Abstract
Background: No comparative data exist with the latest generation self-expanding ACURATE neo2 (Neo2) and the balloon-expandable SAPIEN 3 Ultra (Ultra) transcatheter heart valves (THV).
Aims: We aimed to compare the outcomes after transcatheter aortic valve implantation (TAVI) using the Neo2 and the Ultra THV.
Methods: A total of 1,356 patients at 4 centres were treated either with the Neo2 (n=608) or the Ultra (n=748). The primary endpoint was device success according to the latest Valve Academic Research Consortium definitions. The association of the THV used and the primary endpoint was assessed using inverse probability treatment weighting (IPTW) and 1:1 propensity score matching (PSM), which identified 472 matched pairs.
Results: After PSM, there were no relevant differences between the groups. While rates of moderate to severe paravalvular leakage (PVL) were overall low (0.6% vs 1.1%; p=0.725), elevated transvalvular gradients (≥20 mmHg) were less frequent with the Neo2 (2.4% vs 7.7%; p<0.001), which translated into a significantly higher rate of device success with the Neo2 compared with the Ultra (91.9% vs 85.0%; p<0.001). Consistently, the Neo2 was associated with higher rates of device success in the IPTW analysis (odds ratio [OR] 1.961, 95% confidence interval [CI]: 1.269-3.031; p=0.002). Rates of mild PVL were significantly lower with the Ultra compared with the Neo2 (20.0% vs 32.8%; p<0.001). Clinical events at 30 days were comparable between the 2 groups.
Conclusions: Short-term outcomes after TAVI using the Neo2 or Ultra THV were excellent and, overall, comparable. However, transvalvular gradients were lower with the Neo2, which translated into higher rates of device success. Rates of mild PVL were significantly lower with the Ultra THV.
Introduction
Since the beginning of interventional therapy of severe aortic valve stenosis, transcatheter aortic valve implantation (TAVI) has become a standard therapeutic treatment12. A continuous development of transcatheter heart valve (THV) technology has led to considerable improvement in procedural and clinical outcomes. To date, both self-expanding (SE) and balloon-expandable (BE) THV are being implanted. For both platforms, scientific evidence exists from several randomised and registry-based trials over the years, supporting their broad application. However, there are few data available from randomised clinical trials with direct comparisons of specific THV. Among them, the SCOPE I trial randomised the SE ACURATE neo THV (Neo; Boston Scientific) and the BE SAPIEN 3 THV (S3; Edwards Lifesciences). The Neo system did not meet the prespecified non-inferiority criteria as compared to the S3 THV3 with regard to the primary composite endpoint; this was mainly driven by valve-related dysfunction due to relevant paravalvular leakage (PVL).
For both THV, new iterations have become available recently: the SE ACURATE neo2 (Neo2; Boston Scientific) and the BE SAPIEN 3 Ultra THV (Ultra; Edwards Lifesciences). Both THV showed very promising early results compared to their predecessors456. To date, no direct comparison exists between the 2 latest-generation THV.
Hence, the purpose of this study was to compare the Neo2 and the Ultra THV with regard to 30-day outcomes in a propensity score-matched population.
Methods
Patient population and procedure
A total of 1,356 patients undergoing transfemoral TAVI for severe, native aortic valve stenosis between March 2019 and December 2021 at 4 centres in Germany (Deutsches Herzzentrum München, Munich; Kerckhoff Heart and Lung Center, Bad Nauheim; Elisabeth Hospital Essen, Essen; and Justus-Liebig University of Giessen and Marburg, Giessen) were considered for this retrospective analysis. Of these, 608 patients were treated with the Neo2 from September 2020, while 748 patients were treated with the Ultra THV over the whole inclusion period (Central illustration). The number of patients included from each participating centre is depicted in detail in Supplementary Figure 1. Procedures were performed according to local standards. However, at 1 centre, a minimalistic approach, the “SLIM” (single arterial access and low contrast agent volume) approach was implemented during the inclusion time7. Valve selection was based on the patients' anatomies, the manufacturers' recommendations and was left to the discretion of the responsible operator performing the procedure. The study was approved by each local ethics committee and complied with the Declaration of Helsinki.
