanted with ACURATE neo2. All-cause mortality was 0.8% at 30 days and 5.1% at 1 year. The 1-year rates for stroke and disabling stroke were 3.0% and 1.3%, respectively. Overall, HALT of >50% leaflet involvement of at least one leaflet was present in 9% of patients at 30 days and in 12% of patients at 1 year. No association was observed between the presence of HALT and 1-year clinical or haemodynamic outcomes. Early haemodynamic improvements were maintained up to 1 year (mean aortic valve gradient: 47.6±14.5 mmHg at baseline, 7.6±3.2 mmHg at 1 year; mean aortic valve area: 0.7±0.2 cm2 at baseline, 1.7±0.4 cm2 at 1 year). At 1 year, 99% of patients had mild or no/trace PVL (<1% had moderate PVL; no patient had severe PVL).
Conclusions: The study outcomes confirm favourable performance and safety up to 1 year in patients treated with ACURATE neo2 in routine clinical practice. (ClinicalTrials.gov: NCT04655248)
Introduction
The ACURATE neo2 transcatheter heart valve, and its predecessor the ACURATE neo (both Boston Scientific), have been shown to be safe and efficacious in patients with aortic stenosis (AS) undergoing transcatheter aortic valve implantation (TAVI)12345. The ACURATE platform design – a self-expanding nitinol frame with top-down deployment and porcine pericardial leaflets in a supra-annular position – provides low gradients, large effective orifice areas, and a low risk of periprocedural complications12. However, some head-to-head studies of the first-generation neo device versus second-generation competitor devices found higher rates of paravalvular leak (PVL) in patients treated with the neo device67. Moderate PVL remains a concern in TAVI, as it is associated with increased risk of mortality, cardiovascular mortality, and rehospitalisation8. The design for the neo2 device features several improvements intended to mitigate PVL, including aids to facilitate positioning during implantation and augmented inner and outer sealing skirts4. Post-TAVI hypoattenuated leaflet thickening (HALT), as detected by multidetector computed tomography (CT), is also of concern. HALT can be an early indicator of subclinical leaflet thrombosis, although the clinical impact of this is not well defined9. There are currently no data on the incidence or natural course of subclinical thrombosis for the ACURATE platform.
The ACURATE neo2 Post Market Clinical Follow up (PMCF) Study collected safety and imaging data for the neo2 device in a routine clinical practice setting. Procedural and acute outcomes have been previously reported, including a high rate of procedural success (98.4%), 0.8% all-cause mortality at 30 days (primary safety endpoint), and very low rates of stroke (0.8%) and disabling stroke (0%)5. Patients exhibited early haemodynamic improvements, and PVL at 30 days was moderate in 1.9% of patients (no patients had severe PVL)5. Herein we report the clinical and imaging outcomes from the ACURATE neo2 PMCF Study at 1 year, including a core laboratory-adjudicated assessment of longitudinal improvement in valve haemodynamics and PVL and a core laboratory evaluation of the incidence HALT and its clinical impact.
Methods
STUDY DEVICE AND DESIGN
The ACURATE neo2 bioprosthetic aortic valve comprises a self-expanding nitinol stent frame with porcine pericardial tissue leaflets in the supra-annular position and inner and outer sealing skirts designed to minimise paravalvular leak4. The valve is currently available in 3 sizes (small [S], medium [M], and large [L]), intended for a native aortic annulus diameter range between 21 mm and 27 mm.
The ACURATE neo2 PMCF Study5, a prospective multicentre single-arm post-market surveillance study which enrolled patients with severe AS, did not have specific inclusion criteria beyond those indicated in the neo2 instructions for use. For the purposes of the CT-imaging portion of the study, patients were excluded if they had chronic kidney disease stage IV or V (defined as an estimated glomerular filtration rate <30 mL/min/1.73 m2), uncontrolled atrial fibrillation, or an expected need for chronic anticoagulation therapy post-procedure (short-term anticoagulation post-procedure was permissible, and all imaging assessments were performed 30 days after discontinuation of anticoagulation).
Patient follow-up was conducted per local standard of care. Safety events were evaluated at discharge, 30 days, and 1 year; evaluation will continue annually up to 5 years. Echocardiography was performed predischarge, and at 30 days and 1 year post-procedure for eligible patients; four-dimensional (4D)-CT imaging was performed at 30 days and 1 year. The study is registered at ClinicalTrials.gov (NCT04655248) and adhered to the principles set forth in the Declaration of Helsinki and all applicable local and country regulations.
ENDPOINT EVALUATION
An independent clinical events committee (CEC) adjudicated adverse events (death, stroke, bleeding, major vascular complications, and hospitalisation for valve-related symptoms or worsening congestive heart failure) up to 1 year. Independent core laboratories evaluated echocardiographic measures (MedStar Health Research Institute, Washington, D.C., USA) and 4D-CT imaging (Department of Radiology, The University of British Columbia, Vancouver, BC, Canada). Patients’ health status was evaluated using the EQ-5D Quality of Life questionnaire, and severity of heart failure was categorised according to the New York Heart Association (NYHA) Functional Classification at baseline, 30 days, and 1 year. The study’s primary safety endpoint (all-cause mortality at 30 days) and primary imaging endpoint (HALT at 30 days, as measured by 4D-CT) have been described and reported in a prior publication5.
