These iconic movie characters share one common trait with the ACURATE neo2 (Neo2): resilience − the ability to become stronger, better, and more successful − even after a setback.
In the randomised SCOPE I trial1, the self-expanding (SE) ACURATE neo (Neo; Boston Scientific) failed to meet non-inferiority against the balloon-expandable (BE) SAPIEN 3 (S3; Edwards Lifesciences) due to significant paravalvular leak (PVL). In SCOPE II2, the Neo failed against the CoreValve Evolut (Medtronic), with significantly higher rates of PVL and cardiac death at 30 days2. The causes of failure were targeted by adding 2 main features to the Neo: a newly designed and 60% larger external “active” sealing skirt and a radiopaque positioning marker to optimise deployment. These modifications have done the trick, and the device is again in a position to challenge the best in its class: the Evolut PRO/PRO+ (Pro)3 and S3/S3 Ultra4.
These significant improvements in the Neo2 were heralded in a report showing rates of moderate/severe PVL of 1.7%, 5.3% and 11.3% with the Neo2 (n=120), Pro (n=95) and Neo (n=115), respectively5 (Figure 1).
One of the inherent superiorities of the Neo family is the consistently lower rate of permanent pacemaker implantation (PPMI)2467, driven by a combination of their low radial force and more controlled implantation depth. The former, however, has led to some operators being reluctant to implant the Neo2 in heavily calcified aortic valves (AV), especially those with large eccentric calcific nodules at the annular and subannular levels, as well as in patients with a bicuspid AV (BAV) anatomy. This last point is exemplified by the rate of predilatation, which is practically advocated like a formal recommendation by the manufacturer.
The growing prevalence of transcatheter AV implantation (TAVI) in younger low-risk patients means that its impact on outcomes and quality of life is even more important. In the future, some patients may require coronary intervention or redo-TAVI; therefore, it is vital to maintain long-term access to the coronary ostia after TAVI. The Neo2, like its predecessor, has a wide cell design which facilitates coronary access in conjunction with a technique of commissural alignment8.
In this issue of EuroIntervention, Baggio et al3 report the outcome of the NEOPRO-2 registry, a multicentre, retrospective, non-randomised comparison of the latest 2 generations of SE TAVI devices: the ACURATE neo2 and the Evolut PRO/PRO+. Technical success and in-hospital and 30-day outcomes are reported according to the Valve Academic Research Consortium (VARC)-3 criteria for the whole unmatched cohort (2,175 patients) and among 452 propensity score-matched pairs. The main findings are the high technical success rate for both valves (>93%) and the lower rate of moderate/severe PVL with the Neo2 versus the Pro in the unmatched analysis predischarge (1.7% vs 4.1%; p=0.003) and at 30 days (2.3% vs 4.0%; p=0.037), which was no longer seen at either timepoint in the matched group (2.0% vs 3.1%; p=0.281).
Mild PVL is now under greater scrutiny and a new frontier for investigation, being suspected of impacting late outcomes and possibly mortality910. To this end, also in this issue of EuroIntervention, Pellegrini et al report significantly lower rates of mild PVL with the S3 Ultra compared to the Neo2 in unmatched (19.2% vs 32.6%) and matched cohorts (20.0% vs 32.8%; p<0.001)4, whilst in the NEOPRO-2 registry, mild PVL was comparable (39.3% vs 36.6%). Clearly, these uncertainties will only be resolved with direct randomised comparisons.
Haemodynamic parameters favoured the Pro; however, consistent with previous reports, PPMI was significantly lower with the Neo2 in unmatched (7.7% vs 15.6%) and matched (6.7% vs 16.7%) comparisons47. Thirty-day all-cause death and stroke were comparable, whilst hospitalisation and acute kidney injury were significantly higher with the Neo2, and vascular complications higher with the Pro. Consequently, the VARC-3 early safety composite endpoint at 30 days was higher with the Neo2 (78.7% vs 71.3%; p<0.001) driven by the lower rate of PPMI.
Elevated mean transvalvular gradients (>20 mmHg) were less frequent with the Neo2 (2.4% vs 7.7 %; p<0.001) compared with the S3 Ultra, whilst the mean AV gradient was similar between the Neo2 and PRO (1.9 % vs 1.6%).
New PPMI was less likely with the Neo2 compared to the Pro and similar to the S3 Ultra (8.1% vs 10.3%; p=0.289).
The negative impact of residual higher transvalvular gradients post-TAVI, with premature leaflet deterioration, and the detrimental effect of PPMI on long-term outcome, remain a matter for long-term evaluation.
Can surrogate statistics in a retrospective registry without adjudication replace a fully fledged randomised trial? The answer is obviously no, and the authors acknowledge that.
The 4 statistical approaches classically used to compare data acquired outside the frame of a randomised trial are as follows: crude univariate analysis, a multivariate model with covariate adjustment, nearest neighbour propensity matching with imputation, and inverse probability treatment weighting (IPTW) analysis adjusted for variables derived from the univariable analysis. Of note, the large number of participating centres in the present registry with the expected variable experience may have been an additional confounding factor, better accounted for by an IPTW model, which is relatively robust and has sometimes quite accurately heralded the results of randomised trials11. However, the jury is still out, and an undisputed judgement will only probably be revealed when the results of the ACURATE IDE trial (ClinicalTrials.gov: NCT03735667) comparing the Neo2 versus the Evolut and SAPIEN systems are presented.