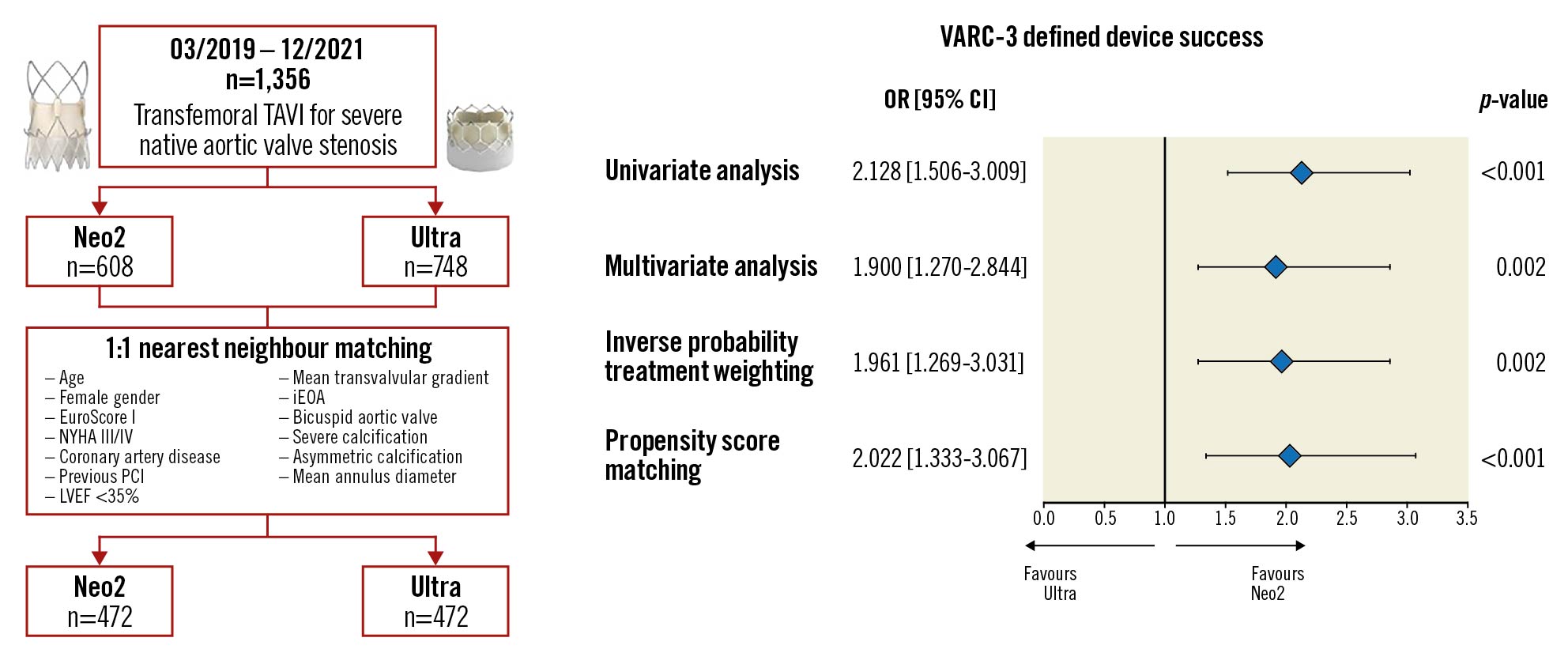
Central illustration. Study flow and variables used for propensity matching and risk of the primary composite endpoint device success according to THV. Device illustrations reproduced with permission from Boston Scientific and Edwards Lifesciences. CI: confidence interval; iEOA: indexed effective orifice area; LVEF: left ventricular ejection fraction; Neo2: ACURATE neo2; NYHA: New York Heart Association Functional Class; OR: odds ratio; PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; Ultra: SAPIEN 3 Ultra: VARC-3: Valve Academic Research Consortium 3
Device description
The SE Neo2 THV was granted the Conformité Européenne (CE) mark in April 2020 based on the results of the Neo2 CE-mark study5. This new iteration was designed to address the major drawback seen with the former Neo THV, i.e., to reduce PVL rates. This has been achieved through a new annular sealing technology designed to conform to irregular, calcified anatomies, which extends to cover the full waist of the stent. Further, the implementation of new radiopaque markers aids reference during positioning. The Neo2 is available in 3 sizes (small, medium and large), covering an annulus range from 21 to 27 mm. Currently, the Neo2 is being investigated in the ACURATE IDE clinical trial (NCT03735667).