STATISTICAL ANALYSES
Safety outcomes were analysed in the intention-to-treat (ITT) population (i.e., all enrolled patients). Outcomes at 1 year were evaluated in patients who experienced a CEC-adjudicated event up to 365 days post-procedure or who were event-free with their last follow-up at least 305 days post-procedure; these are expressed as Kaplan-Meier time-to-event rate estimates to compensate for censored data, including patients who withdrew or were lost to follow-up. Imaging outcomes were analysed in the implanted population (i.e., enrolled patients who were successfully implanted with neo2). Imaging assessments were not mandated in patients who did not have neo2 implanted in the aortic position (these patients were followed for safety up to 30 days only) or in patients who required a second transcatheter valve or conversion to surgery during the index procedure (these patients were followed for safety up to 1 year).
Baseline, procedural, and follow-up data are expressed as mean±standard deviation (n) for continuous variables and as percentage (n/N) for categorical variables. There is no formal statistical testing prespecified for this single-arm study. All statistical analyses are performed using SAS software, version 9.4 or later (SAS Institute).
Results
STUDY PARTICIPANTS AND PATIENT FOLLOW-UP
The study enrolled 250 patients between 16 December 2020 and 10 January 2022. The ITT population was 64% female, with a mean age of 81 years and a baseline Society of Thoracic Surgeons (STS) risk score of 2.9±2.0%. Additional baseline characteristics are presented in Supplementary Table 1; procedural characteristics and outcomes are reported elsewhere5. As shown in Figure 1, ACURATE neo2 was successfully implanted in 246 patients (98.4%; the implanted population); 89% of patients (223/250) completed a 1-year clinical follow-up visit. In the implanted population, 76% (186/246) had 1-year transthoracic echocardiogram and 62% (153/246) had 1-year 4D-CT performed.
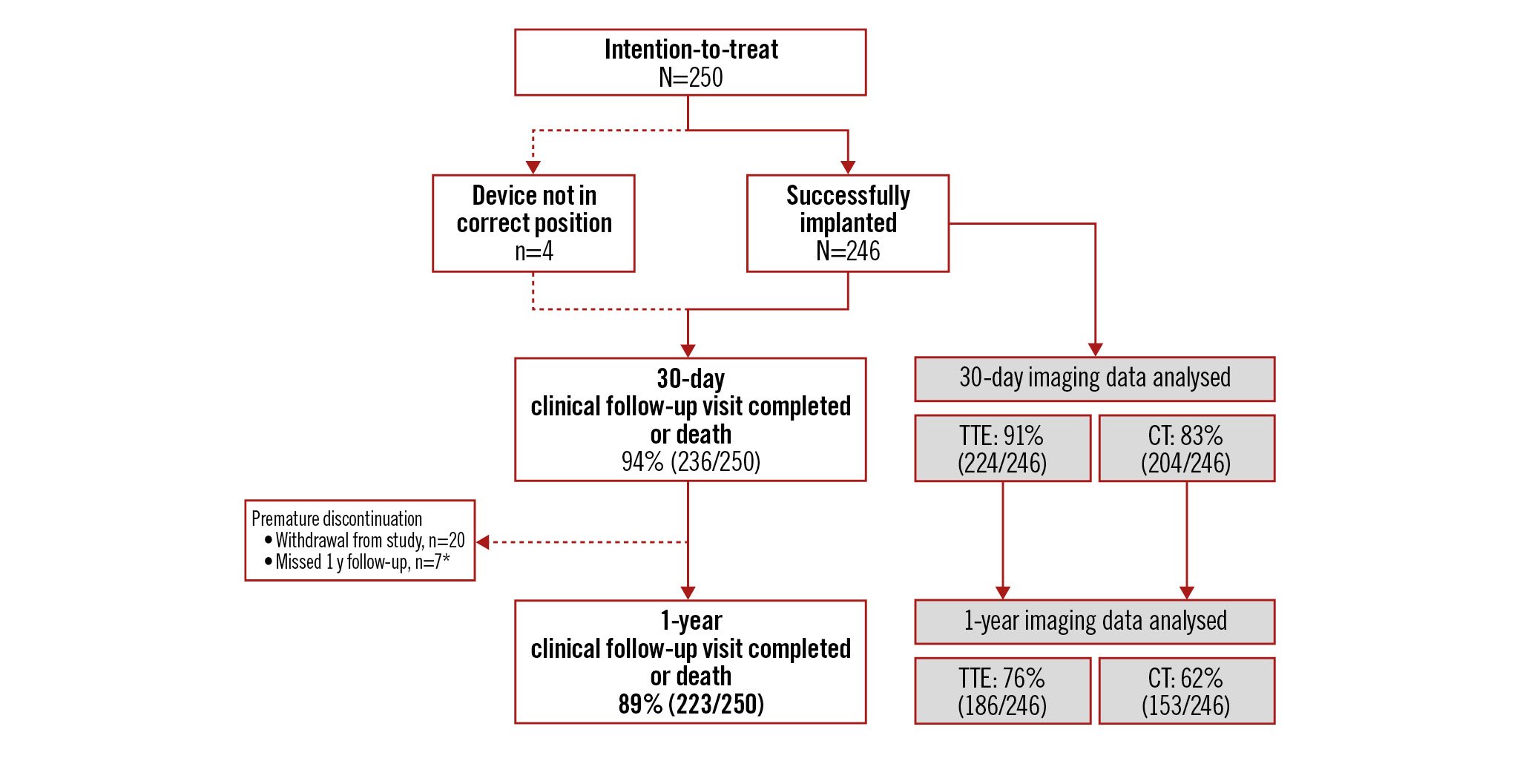
Figure 1. ACURATE neo2 PMCF patient disposition up to 1 year. The ACURATE neo2 Post Market Clinical Follow up (PMCF) Study enrolled 250 patients, 246 of whom (98.4%) were implanted with an ACURATE neo2 valve. *Patient survival confirmed, but no additional follow-up data collected. CT: computed tomography; TTE: transthoracic echocardiogram
CLINICAL SAFETY AND FUNCTIONAL OUTCOMES
At 1 year, the time-to-event rate for all-cause mortality was 5.1% (12/250); the rates for stroke and disabling stroke were 3.0% and 1.3%, respectively (Central illustration). Additional Valve Academic Research Consortium-2 safety outcomes at 1 year are shown in Table 1. Exploratory subgroup analyses indicate that safety outcomes were generally similar between cohorts for age and gender (Supplementary Table 2).
