Abstract
Background: Transcatheter aortic valve replacement (TAVR) with the ACURATE neo device has been associated with a non-negligible incidence of paravalvular aortic regurgitation (AR). The new-generation ACURATE neo2 has been designed to mitigate this limitation.
Aims: The aim of the study was to compare TAVR with the ACURATE neo and neo2 devices.
Methods: The NEOPRO and NEOPRO-2 registries retrospectively included patients undergoing transfemoral TAVR with self-expanding valves at 24 and 20 centres, respectively. Patients receiving the ACURATE neo and neo2 devices (from January 2012 to December 2021) were included in this study. Predischarge and 30-day VARC–3 defined outcomes were evaluated. The primary endpoint was predischarge moderate or severe paravalvular AR. Subgroup analyses per degree of aortic valve calcification were performed.
Results: A total of 2,026 patients (neo: 1,263, neo2: 763) were included. Predischarge moderate or severe paravalvular AR was less frequent for the neo2 group (2% vs 5%; p<0.001), resulting in higher VARC-3 intended valve performance (96% vs 90%; p<0.001). Furthermore, more patients receiving the neo2 had none/trace paravalvular AR (59% vs 38%; p<0.001). The reduction in paravalvular AR with neo2 was mainly observed with heavy aortic valve calcification. New pacemaker implantation and VARC-3 technical and device success rates were similar between the 2 groups; there were more frequent vascular and bleeding complications for the neo device. Similar 1-year survival was detected after TAVR (neo2: 90% vs neo: 87%; p=0.14).
Conclusions: TAVR with the ACURATE neo2 device was associated with a lower prevalence of moderate or severe paravalvular AR and more patients with none/trace paravalvular AR. This difference was particularly evident with heavy aortic valve calcification.
Introduction
Transcatheter aortic valve replacement (TAVR) is an established treatment option for patients with symptomatic severe aortic stenosis12. As TAVR candidates are increasingly younger and at lower surgical risk, it has become crucial to minimise potential procedural complications and to provide surgical-like long-term outcomes. Post-procedural moderate or severe paravalvular aortic regurgitation (AR) is a relevant complication after TAVR that has been found to be associated with adverse short- and long-term outcomes3. In the last few years, the first-generation ACURATE neo (Boston Scientific) transcatheter heart valve (THV) has emerged as a widely adopted self-expanding device for TAVR, associated with good procedural and clinical outcomes456789. However, 2 randomised trials have recently reported a higher rate of moderate or severe paravalvular AR with the ACURATE neo as compared to other new-generation self-expanding and balloon-expandable THVs1011. This complication was more frequent with increased device landing zone calcification5. For this reason, careful patient selection, proper sizing, and appropriate positioning were proposed to optimise procedural outcomes9.
In September 2020, the new-generation ACURATE neo2 THV was commercially released in Europe. This latest iteration of the ACURATE neo platform has been specifically designed to minimise the occurrence of paravalvular AR by utilising a 60% larger sealing skirt. Quantitative aortographic assessments have shown promising results in terms of paravalvular AR reduction with the ACURATE neo2 device12. However, no large, real-world data have compared the performance of ACURATE neo and neo2. With this background, our study aimed to investigate the haemodynamic performance and clinical outcomes after transfemoral TAVR with the ACURATE neo2 compared to the first-generation ACURATE neo THV.
Methods
Study population
The observational, retrospective NEOPRO (A Multicenter Comparison of Acurate NEO Versus Evolut PRO Transcatheter Heart Valves) registry included a total of 1,551 patients who underwent transfemoral TAVR with either ACURATE neo (n=1,263) or Evolut PRO (n=288; Medtronic) devices between January 2012 and March 2018 at 24 centres4. The NEOPRO-2 registry was designed to expand the previous registry to include procedures performed with the new-generation ACURATE neo2 (n=763) and Evolut PRO or PRO+ (n=1,412) devices up to December 2021 at 20 centres. All consecutive patients treated with transfemoral TAVR for symptomatic, severe aortic stenosis of the native aortic valve (AV) with the implantation of the aforementioned self-expanding devices were included in the registries. For the purposes of the present study, only patients treated with ACURATE neo or neo2 THVs were analysed. The number of patients included from each participating centre is detailed in Supplementary Table 1. The treatment period was from January 2012 to March 2018 for the neo THV and from September 2020 to December 2021 for the neo2 device. Data obtained from 29 participating centres were included in the present analysis: 18 centres implanting the ACURATE neo (NEOPRO) and 16 centres using the neo2 device (NEOPRO-2). Local multidisciplinary Heart Teams evaluated each case and confirmed eligibility for transfemoral TAVR. All patients provided written informed consent for the procedure and subsequent data collection per local practice for retrospective data. The study complied with the Declaration of Helsinki and was approved by local ethics committees. Preprocedural screening was performed by means of clinical assessment (patient demographics, symptoms, comorbidities, laboratory examinations, and risk evaluation), echocardiography and multidetector computed tomography.