The balloon-expandable Ultra THV received the CE mark in November 2018. The new features of the Ultra THV have already been described elsewhere4. The main difference, compared to its predecessor, is a revised outer skirt, allowing up to 50% more surface contact area with the native valve anatomy. In contrast to the S3, the Ultra THV is available in 3 sizes (20 mm, 23 mm and 26 mm) covering an annulus range from 18.6 to 26.4 mm.
Definition of endpoints and follow-up
Data were acquired during hospital stay and follow-up through routine visits at the outpatient clinic, review of hospital records, contact with primary care physicians or with the patients and collected in individual institutional databases. Data were then collected in a joint database for statistical analysis. Data collection involved demographic information, procedural data, and clinical and echocardiographic assessment prior to TAVI and before discharge. Adverse events were recorded up to 30 days after TAVI and were categorised according to the novel Valve Academic Research Consortium 3 (VARC-3) criteria8. The primary endpoints were the composite endpoint of technical success and device success. Technical success was achieved at exit from the procedure room in case of freedom from mortality, successful access, delivery of the device and retrieval of the delivery system, correct positioning of a single prosthetic heart valve into the proper anatomical location and freedom from surgery or intervention related to the device or to a major vascular, access-related or cardiac structural complication. Device success was defined as technical success, freedom from mortality, freedom from surgery or intervention related to the device or to a major vascular, access-related or cardiac structural complication, and performance of the valve as intended (mean gradient <20 mmHg, peak velocity <3 m/s, Doppler velocity index ≥0.25 and less than moderate aortic regurgitation) at 30 days. Secondary endpoints comprised VARC-3 clinical endpoints at 30 days.
Haemodynamic valve performance in terms of transvalvular gradients, PVL, indexed effective orifice area (iEOA) and patient-prosthesis mismatch (PPM) was obtained from discharge echocardiography. Severe PPM was defined if the iEOA was <0.65 cm2/m2 for a body mass index <30kg/m2 and <0.60 cm2/m2 for a body mass index ≥30 kg/m2. Calcium volume of the valvular apparatus was measured as previously described in non-contrast and contrast-enhanced multislice computed tomography (MSCT) scans, as per the availability at each centre9. Patients with a calcium volume beyond the 75th percentile were categorised as severely calcified. For patients without an available calcium volume measurement (n=30/1,356), visual grading was used. Asymmetric calcification was considered if its distribution varied significantly between the leaflets as previously described10. The cover index was derived from MSCT measurements in relation to the prosthesis area or perimeter, as appropriate.
Statistical analysis
Continuous variables are expressed as mean with standard deviation (SD) or median with interquartile range (IQR) and were compared using the Student’s t-test or the Mann-Whitney U test, respectively. The influence of the THV on the primary outcome of device success was tested using several approaches: first, the univariate association was analysed; and, second, a multivariable model adjusted for covariates yielding a p-value <0.1 in the univariate analysis was performed. To adjust for a potential centre-specific influence, the variable “participating centre” was entered into the model, independent from its p-value. The variables included were age, diabetes mellitus, chronic obstructive pulmonary disease, previous dialysis, coronary artery disease, peripheral artery disease, mean transvalvular gradients, bicuspid valve, asymmetric calcification, mean annular diameter, use of the Neo2 THV and participating centre. Further, to reduce the imbalance in baseline characteristics and the effect of a potential selection bias, including potential centre-specific influence, an inverse probability treatment weighting (IPTW) analysis was performed, adjusted for variables selected based on their p-value in the univariate analysis and on their potential influence on outcome. The selected variables were age, diabetes mellitus, chronic obstructive pulmonary disease, previous dialysis, coronary artery disease, peripheral artery disease, mean transvalvular gradients, bicuspid valve, asymmetric calcification, mean annular diameter, use of the Neo2 THV and participating centre.