The need for reintervention for valve-related dysfunction was very low (2 patients, 0.8%): in one patient the neo2 valve had embolised during the procedure and a non-study valve was implanted, with surgery performed later to retrieve the embolised valve; one patient exhibited moderate PVL >30 days post-procedure and was treated with balloon valvuloplasty to correct the leak. There were 2 cases of valve thrombosis noted up to 1 year: one in a patient who received a non-study valve following embolisation of the initial neo2 valve and one in a patient who exhibited HALT at 30 days and 1 year and had begun oral anticoagulant therapy shortly before the 1-year follow-up visit.
Data on patients’ change in NYHA Functional Class are shown in Figure 2. Data on Functional Class were available for 184 surviving patients at 1 year. Overall, 73% of these patients improved by at least 1 Functional Class from baseline to 1 year, and 28% improved by at least 2 classes. Patients’ health status showed measurable improvement at 30 days and 1 year. Values for the EQ-5D index score increased significantly from baseline to 30 days (n=224, Δ0.028±0.17; p=0.01) and from baseline to 1 year (n=198, Δ0.037±0.16; p=0.001). There was also significant improvement in the EQ Visual Analogue Scale from baseline to 30 days (n=223, Δ7.2±17.3; p<0.001) and from baseline to 1 year (n=196, Δ7.0±21.9; p<0.001).
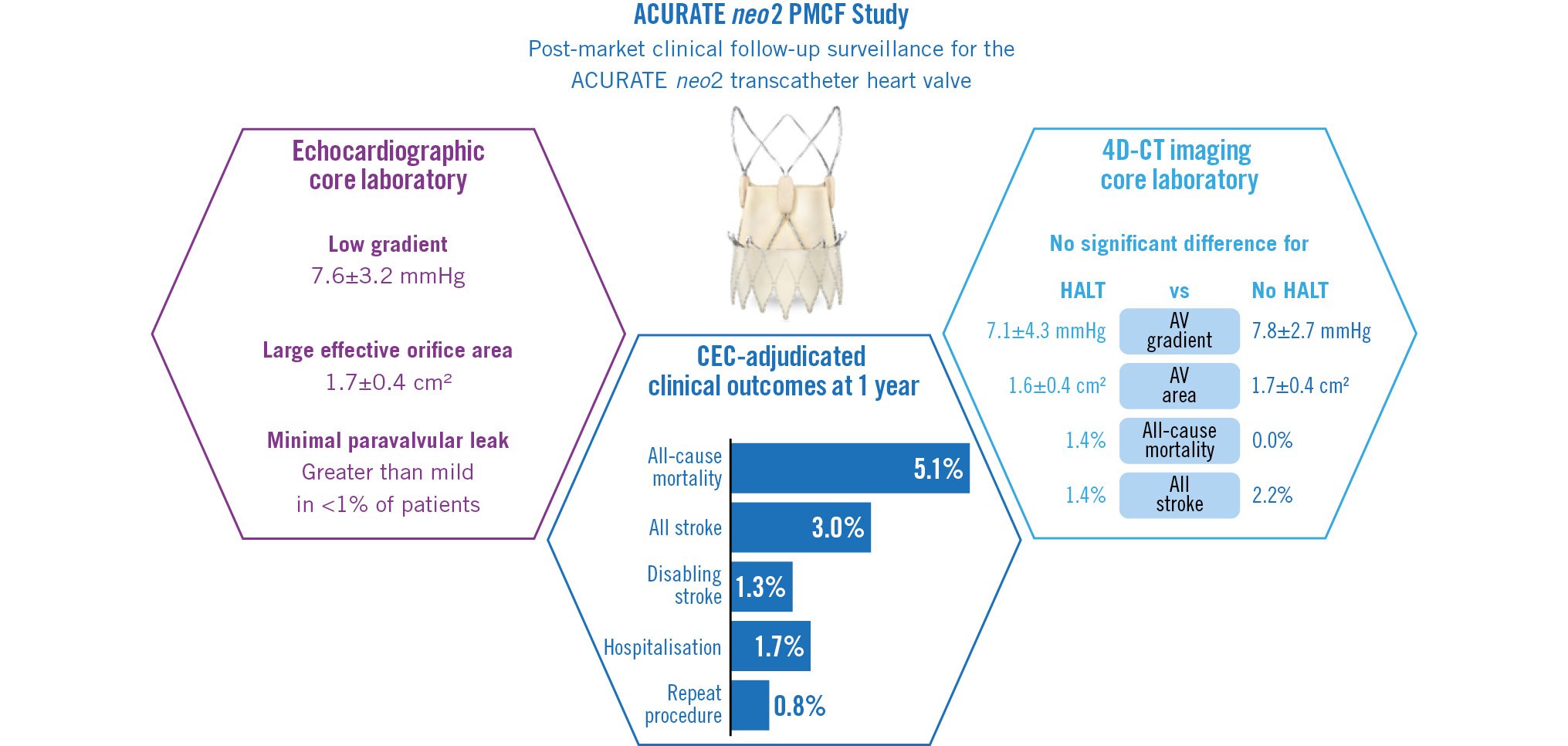
Central illustration. Key outcomes from the ACURATE neo2 PMCF Study. 4D-CT: four-dimensional computed tomography; AV: aortic valve; CEC: clinical events committee; HALT: hypoattenuated leaflet thickening
Table 1. VARC-2 safety up to 1 year.