Device description
The ACURATE neo2 bioprosthesis is a self-expanding THV with a supra-annular leaflet design. Three sizes are currently available (small, medium, and large) and correspond to annular diameters of 21-23, 23-25, and 25-27 mm, respectively. The neo2 THV is implanted using a dedicated transfemoral delivery system inserted through a 14 Fr expandable sheath (iSleeve [Boston Scientific]). The deployment is performed in a top-down sequence, starting with the release of the stabilisation arches, and does not require rapid pacing. The self-expanding nitinol frame is wrapped with a pericardial sealing skirt on the outer and inner surface of the stent body that extends 60% higher from the inflow part of the stent frame as compared to the first-generation ACURATE neo. The skirt’s extended dimensions have the potential of providing a more synchronous adaptation to the native aortic annulus during the different phases of the cardiac cycle, especially in irregular and calcified anatomies. Furthermore, a radiopaque marker has been added to the delivery system to navigate accurate positioning of the THV at the aortic annulus.
Study endpoints and definitions
The primary study endpoint was the occurrence of moderate or severe paravalvular AR at predischarge transthoracic echocardiography. Secondary endpoints were Valve Academic Research Consortium (VARC)-3 defined clinical outcomes at 30 days13, including the need for permanent pacemaker implantation (PPI), and 1-year overall survival.
Paravalvular AR severity was assessed with Doppler echocardiography according to VARC-3 criteria and classified as follows: none or trace, mild, moderate, and severe13. Native aortic valve and left ventricular outflow tract calcifications from multidetector computed tomography scans were classified and graded using a semiquantitative scoring system, as previously described614.
Statistical analysis
Continuous variables are reported as mean±standard deviation or median (interquartile range [IQR]) and compared with the Student’s unpaired t-test (parametric test) or the Wilcoxon rank-sum test (non-parametric test), according to their distribution. Categorical variables were reported as absolute and relative frequencies and compared with the χ2 test with Yates’ correction for continuity or Fisher’s exact test, as appropriate. Survival curves with their 95% confidence interval (CI) were plotted using the Kaplan-Meier estimator and compared with the log-rank test. A subgroup analysis testing the primary and secondary endpoints across different degrees of aortic valve calcification was also performed. For all analyses, a two-sided p-value <0.05 was considered to be significant. Statistical analyses were performed using R, version 4.0.2 (R Foundation).
Results
Baseline patient characteristics
A total of 2,026 patients in the NEOPRO and NEOPRO-2 registries underwent transfemoral TAVR with the self-expanding ACURATE neo or neo2 THVs and were included in this study. Of these, 1,263 patients received the first-generation ACURATE neo, whereas 763 were treated using the new ACURATE neo2 device. Baseline characteristics of the study population are reported in Table 1. The mean age was 82.0±5.8 years, and 66% of patients were women. Patients treated with the ACURATE neo were more frequently in New York Heart Association (NYHA) Functional Class III or IV (78% vs 55%; p<0.001), and were deemed at higher surgical risk (STS score: 4.1 [IQR 2.9-6.1] vs 3.5 [IQR 2.5-5.0], EuroSCORE II: 4.4 [IQR 2.7-7.2] vs 3.1 [IQR 2.1-5.1]; p<0.001). Patients receiving the ACURATE neo2 were characterised by smaller anatomies of the aortic valve (area and perimeter) and of the TAVR femoral access (minimal diameter: 7.14±1.13 vs 7.95±1.37; p<0.001). The severity of aortic valve calcification was higher in the neo group, whereas mild and moderate left ventricular outflow tract calcifications were more frequent in neo2 patients.
Table 1. Baseline characteristics.