Lastly, nearest neighbour propensity score matching (PSM) was performed as previously described (calliper width 0.1)11. Baseline demographic, clinical and echocardiographic characteristics as well as MSCT measurements showing significant differences between both treatment groups or with a known influence on outcome were included in the matching algorithm. Missing data were imputed using the R package “mice” (R Foundation for Statistical Computing) before matching and weighting. The Central illustration summarises the study flow and variables used for PSM.
A 2-sided p-value of <0.05 was considered statistically significant for all analyses. SPSS Statistics, version 27.0.1.0 (IBM) and RStudio, version 1.4.1103 (R Foundation for Statistical Computing) including the package “MatchIt” were used for all analyses.
Results
Patient population
A total of 1,356 patients (Neo2: n=608; Ultra: n=748) were included in the present analysis. The median age was 81.7 years (IQR 77.9, 85.0), 50.7% were female, and the median logistic EuroSCORE was 13.29% (IQR 7.81, 22.65). As displayed in Table 1, patients treated with the Neo2 THV had a higher logistic EuroSCORE compared to patients treated with the Ultra THV (14.37% [IQR 8.05, 23.42] vs 12.34% [IQR 7.63, 21.31]; p=0.008) and presented more frequently with New York Heart Association (NYHA) Class III/IV symptoms (420 [69.1%] vs 429 [57.4%]; p<0.001), while patients treated with the Ultra THV were more often female (398 [53.2%] vs 289 [47.5%]; p=0.038) and more frequently had coronary artery disease (558 [74.6%] vs 376 [61.8%]; p<0.001), including prior percutaneous coronary interventions (310 [41.4%] vs 216 [35.5%]; p=0.029). Aortic valve stenosis was more severe in patients treated with the Ultra THV, in terms of higher mean transvalvular gradients (44.00 mmHg [IQR 37.00, 54.00] vs 42.00 mmHg [IQR 31.30, 50.00]; p<0.001) and smaller iEOA (0.36 cm2 [IQR 0.30, 0.42] vs 0.38 cm2 [IQR 0.32, 0.44]; p<0.001). Furthermore, significant anatomical differences were found between the treatment groups, with higher rates of bicuspid valves (97 [13.0%] vs 20 [3.3%]; p<0.001), severe calcification (206/747 [27.6%] vs 126/606 [20.8%]; p=0.004), asymmetric calcification (336 [44.9%] vs 123 [20.2%]; p<0.001) and larger aortic annuli (24.85 mm [IQR 23.35, 26.20] vs 23.65 mm [IQR 22.39, 25.05; p<0.001]) in the Ultra THV treatment group.
PSM yielded 472 well-balanced pairs of patients treated either with the Neo2 or the Ultra THV with a standardised mean difference of 0.0573. After matching, no further statistically significant differences regarding baseline characteristics or anatomical variables persisted, particularly in terms of bicuspid aortic valve, severe aortic valve calcification and asymmetric calcification, with the exception of patients treated with the Ultra THV who presented with larger mean annulus diameters by 1 mm (24.85 mm [IQR 23.35, 26.16] vs 23.80 mm [IQR 22.35, 25.21]; p<0.001). Detailed information on the distribution and balance of the propensity score across treatment and control cases is depicted in Supplementary Table 1 and Supplementary Figure 2.
Table 1. Baseline characteristics of patients for the entire population and matched population according to implanted THV.