| Event | 30 days | 1 year |
|---|---|---|
| All-cause mortality | 0.8 (2) | 5.1 (12) |
| Cardiovascular mortality | 0.8 (2) | 3.4 (8) |
| All stroke | 0.8 (2) | 3.0 (7) |
| Disabling stroke | 0 (0) | 1.3 (3) |
| Non-disabling stroke | 0.8 (2) | 1.7 (4) |
| Bleeding | ||
| Life-threatening or disabling | 2.9 (7) | 3.7 (9) |
| Major | 2.4 (6) | 2.8 (7) |
| Myocardial infarction (>72h post-procedure) | 0 (0) | 0.4 (1) |
| Repeat procedure for valve-related dysfunction | 0.4 (1)a | 0.8 (2)b |
| Hospitalisation for valve-related symptoms or worsening congestive heart failure | 0 (0) | 1.7 (4) |
| Prosthetic aortic valve thrombosis | 0.4 (1)c | 0.9 (2)d |
| Prosthetic aortic valve endocarditis | 0.4 (1) | 1.7 (4) |
| Newly implanted permanent pacemaker | ||
| Among all patients | 6.1 (15) | 7.8 (19) |
| Among pacemaker-naïve patients (n=235) | 6.5 (15) | 8.3 (19) |
| Values reported are binary rates at 30 days and Kaplan-Meier time-to-event rate estimates at 1 year, expressed as % (n). a Surgical removal of an embolised ACURATE neo2 valve. b Moderate PVL recorded >30 days post-procedure, corrected by balloon valvuloplasty. c Thrombus noted predischarge in non-study valve implanted subsequent to ACURATE neo2 embolisation. d Diagnosis of HALT at 30 days and 1 year, with oral anticoagulant therapy initiated shortly before 1-year visit. HALT: hypoattenuated leaflet thickening; PVL: paravalvular leak; VARC: Valve Academic Research Consortium | ||
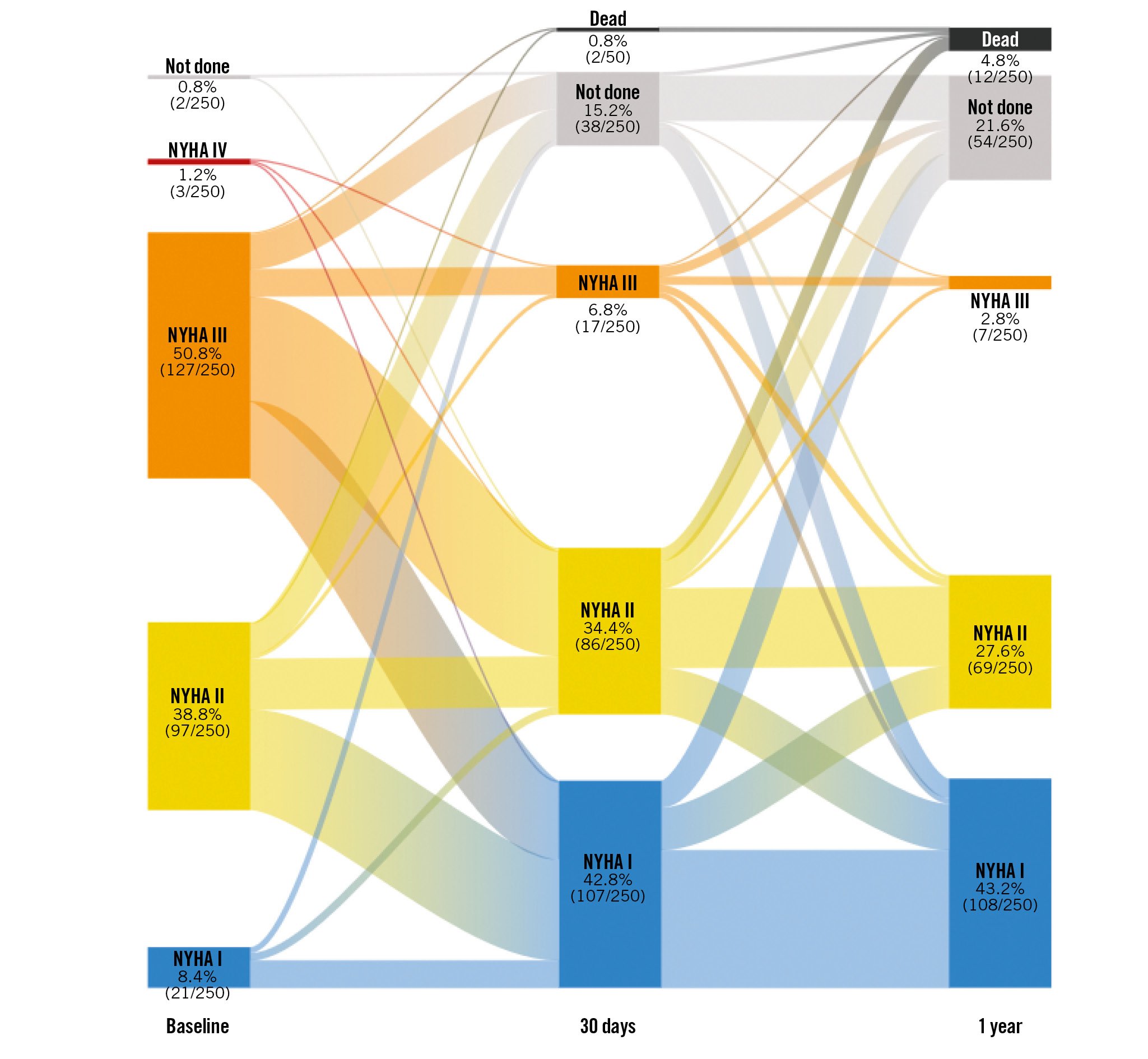
Figure 2. Post-TAVI improvement in functional status. Patients exhibited progressive improvement in NYHA Functional Class after TAVI with ACURATE neo2. Among patients with evaluable data, improvement from baseline by at least 1 Functional Class was seen in 71% of patients at 30 days and 73% at 1 year. NYHA: New York Heart Association; TAVI: transcatheter aortic valve implantation