|
Total |
ACURATE neo (1,263) |
ACURATE neo2 (763) |
p-value |
||
|---|---|---|---|---|---|
|
Clinical characteristics |
|||||
|
Age, years |
82±5.8 |
82±5.8 |
82±5.9 |
0.822 |
|
|
Male |
694 (34) |
444 (35) |
250 (33) |
0.303 |
|
|
BMI |
27±5 |
27±5 |
27±5 |
0.281 |
|
|
BSA |
1.82±0.21 |
1.82±0.21 |
1.82±0.22 |
0.314 |
|
|
Hypertension |
1,726 (87) |
1,079 (88) |
647 (85) |
0.055 |
|
|
Diabetes mellitus |
598 (30) |
379 (30) |
219 (29) |
0.571 |
|
|
Atrial fibrillation |
648 (32) |
408 (33) |
240 (32) |
0.772 |
|
|
Previous stroke |
209 (10) |
126 (10) |
83 (11) |
0.719 |
|
|
Peripheral vascular disease |
271 (13) |
156 (12) |
115 (15) |
0.093 |
|
|
Previous myocardial infarction |
220 (11) |
138 (12) |
82 (11) |
0.689 |
|
|
Previous PCI |
582 (29) |
370 (29) |
212 (28) |
0.496 |
|
|
Previous CABG |
194 (10) |
147 (12) |
47 (6) |
<0.001 |
|
|
COPD |
369 (18) |
244 (19) |
125 (16) |
0.114 |
|
|
eGFR, ml/min/1.73m2 |
60±25 |
58±22 |
64±29 |
<0.001 |
|
|
Prior PM/ICD |
219 (11) |
158 (12) |
61 (8) |
0.002 |
|
|
NYHA Class III/IV |
1,397 (69) |
981 (78) |
416 (55) |
<0.001 |
|
|
EuroSCORE II |
3.9 [2.5-6.6] |
4.4 [2.7-7.2] |
3.1 [2.1-5.1] |
<0.001 |
|
|
STS score (mortality) |
4.0 [2.8-5.8] |
4.1 [2.9-6.1] |
3.5 [2.5-5.0] |
<0.001 |
|
|
Echocardiographic data |
|||||
|
Mean aortic gradient, mmHg |
44±16 |
43±17 |
45±14 |
0.057 |
|
|
AVA, cm2 |
0.71±0.22 |
0.71±0.19 |
0.71±0.26 |
0.523 |
|
|
Indexed AVA, cm2/m2 |
0.39±0.10 |
0.39±0.11 |
0.39±0.09 |
0.891 |
|
|
LVEF, % |
57±11 |
57±12 |
58±10 |
0.032 |
|
|
CT analysis |
|||||
|
Aortic valve area, mm2 |
429±64 |
432±67 |
424±59 |
0.015 |
|
|
Aortic valve perimeter, mm |
74±6 |
75±6 |
74±5 |
<0.001 |
|
|
Aortic valve calcification |
None or mild |
470 (29) |
286 (29) |
184 (29) |
0.02 |
|
Moderate |
739 (46) |
428 (44) |
311 (50) |
||
|
Heavy |
396 (25) |
263 (27) |
133 (21) |
||
|
LVOT calcification |
None |
693 (53) |
541 (56) |
152 (46) |
0.01 |
|
Mild |
379 (29) |
265 (27) |
114 (35) |
||
|
Moderate |
152 (12) |
107 (11) |
45 (14) |
||
|
Severe |
78 (6) |
62 (6) |
16 (5) |
||
|
Femoral access*, mm |
7.78±1.36 |
7.95±1.37 |
7.14±1.13 |
<0.001 |
|
|
*Minimal lumen diameter of the femoral artery used to deliver the valve. Values are n (%), mean±standard deviation, or median [interquartile range]. AVA: aortic valve area; BMI: body mass index; BSA: body surface area; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CT: computed tomography; eGFR: estimated glomerular filtration rate; ICD: implantable cardioverter defibrillator; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; PM: pacemaker; STS: Society of Thoracic Surgeons |
|||||
Procedural results
Procedural characteristics are depicted in Table 2. Valve sizes were equally distributed within the 2 groups. Implantation of the neo THV was more frequently performed under general anaesthesia (13% vs 1%; p<0.001) and completed with final post-dilatation (42% vs 31%; p<0.001). The prevalence of procedural complications (namely: death, valve embolisation, the need for a second THV, annular rupture, pericardial tamponade, aortic dissection, coronary occlusion, and conversion to open-heart surgery) was low with no differences between the neo and neo2 groups.
Table 2. Procedural characteristics.
|
Total |
ACURATE neo (1,263) |
ACURATE neo2 (763) |
p-value |
||
|---|---|---|---|---|---|
|
Valve size, mm |
23 |
533 (26) |
348 (28) |
185 (24) |
0.255 |
|
25 |
847 (42) |
520 (41) |
327 (43) |
||
|
27 |
645 (32) |
394 (31) |
251 (33) |
||
|
General anaesthesia |
175 (9) |
168 (13) |
7 (1) |
<0.001 |
|
|
Predilatation |
1,706 (84) |
1,051 (83) |
655 (86) |
0.141 |
|
|
Post-dilatation |
759 (38) |
526 (42) |
233 (31) |
<0.001 |
|
|
Death |
2 (0.3) |
0 (0.0) |
2 (0.3) |
1.000 |
|
|
Valve embolisation |
21 (1) |
13 (1) |
8 (1) |
1.000 |
|
|
Second THV implanted |
20 (1) |
14 (1) |
6 (1) |
0.634 |
|
|
Annular rupture |
5 (0.2) |
4 (0.3) |
1 (0.1) |
0.656 |
|
|
Pericardial tamponade |
27 (1) |
20 (2) |
7 (1) |
0.286 |
|
|
Aortic dissection |
1 (0.0) |
1 (0.1) |
0 (0.0) |
1.000 |
|
|
Coronary occlusion |
4 (0.2) |
2 (0.2) |
2 (0.3) |
1.000 |
|
|
Conversion to open-heart surgery |
16 (1.0) |
13 (1.0) |
3 (0.4) |
0.191 |
|
|
Values are expressed as n (%). THV: transcatheter heart valve |
|||||
Clinical outcomes
Clinical outcomes assessed at 30 days post-TAVR are shown in Table 3 and the Central illustration. VARC-3 defined technical (neo: 91% vs neo2: 93%; p=0.117) and device success (neo: 81% vs neo2: 84%; p=0.119) were similar in the 2 groups. The VARC-3 defined intended performance of the valve was more frequently met in the neo2 group (96% vs 90%; p<0.001). This result was mainly driven by a lower rate of moderate or severe paravalvular AR in the neo2 group (2% vs 5%; p<0.001). The prevalence of none/trace paravalvular AR was significantly higher after TAVR with the neo2 THV (59% vs 38%; p<0.001). Slightly increased mean aortic gradients were found in the neo2 group (neo: 8.0±3.3 mmHg vs neo2: 8.9±4.1 mmHg; p<0.001), and no differences were found in terms of aortic valve area. The need for a new PPI was similar in the 2 groups (neo: 9% vs neo2: 8%; p=0.46). Patients receiving the neo2 THV experienced fewer vascular (p<0.001) and bleeding (p=0.020) complications.