| Entire population | Matched population | |||||
|---|---|---|---|---|---|---|
| Neo2 n=608 | Ultra n=748 | p-value | Neo2 n=472 | Ultra n=472 | p-value | |
| Age, years | 82.00 [78.72, 85.00] | 81.37 [77.05, 85.00] | 0.032 | 82.00 [78.65, 85.00] | 81.60 [77.61, 85.07] | 0.584 |
| Female gender | 289 (47.5) | 398 (53.2) | 0.038 | 239 (50.6) | 246 (52.1) | 0.696 |
| BMI, kg/m2 | 26.30 [23.67, 29.95] | 26.42 [24.15, 29.38] | 0.896 | 26.30 [23.74, 29.90] | 26.36 [24.09, 29.62] | 0.943 |
| Logistic EuroSCORE, % | 14.37 [8.05, 23.42] | 12.34 [7.63, 21.31] | 0.008 | 13.84 [7.94, 22.97] | 12.49 [7.85, 21.84] | 0.184 |
| EuroSCORE II, % | 3.02 [2.11, 5.01] | 3.01 [1.90, 5.18] | 0.508 | 2.96 [2.04, 4.99] | 3.11 [2.00, 5.18] | 0.827 |
| NYHA III/IV | 420 (69.1) | 429 (57.4) | <0.001 | 305 (64.6) | 297 (62.9) | 0.636 |
| Arterial hypertension | 530 (87.2) | 659 (88.1) | 0.619 | 412 (87.3) | 428 (90.7) | 0.119 |
| Hypercholesterolaemia | 368 (60.5) | 476 (63.6) | 0.260 | 295 (62.5) | 305 (64.6) | 0.543 |
| Diabetes mellitus | 207 (34.0) | 234 (31.3) | 0.294 | 154 (32.6) | 155 (32.8) | 0.999 |
| Coronary artery disease | 376 (61.8) | 558 (74.6) | <0.001 | 340 (72.0) | 336 (71.2) | 0.829 |
| Previous PCI | 216 (35.5) | 310 (41.4) | 0.029 | 193 (40.9) | 190 (40.3) | 0.895 |
| Previous CABG | 55 (9.0) | 53 (7.1) | 0.191 | 49 (10.4) | 37 (7.8) | 0.213 |
| Previous myocardial infarction | 58 (9.5) | 87 (11.6) | 0.251 | 50 (10.6) | 54 (11.4) | 0.755 |
| Previous stroke | 77 (12.7) | 94 (12.6) | 0.999 | 57 (12.1) | 55 (11.7) | 0.920 |
| COPD | 74 (12.2) | 86 (11.5) | 0.735 | 57 (12.1) | 56 (11.9) | 0.999 |
| Peripheral artery disease | 84 (13.8) | 130 (17.4) | 0.085 | 61 (12.9) | 81 (17.2) | 0.083 |
| On dialysis | 13 (2.1) | 6 (0.8) | 0.060 | 10 (2.1) | 5 (1.1) | 0.298 |
| eGFR, mL/min/1.73m2 | 65.00 [47.00, 83.00] | 64.00 [48.50, 79.50] | 0.684 | 65.00 [47.00, 84.25] | 62.00 [47.70, 79.00] | 0.198 |
| Previous pacemaker | 75 (12.3) | 71 (9.5) | 0.095 | 57 (12.1) | 46 (9.7) | 0.296 |
| Atrial fibrillation | 256 (42.1) | 291 (38.9) | 0.243 | 190 (40.3) | 191 (40.5) | 0.999 |
| Right bundle-branch block | 56 (9.2) | 85 (11.4) | 0.211 | 50 (10.6) | 60 (12.7) | 0.361 |
| Left bundle-branch block | 61 (10.0) | 57 (7.6) | 0.122 | 48 (10.2) | 36 (7.6) | 0.208 |
| LVEF <35% | 17 (2.8) | 42 (5.6) | 0.011 | 17 (3.6) | 13 (2.8) | 0.579 |
| Mean transvalvular gradient, mmHg | 42.00 [31.30, 50.00] | 44.00 [37.00, 54.00] | <0.001 | 43.00 [34.00, 52.00] | 42.50 [34.75, 51.00] | 0.940 |
| Indexed effective orifice area, cm2 | 0.38 [0.32, 0.44] (n=601) | 0.36 [0.30, 0.42] (n=721) | <0.001 | 0.37 [0.31, 0.43] | 0.36 [0.31, 0.43] | 0.518 |
| Bicuspid aortic valve | 20 (3.3) | 97 (13.0) | <0.001 | 20 (4.2) | 25 (5.3) | 0.542 |
| Severe aortic valve calcification | 126/606 (20.8) | 206/747 (27.6) | 0.004 | 114 (24.3) | 115 (24.4) | 0.954 |
| Asymmetric calcification | 123 (20.2) | 336 (44.9) | <0.001 | 120 (25.4) | 135 (28.6) | 0.305 |
| Mean annulus diameter, mm | 23.65 [22.39, 25.05] | 24.85 [23.35, 26.20] | <0.001 | 23.80 [22.35, 25.21] | 24.85 [23.35, 26.16] | <0.001 |