ECHOCARDIOGRAPHIC OUTCOMES
Core laboratory-adjudicated echocardiographic analyses indicate that haemodynamic improvements occurred early and were maintained up to 1 year. The mean aortic valve area was 0.7±0.2 cm2 at baseline, 1.6±0.4 cm2 at discharge and 30 days, and 1.7±0.4 cm2 at 1 year. The mean aortic valve gradient was 47.6±14.5 mmHg at baseline, 9.7±5.4 mmHg at discharge, 8.6±3.9 mmHg at 30 days, and 7.6±3.2 mmHg at 1 year (Figure 3A). Paired analyses of patients with data available at discharge, 30 days, and 1 year (n=116) indicate significant improvements in valve area and gradient post-TAVI (Figure 3B, Figure 3C). Patients exhibited substantial improvement in PVL overall, and in the paired population of patients with data available at all 3 post-TAVI time points (Figure 4). At 1 year, 84% of patients had no/trace PVL, 15% had mild PVL, and <1% had moderate PVL. There was no severe PVL detected at any point in the study. In the paired analyses, 13% of patients improved at least one PVL grade between their discharge and 1-year visit.
ACURATE neo2 valve performance was also evaluated in a post hoc analysis of bioprosthetic valve dysfunction, using the definitions outlined in the recent VARC-3 guidelines10. Per this definition, there were no cases of moderate or severe haemodynamic valve deterioration observed in the study up to 1 year.
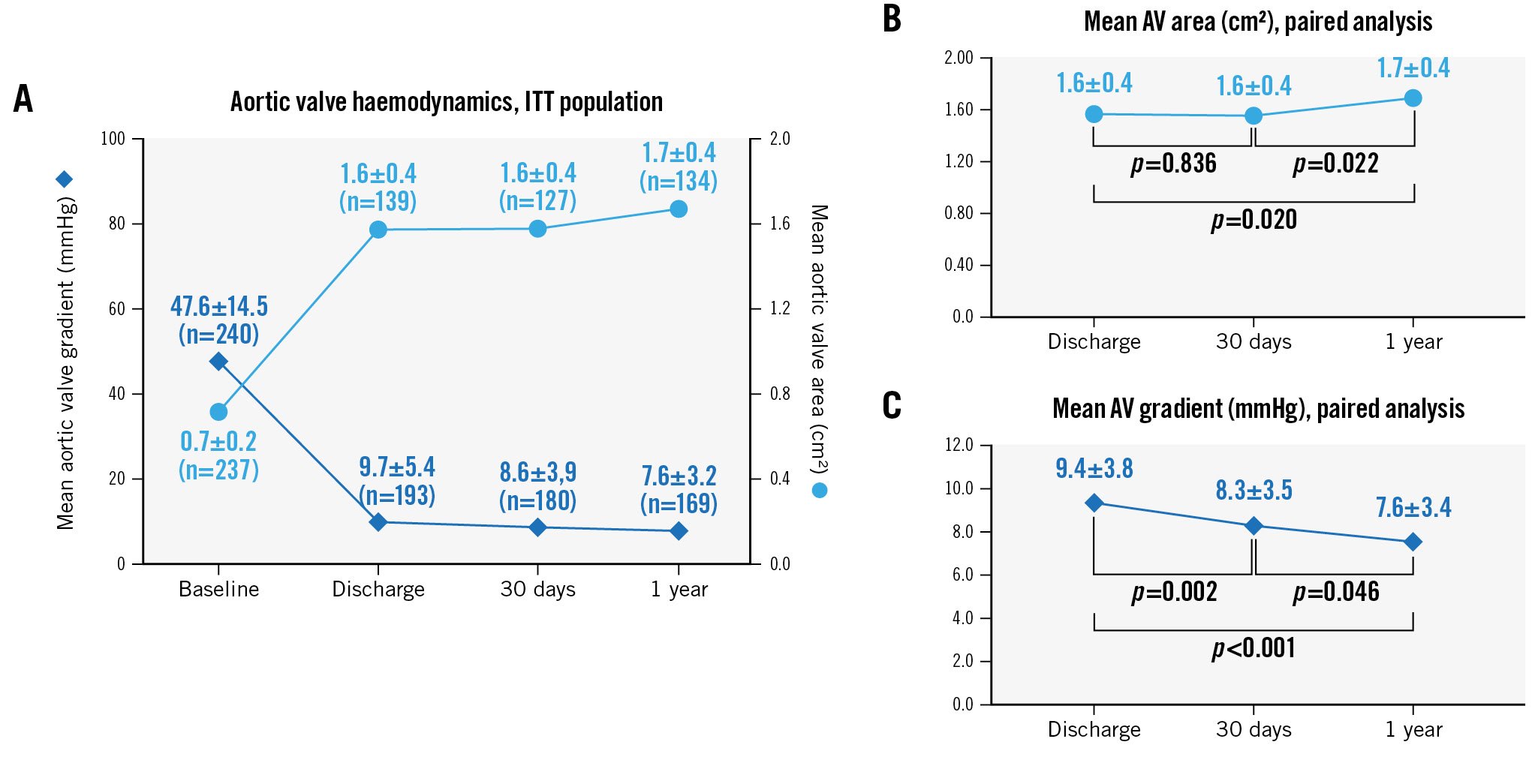