Table 3. 30-day outcomes.
|
Total |
ACURATE neo (1,263) |
ACURATE neo2 (763) |
p-value |
||
|---|---|---|---|---|---|
|
All-cause death |
61 (3) |
39 (3) |
22 (3) |
0.903 |
|
|
VARC 3 – technical success |
1,859 (92) |
1,149 (91) |
710 (93) |
0.117 |
|
|
VARC 3 – device success |
1,630 (82) |
1,024 (81) |
606 (84) |
0.119 |
|
|
VARC 3 – intended performance |
1,286 (93) |
572 (90) |
714 (96) |
<0.001 |
|
|
PM implantation |
147 (8) |
96 (9) |
51 (8) |
0.460 |
|
|
Acute kidney injury (stage 2-3) |
58 (3) |
37 (3) |
21 (3) |
0.953 |
|
|
Vascular complications |
None |
1,700 (87) |
1,032 (83) |
668 (94) |
<0.001 |
|
Minor |
156 (8) |
138 (11) |
18 (2) |
||
|
Major |
98 (5) |
75 (6) |
23 (3) |
||
|
Bleeding complications |
None |
1,638 (86) |
1,011 (85) |
627 (88) |
0.020 |
|
Type 1 |
104 (6) |
65 (6) |
39 (6) |
||
|
Type 2 |
79 (4) |
56 (5) |
23 (3) |
||
|
Type 3 |
74 (4) |
56 (5) |
18 (2) |
||
|
Type 4 |
2 (0.1) |
0 (0.0) |
2 (0.3) |
||
|
Mean aortic gradient, mmHg |
8.5±3.8 |
8±3.3 |
8.9±4.1 |
<0.001 |
|
|
AVA, cm2 |
1.8±0.4 |
1.8±0.4 |
1.8±0.4 |
0.826 |
|
|
Indexed AVA, cm2/m2 |
0.96±0.20 |
0.96±0.20 |
0.96±0.20 |
0.769 |
|
|
Moderate or severe paravalvular AR* |