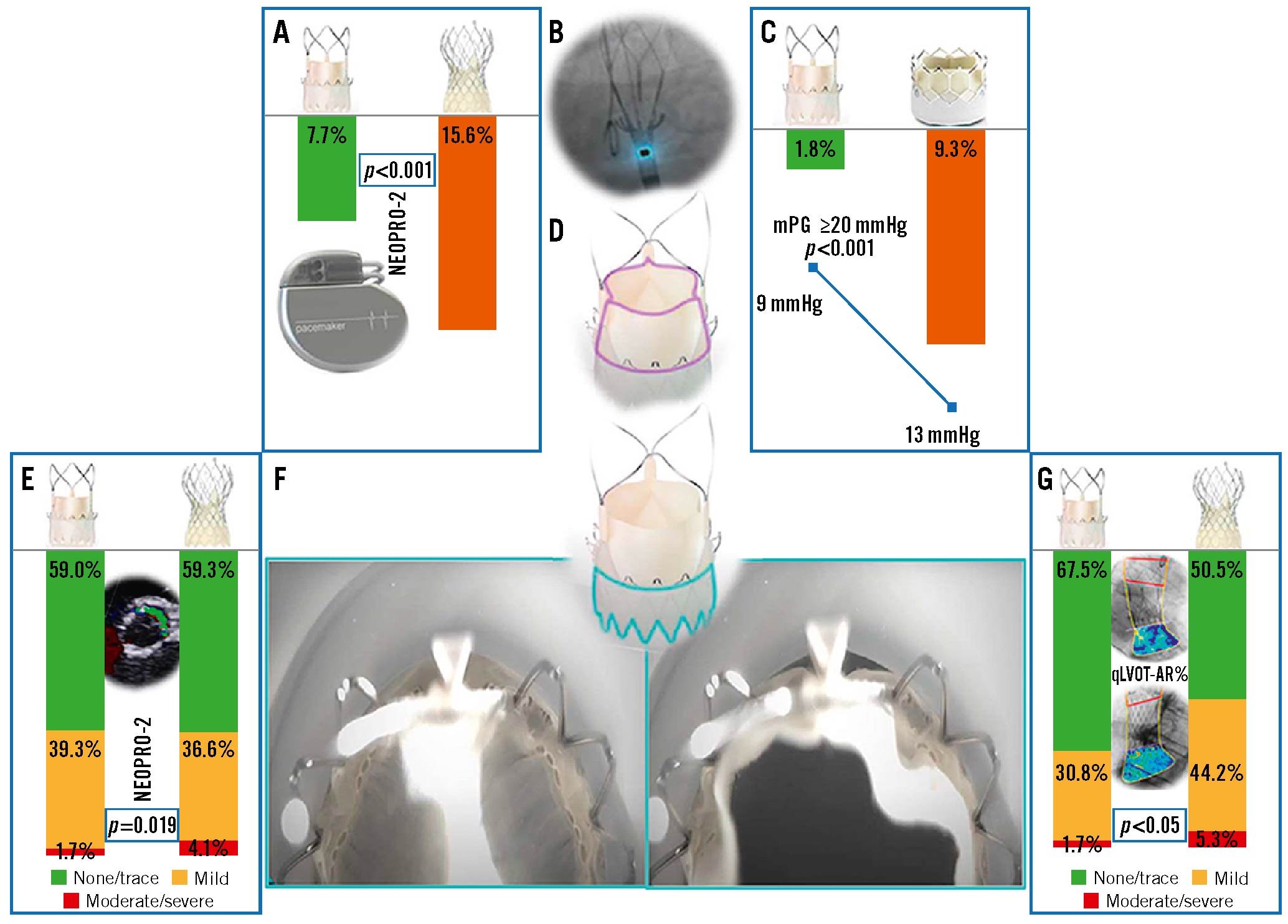
Figure 1. Does the ACURATE neo2 combine the best of 2 worlds: SE and BE? A) PPMI incidence between the Neo2 and the Pro, B) radiopaque markers within the Neo2 system, C) the difference in mean pressure gradient and ≥20 mmHg between the Neo2 and the S3 Ultra, D) the supra-annular design of the Neo2, E) PVL frequency between the Neo2 and the Pro, F,G) the Neo2 skirt with active function during systole and diastole, H) frequency of AR between the Neo2 and the Pro using quantitative videodensitometry. BE: balloon-expandable; mPG: mean pressure gradient; Neo2: ACURATE neo2; PPMI: permanent pacemaker implantation; Pro: Evolut PRO/PRO+; PVL: paravalvular leak; qLVOT-AR: quantitative left ventricular outflow tract aortic regurgitation; S3 Ultra: SAPIEN 3 Ultra; SE: self-expanding
Conflict of interest statement
The authors have no conflicts of interest to declare.