Figure 3. Change in valve haemodynamics. Core laboratory-adjudicated echocardiographic data in the full ITT population (N=250) indicate early haemodynamic improvements are maintained up to 1-year follow-up (A). Paired analyses of patients with data available at discharge, 30 days, and 1 year (n=116) indicate significant improvement post-procedure in the (B) mean aortic valve area (effective orifice area) and (C) mean aortic valve gradient (reported as time-velocity integral [TVI] ratio). P-values are derived from a paired t-test analysis. AV: aortic valve; ITT: intention-to-treat
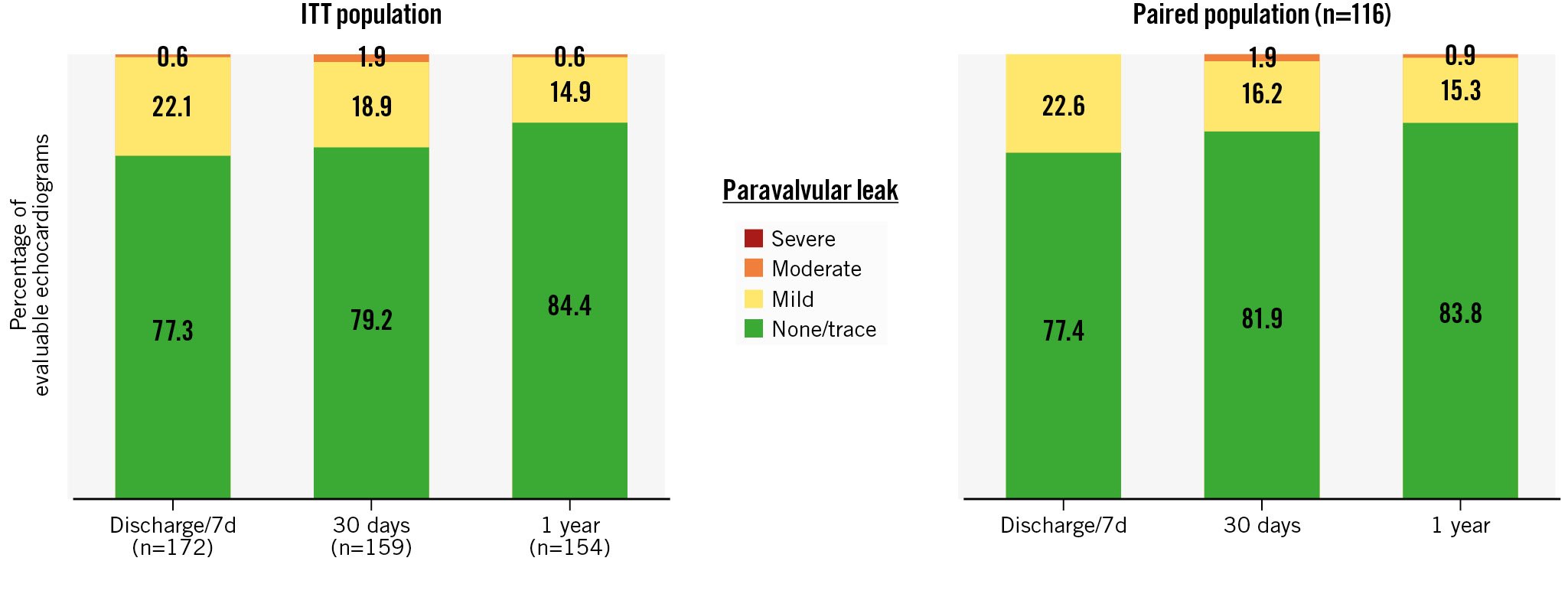
Figure 4. Improvement in paravalvular leak. At 1 year, 99% of patients in the ITT population exhibited mild or less PVL, and <1% had moderate PVL (no patients exhibited greater than moderate PVL at any time post-procedure). A paired analysis of patients who had core laboratory-adjudicated echocardiographic data available at baseline, 30 days, and 1 year (n=116) showed similar outcomes. ITT: intention-to-treat; PVL: paravalvular leak
4D-CT IMAGING OUTCOMES
For the primary imaging endpoint, some degree of HALT was detected at 30 days in 24.5% (50/204) of patients with imaging data available, and 9.3% (19/204) of patients exhibited HALT severity of >50% leaflet involvement of at least one leaflet. At 1 year, 153 patients had evaluable 4D-CT imaging data available (Supplementary Table 3). Some degree of HALT was detected in 49/153 patients (32.0%), and 18 patients (11.8%) exhibited HALT severity of >50%. There was evidence of restricted leaflet mobility in one leaflet in 13.1% of patients, in two leaflets in 10.5% of patients, and in all three leaflets in 7.2% of patients. While anticoagulation was an exclusion criterion, there were patients who were prescribed oral anticoagulant (OAC) therapy per standard of care. There were 11 patients who had a 30-day CT scan while on OAC therapy and 15 patients who had a 1-year CT scan while on OAC therapy; none of these patients had HALT.