75 (4) |
62 (5) |
13 (2) |
<0.001 |
|
|
*Predischarge assessment. Values are n (%) or mean±standard deviation. AR: aortic regurgitation; AVA: aortic valve area; PM: pacemaker; VARC: Valve Academy Research Consortium |
|||||
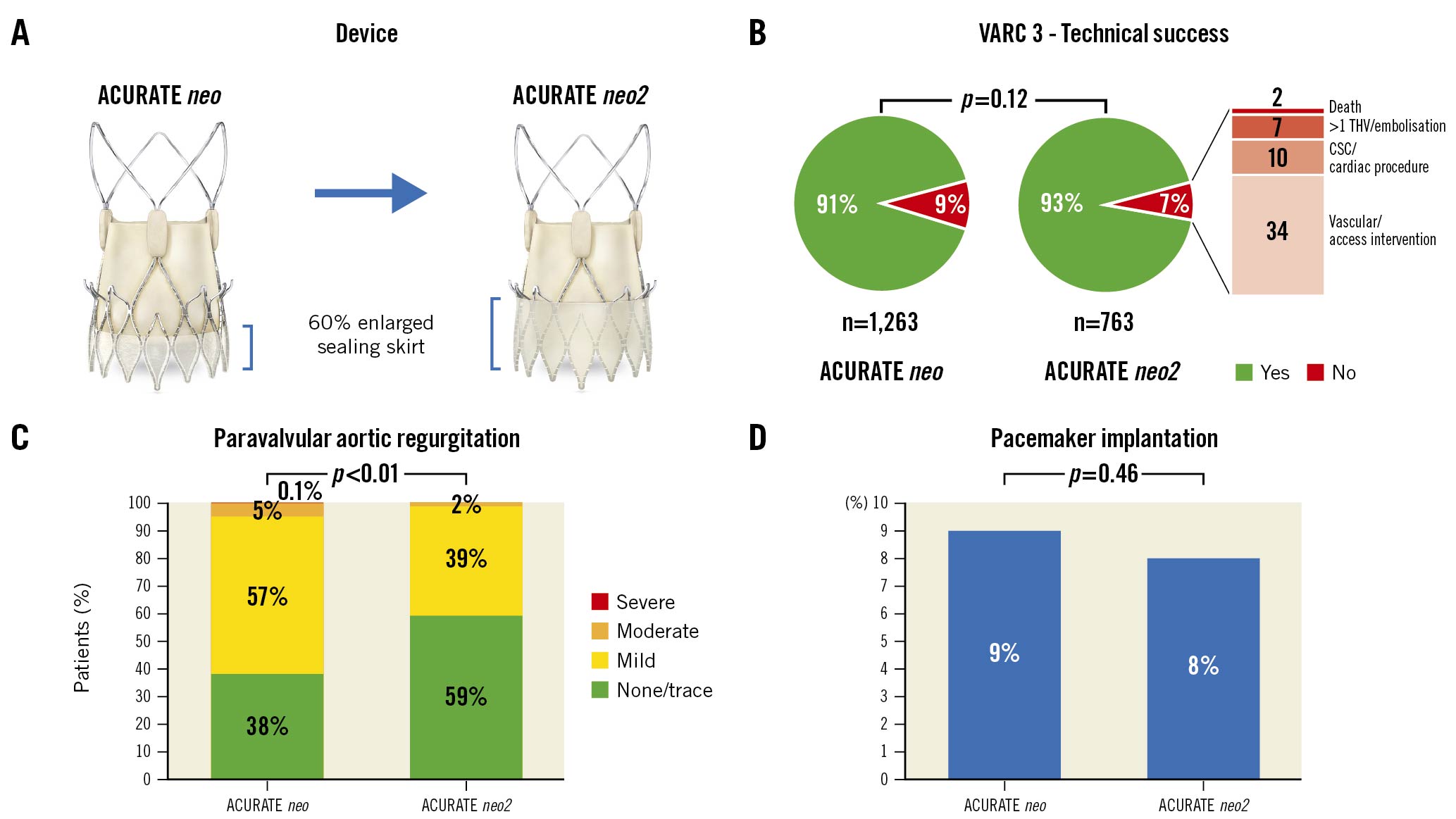
Central Illustration. Comparison of the ACURATE neo and the ACURATE neo2 THVs. A) Device characteristics, B) VARC-3 technical success, C) predischarge paravalvular aortic regurgitation, and D) the need for permanent pacemaker implantation after transcatheter aortic valve replacement. Device illustrations reproduced with permission from Boston Scientific. CSC: cardiac structural complication; THV: transcatheter heart valve; VARC: Valve Academic Research Consortium
Patients were followed up for a median time of 83 [IQR 30-261] days. As shown in the Kaplan-Meier analysis (Figure 1), all-cause mortality at 1-year follow-up was not significantly different between the neo2 and neo groups (90%, 95% CI: 83-98 vs 87%, 95% CI: 84-90; p=0.14).
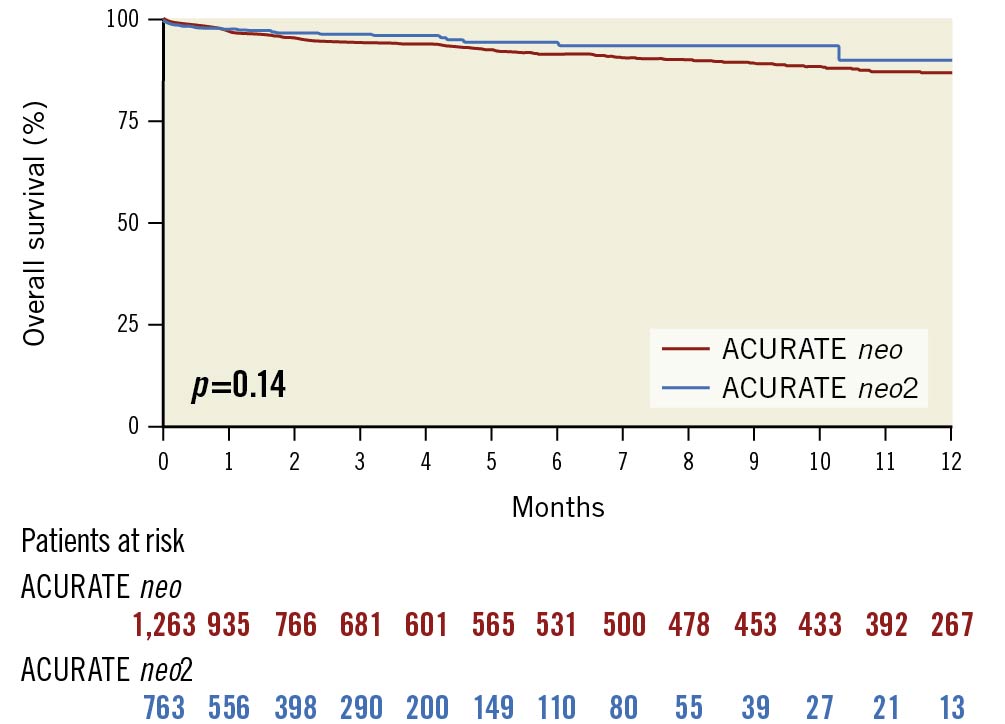
Figure 1. Kaplan-Meier analysis of overall survival in ACURATE neo vs ACURATE neo2 transcatheter aortic valves.