| Data are median [interquartile range] or n (%). In case of missing data, numbers of available measurements are given. BMI: body mass index; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; LV: left ventricular; NYHA: New York Heart Association Functional Class; PCI: percutaneous coronary intervention; THV: transcatheter heart valve | ||||||
Procedural outcome and device success
Procedural characteristics of the entire and matched population are displayed in Table 2. Most (>99%) procedures were performed under conscious sedation. The small, medium and large sizes of the Neo2 THV were implanted in 21.9%, 43.4% and 34.7% of patients, respectively, with an overall cover index calculated by perimeter of 6.04% (IQR 3.91, 8.15). The Ultra THV sizes 20 mm, 23 mm and 26 mm were deployed in 1.5%, 28.7% and 69.8% of cases, respectively, with a median cover index calculated by area of 2.65% (IQR -0.23, 6.10). Patients treated with the Neo2 presented higher rates of pre- and post-dilatation compared with the Ultra THV in the entire population (predilatation: 534 [87.8%] vs 268 [35.8%]; p<0.001; post-dilatation: 250 [41.1%] vs 111 [14.8%]; p<0.001) as well as in the matched cohort (predilatation: 434 [91.9%] vs 148 [31.4%]; p<0.001; post-dilatation: 211 [44.7%] vs 69 [14.6%]; p<0.001). Furthermore, a significantly lower amount of contrast agent was used with the Neo2 compared with the Ultra THV in the entire population (40.00 ml [IQR 20.00, 116.00] vs 115.00 ml [IQR 36.00, 160.00]; p<0.001) as well as in the matched population (40.00 ml [IQR 22.00, 130.00] vs 117.50 ml [IQR 37.75, 160.00]; p<0.001), while the fluoroscopy time differed only in the entire population (9.40 min [IQR 7.01, 13.29] vs 10.21 min [IQR 7.10, 14.62]; p=0.033).
Technical success was comparable between both groups in the entire and matched populations. On the contrary, the crude rate of the composite endpoint device success was 91.6% with the Neo2 THV, which was significantly higher than with the Ultra THV (83.7%, OR 2.128, 95% CI: 1.506-3.009; p<0.001) (Table 2, Central illustration). The significant risk reduction persisted after multivariate adjustment (OR 1.900, 95% CI: 1.270-2.844; p=0.002) (Central illustration, Supplementary Table 2), as well as after IPTW analysis (OR 1.961, 95% CI: 1.269-3.031; p=0.002) (Central illustration). Consistently, after PSM, device success rates were higher with the Neo2 compared to the Ultra (434 [91.9%] vs 401 [85.0%]; OR 2.022, 95% CI: 1.333-3.067; p<0.001) (Table 2, Central illustration). This finding was mainly driven by elevated mean transvalvular gradients (≥20 mmHg), which were higher in patients treated with the Ultra compared with the Neo2 THV, both in the entire and matched populations (entire population: 69 [9.3%] vs 11 [1.8%]; p<0.001; matched population: 36 [7.7%] vs 11 [2.4%]; p<0.001). Further, the Ultra cohort presented smaller iEOA in the entire (0.78 cm2 [0.68, 0.90] vs 0.92 cm2 [0.79, 1.05]; p<0.001) and matched populations (0.78 cm2 [0.67, 0.91] vs 0.92 cm2 [0.79, 1.05]; p<0.001) compared to the Neo2 cohort, resulting in higher rates of severe PPM (entire population: 39 [14.9%] vs 10 [2.2%]; p<0.001 and matched population: 25 [15.0%] vs 10 [2.9%]; p<0.001).