To evaluate the change in HALT severity, a paired analysis was performed for patients with HALT data available at 30 days and 1 year (n=135; none of these patients were on OAC therapy). HALT severity spontaneously improved in 12% of these patients, remained unchanged in 67% of patients, and worsened in 21% of patients. There did not appear to be any clinical implications of HALT and restricted leaflet mobility, as there was no association observed between HALT at 30 days or 1 year and 1-year clinical or haemodynamic outcomes (Table 2).
Table 2. Clinical and haemodynamic outcomes at 1 year by HALT status.
| HALT at 30 d or 1 y N=72 | No HALT at 30 d or 1 y N=93 | p-value | |
|---|---|---|---|
| VARC-2 safety event | |||
| All-cause mortality | 1.4 (1) | 0 (0) | 0.25 |
| Cardiovascular mortality | 1.4 (1) | 0 (0) | 0.25 |
| All stroke | 1.4 (1) | 2.2 (2) | 0.74 |
| Disabling stroke | 0 (0) | 0 (0) | -- |
| Non-disabling stroke | 1.4 (1) | 2.2 (2) | 0.74 |
| Repeat procedure for valve-related dysfunction | 0 (0) | 0 (0) | -- |
| Hospitalisation for valve-related symptoms or worsening congestive heart failure | 1.4 (1) | 1.1 (1) | 0.84 |
| Prosthetic aortic valve thrombosis | 1.5 (1) | 0 (0) | 0.25 |
| Prosthetic aortic valve endocarditis | 1.4 (1) | 1.1 (1) | 0.83 |
| Newly implanted permanent pacemaker | 1.6 (1) | 3.2 (3) | 0.46 |
| Echocardiographic measurement | n=27 | n=91 | |
| Mean aortic valve gradient, mmHg | 7.1±4.3 | 7.8±2.7 | 0.36 |
| Mean aortic valve area, cm2 | 1.6±0.4 | 1.7±0.4 | 0.53 |
| Safety event rates are reported as landmark time-to-event rate estimates (7 d to 365 d post-procedure), expressed as % (n). Echocardiographic measures are core laboratory adjudicated, and only subjects with HALT recorded at 30 days and 1 year are included in the analysis; aortic valve area is reported as the time-velocity integral ratio. HALT: hypoattenuated leaflet thickening; VARC: Valve Academic Research Consortium | |||
Discussion
Results from the ACURATE neo2 PMCF Study at 1 year indicate sustained safety with ACURATE neo2. The rates for mortality and disabling stroke observed in the present study (5.1% and 1.3%, respectively) are comparable to or lower than other large “real-world” TAVI registries of second- and third-generation competitor devices. In the S3U registry, the 1-year rates for mortality and disabling stroke were 6.3% and 2.0%, respectively, for patients treated with SAPIEN 3 and 4.9% and 1.0%, respectively, for those treated with SAPIEN 3 Ultra (both Edwards Lifesciences)11. Patients treated with Evolut PRO/PRO+ (Medtronic) in the NEOPRO/NEOPRO-2 registries had a 1-year mortality rate of 10%12; those from the FORWARD PRO registry had a 9.7% mortality rate and 4.6% disabling stroke rate at 1 year13. Patients treated with neo2 had low rates for pacemaker implantation (7.8%, compared with 12.1% for SAPIEN 3 and 8.5% for SAPIEN 3 Ultra in the S3U registry, and 22.7% in FORWARD PRO)1113 and for rehospitalisation (1.7%, compared with 3.0% for SAPIEN 3 and 2.2% for SAPIEN 3 Ultra)11.
A strength of the present study is the longitudinal assessment of valve haemodynamics and PVL, with data adjudicated by an echocardiographic core laboratory. Overall, the magnitude of improvement from baseline in valve area and gradient were in line with expectations and were maintained up to 1-year follow-up. Patients showed significant interindividual improvement in gradient, not only from baseline but between discharge and 30 days, and 30 days and 1 year. The rate of PVL in the present study was very low, as observed in other recent studies of neo2451415, and can be attributed primarily to the device’s augmented inner and outer sealing skirts. In paired analyses, the proportion of patients with mild or moderate PVL declined during 1 year of follow-up (22.6% at discharge, 18.1% at 30 days, 16.2% at 1 year), and the grade of PVL improved between discharge and 1 year in 13% of patients. These results are consistent with other studies of self-expanding valves with longitudinal echocardiographic follow-up: in a paired analysis from the FORWARD PRO Study, there was a decrease in PVL severity over time, with 37.7% of patients exhibiting mild or greater PVL at discharge compared to 30.9% at 1 year13, and in the PORTICO-1 study, 17.9% of patients with mild PVL at 30 days improved to no/trace PVL at 1 year16.