Subgroup analysis on aortic valve calcification severity
As shown in Table 4, clinical outcomes were further analysed stratifying the overall population for baseline aortic valve calcification grades (none or mild, moderate, and heavy). The significant reduction of moderate or severe paravalvular AR in the neo2 group was observed in the subgroup of patients with heavy aortic valve calcification (2% vs 9%; p=0.018); consequently, VARC-3 intended performance of the valve was more frequently met among patients receiving neo2 THV in the heavy aortic valve calcification subgroup (97% vs 88%; p=0.005). No significant differences were observed for these 2 endpoints between the neo and neo2 groups in the other calcification subgroups (Table 4).
Table 4. 30-day outcomes stratified per aortic valve calcification grade.
|
None or mild calcification |
Moderate calcification |
Heavy calcification |
p-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
ACURATE neo |
ACURATE neo2 (184) |
p-value |
ACURATE neo |
ACURATE neo2 (311) |
p-value |
ACURATE neo |
ACURATE neo2 (133) |
p-value |
||
|
All-cause death |
9 (3) |
6 (3) |
1.000 |
11 (3) |
9 (3) |
0.962 |
9 (4) |
0 (0) |
0.070 |
0.418 |
|
VARC 3 - technical success |
250 (87) |
167 (91) |
0.332 |
390 (91) |
287 (92) |
0.669 |
240 (91) |
128 (96) |
0.105 |
0.404 |
|
VARC 3 - device success |
229 (80) |
150 (84) |
0.311 |
346 (81) |
244 (83) |
0.524 |
203 (78) |
100 (85) |
0.172 |
0.691 |
|
VARC 3 - intended performance |
262 (97) |
178 (97) |
0.937 |
377 (93) |
293 (95) |
0.298 |
214 (88) |
127 (97) |
0.005 |
0.899 |
|
PM implantation |
21 (9) |
11 (7) |
0.605 |
40 (10) |
23 (9) |
0.535 |
17 (8) |
6 (6) |
0.705 |
0.982 |
|
Mean aortic gradient, mmHg |
7.6±3.4 |
8.5±4.2 |
0.023 |
8.0±3.2 |
9.0±4.1 |
0.001 |
8.2±3.6 |
8.9±3.9 |
0.023 |
0.141 |
|
AVA, cm2 |
1.7±0.4 |
1.7±0.4 |
0.867 |
1.7±0.4 |
1.7±0.4 |
0.731 |
1.8±0.4 |
1.9±0.4 |
0.867 |
0.135 |
|
Indexed AVA, cm2/m2 |
0.96±0.20 |
0.96±0.20 |
0.979 |
0.93±0.20 |
0.94±0.20 |
0.589 |
0.96±0.20 |
1.0±0.2 |
0.979 |
0.208 |
|
Moderate or severe paravalvular AR* |
6 (2) |
1 (0.5) |
0.317 |
21 (5) |
7 (2) |
0.077 |
23 (9) |
3 (2) |
0.018 |
0.671 |
|
*Predischarge assessment. Values are n (%) or mean±standard deviation. AR: aortic regurgitation; AVA: aortic valve area; PM: pacemaker; VARC: Valve Academic Research Consortium |
||||||||||
All the remaining clinical outcomes were consistent with the primary analysis with no significant differences between both groups across all aortic valve calcification subgroups. In detail, the rates of new PPI were similar between neo and neo2 THVs in each subgroup (none/mild: 9% vs 7%, moderate: 10% vs 9%, heavy: 8% vs 6%; all p-values>0.05, p for interaction=0.982).
Discussion
The main findings of our multicentre, observational, real-world comparison between ACURATE neo and neo2 devices in a total of 2,026 patients undergoing transfemoral TAVR (from the NEOPRO/NEOPRO-2 registries) are as follows: 1) the latest-generation ACURATE neo2 THV was associated with a significant reduction in post-procedural moderate or severe paravalvular AR as compared to the first-generation neo THV; 2) a similar need for new PPI was observed between neo2 and neo devices; 3) the superior performance of the ACURATE neo2 THV was particularly evident in severely calcified aortic valve anatomies; 4) TAVR with the ACURATE neo2 in combination with the expandable iSleeve is associated with reduced rates of vascular complications.