Table 2. Procedural and post-procedural characteristics for the entire population and matched population according to implanted THV.
| Entire population | Matched population | |||||
|---|---|---|---|---|---|---|
| Neo2 n=608 | Ultra n=748 | p-value | Neo2 n=472 | Ultra n=472 | p-value | |
| Procedural characteristics | ||||||
| Conscious sedation | 606 (99.7) | 742 (99.2) | 0.308 | 471 (99.8) | 467 (98.9) | 0.217 |
| Predilatation | 534 (87.8) | 268 (35.8) | <0.001 | 434 (91.9) | 148 (31.4) | <0.001 |
| Post-dilatation | 250 (41.1) | 111 (14.8) | <0.001 | 211 (44.7) | 69 (14.6) | <0.001 |
| Procedural time, min | 44.00 [35.00, 59.00] | 46.00 [35.00, 58.00] | 0.867 | 45.00 [36.00, 59.00] | 46.00 [35.00, 57.00] | 0.472 |
| Contrast agent, ml | 40.00 [20.00, 116.00] | 115.00 [36.00, 160.00] | <0.001 | 40.00 [22.00, 130.00] | 117.50 [37.75, 160.00] | <0.001 |
| Fluoroscopy time, min | 9.40 [7.01, 13.29] | 10.21 [7.10, 14.62] | 0.033 | 9.82 [7.30, 13.81] | 10.20 [6.90, 14.11] | 0.974 |
| Cover index by area | 8.00 [5.61, 10.00] | 2.65 [-0.23, 6.10] | <0.001 | 7.83 [5.60, 9.67] | 2.42 [-0.38, 6.04] | <0.001 |
| Cover index by perimeter | 6.04 [3.91, 8.15] | 0.71 [-2.35, 4.12] | <0.001 | 6.00 [3.90, 7.93] | 0.59 [-2.69, 4.19] | <0.001 |
| Technical success (VARC-3) | 575 (94.6) | 714 (95.5) | 0.529 | 448 (94.9) | 450 (95.3) | 0.880 |
| Device success (VARC-3) | 557 (91.6) | 626 (83.7) | <0.001 | 434 (91.9) | 401 (85.0) | 0.001 |
| Procedural mortality | 0 (0.0) | 4 (0.5) | 0.132 | 0 (0.0) | 2 (0.4) | 0.499 |
| Correct implant position | 602 (99.0) | 747 (99.9) | 0.050 | 467 (98.9) | 472 (100.0) | 0.062 |
| Multiple valves | 3 (0.5) | 1 (0.1) | 0.331 | 3 (0.6) | 1 (0.2) | 0.624 |
| Conversion to surgery | 1 (0.2) | 6 (0.8) | 0.138 | 1 (0.2) | 3 (0.6) | 0.624 |
| Post-procedural characteristics | ||||||
| Moderate to severe PVL* | 4 (0.7) | 6 (0.8) | 1.000 | 3 (0.6) | 5 (1.1) | 0.723 |
| Mean gradient ≥20 mmHg | 11 (1.8) | 69 (9.3) | <0.001 | 11 (2.4) | 36 (7.7) | <0.001 |
| Indexed effective orifice area, cm²** | 0.92 [0.79, 1.05] (n=453) | 0.78 [0.68, 0.90] (n=261) | <0.001 | 0.92 [0.79, 1.05] (n=342) | 0.78 [0.67, 0.91] (n=167) | <0.001 |
| Severe PPM** | 10 (2.2) (n=453) | 39 (14.9) (n=261) | <0.001 | 10 (2.9) (n=342) | 25 (15.0) (n=167) | <0.001 |
| Annular rupture | 1 (0.2) | 2 (0.3) | 1.000 | 1 (0.2) | 0 (0.0) | 0.999 |
| Data are median [interquartile range] or n (%). *As assessed by echocardiography at discharge, for missing data aortic regurgitation was assessed by angiography (n=10/1,356). **Available for 714/1,356 in the entire population and for 509/944 in the matched population. PPM: patient-prosthesis mismatch; PVL: paravalvular leakage; THV: transcatheter heart valve; VARC-3: updated Valve Academic Research Consortium 3 | ||||||
In-hospital and 30-day outcomes
Table 3 displays clinical outcomes during hospital stay and at 30 days for the entire and the matched populations. There were no significant differences with respect to in-hospital events between the treatment groups. Notably, despite differences in the use of contrast agent, rates of acute kidney injury stage 2-4 were low and comparable in both groups. Haemodynamic performance as measured by echocardiography improved substantially after TAVI as shown in Figure 1 with a reduction in mean transvalvular gradients for both platforms. However, transprosthetic gradients were significantly lower with the Neo2 THV compared with the Ultra THV after TAVI in the entire (9±4 mmHg vs 13±5 mmHg; p<0.001) and matched populations (9±4 mmHg vs 13±4 mmHg; p<0.001) (Figure 1A, Figure 1B). Rates of moderate PVL were overall low and similar for both THV before and after matching, with no case of severe PVL in either group (Figure 1C, Figure 1D). Conversely, rates of mild PVL were lower in Ultra compared with Neo2 recipients in the entire (19.2% vs 32.6%; p<0.001) and matched populations (20.0% vs 32.8%; p<0.001).