The present study also includes core laboratory evaluation of the incidence of HALT and an assessment of its clinical impact. A recent meta-analysis found a median 6% of patients exhibited evidence of leaflet thrombosis at 30 days post-TAVR17. However, the incidence in individual studies is considerably higher when patients’ use of oral anticoagulant therapy is taken into account. The 30-day and 1-year HALT rates in the present study, which excluded any patients on anticoagulant therapy, were 24.5% and 32.0%, respectively. These rates fall within the range of those observed among patients not treated with anticoagulant therapy in other contemporary studies (11.0% at 30 days and 24.4% at 1 year in PARTNER 318; 15.8% at 30 days in the LRT study19; 17.3% at 30 days and 30.9% at 1 year in the Evolut Low Risk trial20). Studies have shown that HALT is dynamic, and while the use of oral anticoagulants increases the likelihood that leaflet thrombosis will be resolved17, in many cases spontaneous resolution of HALT occurs1820 as was the case for some of the patients in the present study. The reported impact of HALT on valve haemodynamics and clinical outcomes is also variable. Data from PARTNER 3, LRT, and the SAVORY registry suggest there are no significant changes in aortic valve gradient or effective orifice area observed over time in patients with HALT182122. While PARTNER 3 and the SAVORY registry did not find any association between HALT and the individual endpoints of death, myocardial infarction, or stroke up to 1 year1822, the aforementioned meta-analysis identified a 2.6-fold relative risk increase for stroke or transient ischaemic attack in patients with leaflet thrombosis17, and patients with HALT in the LRT study had a numerically higher stroke rate at 1 year, although this was not statistically significant21. The present study found no association between HALT at 30 days or 1 year and 1-year clinical or haemodynamic outcomes; however, the overall stroke rate in the study was low, and the sample size was relatively small.
Limitations
In this single-arm study, clinical follow-up was limited to 1 year. For some clinical and imaging endpoints, the evaluable sample size was reduced because of lack of follow-up or unanalysable data (e.g., poor image quality). These factors constrained the ability to determine the extent of the correlation between HALT and clinical outcomes. Additional follow-up data are needed to evaluate the long-term performance of neo2 and assess the impact of HALT on valve performance and durability over time.
Conclusions
The ACURATE neo2 PMCF Study results confirm that treatment with ACURATE neo2 is safe and efficacious in patients with aortic stenosis undergoing transfemoral TAVI in a routine clinical setting. Good clinical and haemodynamic outcomes were maintained up to 1 year of follow-up, with very low rates of new pacemaker implantation and minimal reintervention for valve-related dysfunction. At 1 year, 12% of patients had 4D-CT evidence of HALT severity >50%; however, there was no observed impact of HALT on 1-year clinical or haemodynamic outcomes.
Impact on daily practice
Data from the ACURATE neo2 Post Market Clinical Follow up Study suggest patients treated with the ACURATE neo2 valve can expect favourable clinical outcomes, including low rates of reintervention and rehospitalisation. Early improvements in valve haemodynamics were maintained up to 1 year, with minimal paravalvular leak (greater than mild in <1% of patients). There was no association observed between HALT at 30 days or 1 year and 1-year clinical or haemodynamic outcomes. Additional data are needed to evaluate the long-term performance of the ACURATE neo2 valve. The ongoing ACURATE IDE study includes a randomised controlled cohort to assess the safety and effectiveness of the neo2 valve versus other contemporary TAVI devices in an all-risk patient population and will follow patients for 10 years.
Acknowledgements
The authors thank Michele Nozza, MSc (Boston Scientific), for study management, Songtao Jiang, MS (Boston Scientific), for providing statistical analysis, and MaryEllen Carlile Klusacek, PhD (Boston Scientific), for their assistance in manuscript preparation.
Funding
The ACURATE neo2 PMCF Study was sponsored and funded by Boston Scientific Corporation (Marlborough, MA, USA). The data and study protocol for this clinical trial may be made available to other researchers in accordance with Boston Scientific’s data sharing policy on the Boston Scientific website.
Conflict of interest statement
W. Kim reports personal fees from Abbott, Boston Scientific, Edwards Lifesciences, Meril Life Sciences, Shockwave Medical; and institutional fees from Boston Scientific. H. Möllmann reports speaker/proctor/advisor honoraria from Abbott, Boston Scientific, and Edwards Lifesciences. T.K. Rudolph has received speaker honoraria from Boston Scientific, Edwards Lifesciences, JenaValve, and Medtronic; and institutional grants from Boston Scientific and Edwards Lifesciences. N.M. Van Mieghem reports consulting fees/honoraria from Daiichi Sankyo, Abiomed, Anteris, Amgen, and Teleflex; research grants from Medtronic, Boston Scientific, ACIST, PulseCath, Edwards Lifesciences, and Abbott. M. Hilker has participated on advisory boards for and received proctor fees from Boston Scientific. I. Amat-Santos is a proctor for Boston Scientific. A.S. Petronio reports consulting and research funds from Medtronic; is a consultant for Abbott; and speaker fees from Edwards Lifesciences, Medtronic, and Abbott. P. Stella reports proctoring for Boston Scientific and Edwards Lifesciences. M. Götberg serves as a proctor for and reports consulting honoraria from Boston Scientific. A. Rück reports institutional research grants from Boston Scientific; and personal lecture fees and consulting fees from Boston Scientific, Edwards Lifesciences, Abbott, and Anteris Medical. R. Trillo serves as a proctor for Medtronic and Boston Scientific. C. Appleby received speaker honoraria from Boston Scientific. M. Barbanti has received speaker honoraria from Edwards Lifesciences, Medtronic, and Boston Scientific. P. Blanke is a consultant to Edwards Lifesciences; and has provided institutional CT Core Laboratory services for Boston Scientific, Medtronic, Edwards Lifesciences and Abbott, for which no direct personal compensation was received. F.M. Asch has no personal conflicts of interest but directs an academic echocardiography core lab (MedStar Health) with institutional contracts with Boston Scientific, Edwards Lifesciences, Abbott, Medtronic, Biotronik, and Foldax. R. Modolo and D.J. Allocco are employees and shareholders of Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