In our study, procedural outcomes after ACURATE neo2 THV implantation suggest acceptable safety and efficacy with rates of 3% for 30-day mortality, 93% for VARC-3 technical success, 84% for VARC-3 device success, and 96% for VARC-3 intended performance of the valve. These results compare favourably with available evidence on the first-generation neo device reporting an equal 30-day mortality rate (3%) and similar rates of procedural complications51011. Interestingly, we observed fewer vascular and bleeding complications in the neo2 group as compared to the neo group. It must be acknowledged that all the procedures were transfemoral with smaller accesses for neo2 recipients (minimal lumen diameter 7.14±1.13 vs 7.95±1.37; p<0.001), similar baseline patient characteristics (age: 82±6 years, atrial fibrillation: 33% vs 32%, peripheral vascular disease: 12% vs 15%), and lower surgical risk scores for the neo2 group, in accordance with TAVR indication expanding to lower-risk patients over time. Even if speculative, the fewer bleeding and vascular complications observed with the implantation of the neo2 THV may be explained by a combination of the redesigned expandable introducer with a low-profile (iSleeve; Boston Scientific), increased operator experience with the ACURATE system, and improved vascular access/complication management.
The only VARC-3 defined outcome that was significantly different between the neo and neo2 devices was the intended performance of the valve (neo2: 96% vs neo: 90%; p<0.001). This composite endpoint is achieved with a mean transvalvular gradient <20 mmHg, peak velocity <3 m/s, Doppler velocity index ≥0.25, and less than moderate AR13. The improved performance of the neo2 THV was driven by a reduction in moderate or severe paravalvular AR compared to the first-generation neo THV. In the overall population, the 5% rate of moderate or severe paravalvular AR that occurred after the ACURATE neo implantation was significantly higher than the 2% rate observed with the neo2 THV (p<0.001). The rate of paravalvular AR in our neo group (5%) is lower than those reported by the randomised SCOPE 1 (9.4%) and SCOPE 2 (9.6%) trials1011. This finding may be explained by differences in outcome adjudication (core lab vs centre-reported) and baseline patient characteristics. Although these trials excluded severe eccentric aortic valve calcifications, they did not report the degrees of overall calcifications which have been demonstrated by Kim et al as having a significant impact on moderate or severe paravalvular AR rates (mild: 0.8%, moderate: 5%, severe: 13%)5. Accordingly, our subgroup analysis reported a similarly increasing trend for paravalvular AR in the neo group, starting from 2% for none/mild aortic valve calcifications, up to 5% for moderate and 9% for heavy calcifications. On the contrary, the rate of moderate or severe paravalvular AR after TAVR with the neo2 THV was consistent among these 3 calcification subgroups (none/mild: 0.5%, moderate: 2%, heavy: 2%). Similar rates of moderate or severe paravalvular AR (1.7-2.5%) were reported in other exploratory analyses evaluating TAVR with the ACURATE neo2 device1215. Furthermore, 59% of patients treated with the new-generation neo2 device showed none/trace paravalvular AR after TAVR, which is significantly higher than the 38% obtained with the neo THV (p<0.001). This finding also compares favourably with the frequency of none/trace paravalvular AR observed after neo implantation in the SCOPE 1 (40%) and SCOPE 2 (27%) trials1011. These results are promising as they demonstrate how the engineering refinements translate into better performance of the ACURATE neo2 THV. Moreover, they indicate that the caveat of avoiding patients with severe aortic calcifications may no longer be appropriate for the ACURATE neo2 system9. Pending further supporting evidence, it seems that this new-generation device can provide favourable performance with a low rate of significant paravalvular AR, even in the more challenging calcific anatomies. The observed gradient with neo2 in our population (8.9±4.1 mmHg) is similar to the one reported in patients with small annuli included in the TAVI-SMALL registry and receiving the neo device (9.6±0.3 mmHg)16. An inverse correlation between annular dimensions and post-procedural gradients has been previously demonstrated with better haemodynamic performance of self-expanding supra-annular THVs in this anatomical setting1718.
Another relevant clinical outcome after TAVR is represented by the need for new PPI. Previous studies investigating the ACURATE neo system reported a 10-11% rate of PPI51011,which was found to be independent of device landing zone calcification5. Our analysis confirms these findings for the neo2 THV, showing a rate of new PPI equal to 8% with no significant differences across the aortic valve calcification subgroups. These results are even more meaningful when compared with the 17-18% of PPI after TAVR with the self-expanding CoreValve Evolut platform (Medtronic)1019. A stable and precise (less protrusion in the left ventricular outflow tract) valve implantation with top-down deployment and radiopaque positioning markers, a moderate device radial force, and the temporal shift from the left anterior oblique to cusp overlap view for the THV implantation are the technical factors explaining this relatively low PPI rate. Keeping this figure as low as possible has a great clinical value and may be used to guide device selection in patients at high risk of permanent conduction disturbances after TAVR20.
At 1-year follow-up, we did not observe a significant difference in all-cause mortality between the neo2 and neo groups (10% vs 13%; p=0.14). The 1-year mortality rate observed in this real-world experience with ACURATE neo is in line with rates recently reported by the randomised SCOPE 1 (11%) and SCOPE 2 (13%) trials1011. Given the prognostic impact of moderate or severe paravalvular AR3 and its significant reduction using the ACURATE neo2, further analyses with larger sample sizes and longer follow-up are eagerly awaited to better explore long-term outcomes after TAVR with this new self-expanding platform.
Limitations
The main limitation of this study is related to its retrospective observational design, with no core laboratory analysis of procedural results and echocardiographic readings or independent adjudication of clinical events. The investigated THVs were implanted in consecutive time periods, and unmeasured confounding factors (e.g., operator experience, valve preference) may have affected the presented results. The use of multiple different sheaths with the ACURATE neo may be a major contributor to the differences in peripheral vascular characteristics compared to the neo2, which was implanted through a redesigned expandable introducer with a low-profile (iSleeve; Boston Scientific). Given the recent release of the ACURATE neo2 device (September 2020), available follow-up time is currently limited, and future studies will be needed to assess if lower paravalvular AR is sustained over time and how it impacts on long-term clinical outcomes. Whilst waiting for the results of ongoing registries (Early neo2 Registry of the Acurate neo2 TAVI Prosthesis [ClinicalTrials.gov: NCT04810195]) and randomised controlled trials (ACURATE IDE: Safety and Efficacy Study of Acurate Valve for Transcatheter Aortic Valve Replacement [ClinicalTrials.gov: NCT03735667]), these exploratory analyses can provide immediate assistance in THV selection for TAVR.
Conclusions
The latest-generation ACURATE neo2 THV is associated with a lower rate of moderate or severe paravalvular AR, when compared to the first-generation ACURATE neo, in patients undergoing transfemoral TAVR. As a result, a greater percentage of patients receiving the neo2 THV have none/trace paravalvular AR. The superior performance of the neo2 device is particularly evident among patients with heavy aortic valve calcification.
Impact on daily practice
TAVR with the ACURATE neo THV was associated with a non-negligible rate of moderate or severe paravalvular AR, which is known to have an adverse prognostic impact. TAVR with the ACURATE neo2 device is associated with a lower rate of moderate or severe paravalvular AR when compared with the first-generation ACURATE neo. The superior performance of the neo2 device is particularly evident among patients with heavy aortic valve calcification. Further studies with larger sample sizes and longer follow-up are needed to confirm these preliminary findings.
Guest editor
This paper was guest edited by Franz-Josef Neumann, MD, Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Conflict of interest statement
A. Latib has served on advisory boards or as a consultant for Medtronic, Boston Scientific, Philips, Edwards Lifesciences, and Abbott. W-K. Kim is a proctor for Boston Scientific and Abbott Vascular; and has received speaker fees from Edwards Lifesciences. U. Schäfer is proctor for Boston Scientific and Medtronic and has received lecture fees and travel support from both companies. M. Barbanti has served as a consultant for Edwards Lifesciences. D. Hildick-Smith is an advisor and a proctor for Boston Scientific, Symetis, and Medtronic. A. Wolf is a proctor for Medtronic and Boston Scientific. N. Van Mieghem has received research grant support from Boston Scientific, Abbott Vascular, Medtronic, Claret, and Essential Medical. S. Toggweiler is a consultant and proctor for Boston Scientific, Medtronic, Abbott, and Biosensors; is a consultant for Medira, Atheart Medical, Veosource, Shockwave, and Teleflex; has received institutional research grants from Boston Scientific and Fumedica AG; and holds equity in Hi-D Imaging. D. Mylotte is a proctor for Medtronic and Microport. V. Veulemans has received lecture fees and travel support from Medtronic and Edwards Lifesciences. S. Windecker has received grants from Abbott Vascular, Biotronik, Boston Scientific, Edwards Lifesciences, and Medtronic. D. Siqueira is proctor for Medtronic, Symetis, and Edwards Lifesciences. M. Adamo reports speaker honoraria from Abbott Vascular and Medtronic. J-M. Sinning is a proctor for Medtronic and Boston Scientific; and has received speaking honoraria and research grants from Medtronic, Edwards Lifesciences, and Boston Scientific. D.A. Wood has received grant support from Boston Scientific; and is a consultant for Medtronic. L. Sondergaard has received consultant fees and institutional research grants from Boston Scientific. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.