Follow-up at 30 days was complete for 95.0% of the entire population, specifically for matched patients in 98.3% (464 of 472) of patients treated with the Neo2 THV and for 98.5% (465 of 472) of patients treated with the Ultra THV. As shown in Table 3, clinical event rates at 30 days were low and did not differ between Neo2 and Ultra recipients.
Table 3. In-hospital and 30-day clinical outcomes for the entire population and matched population according to implanted THV.
| Entire population | Matched population | |||||
|---|---|---|---|---|---|---|
| Neo2 n=608 | Ultra n=748 | p-value | Neo2 n=348 | Ultra n=348 | p-value | |
| In-hospital clinical outcomes | ||||||
| All-stroke | 17 (2.8) | 24 (3.2) | 0.778 | 16 (3.4) | 11 (2.3) | 0.435 |
| New permanent pacemaker implantation* | 40/553 (7.5) | 66/677 (9.7) | 0.170 | 33/415 (8.0) | 42/426 (9.9) | 0.332 |
| Major vascular complication (VARC-3) | 39 (6.4) | 66 (8.8) | 0.122 | 29 (6.1) | 45 (9.5) | 0.069 |
| Bleeding type 3 and 4 (VARC-3) | 26 (4.3) | 33 (4.4) | 0.999 | 18 (3.8) | 17 (3.6) | 0.999 |
| Cardiac structural complication (VARC-3) | 5 (0.8) | 12 (1.6) | 0.298 | 4 (0.8) | 5 (1.1) | 0.999 |
| Myocardial infarction | 0 (0.0) | 3 (0.4) | 0.326 | 0 (0.0) | 2 (0.4) | 0.479 |
| Coronary obstruction requiring PCI | 1 (0.2) | 3 (0.4) | 0.768 | 1 (0.2) | 2 (0.4) | 0.999 |
| AKIN 2/3/4 | 18 (3.0) | 23 (3.1) | 0.999 | 15 (3.2) | 15 (3.2) | 0.999 |
| In-hospital mortality | 7 (1.2) | 7 (0.9) | 0.904 | 5 (1.1) | 4 (0.8) | 0.999 |
| 30-day clinical outcomes | Neo2 n=598** | Ultra n=734** | p-value | Neo2 n=464** | Ultra n=465** | p-value |
| All-cause mortality | 11 (1.8) | 18 (2.5) | 0.566 | 8 (1.7) | 11 (2.4) | 0.646 |
| All-stroke | 18 (3.0) | 23 (3.1) | 0.999 | 16 (3.4) | 11 (2.4) | 0.435 |
| Cardiovascular rehospitalisation | 5 (0.8) | 7 (1.0) | 0.999 | 5 (1.1) | 3 (0.6) | 0.723 |
| New pacemaker implantation* | 40/522 (7.7) | 70/664 (10.5) | 0.090 | 33/406 (8.1) | 43/419 (10.3) | 0.289 |
| Repeat procedure | 0 (0.0) | 1 (0.1) | 0.999 | 0 (0.0) | 0 (0.0) | – |
| Data are median [interquartile range] or n (%). *Excluding patients with pacemaker at baseline. **Patients with available follow-up at 30 days in the entire population 1,332/1,356 and in the matched population 929/944. AKIN: Acute Kidney Injury Network classifcation; CHF: congestive heart failure; PCI: percutaneous coronary intervention; THV: transcatheter heart valve | ||||||
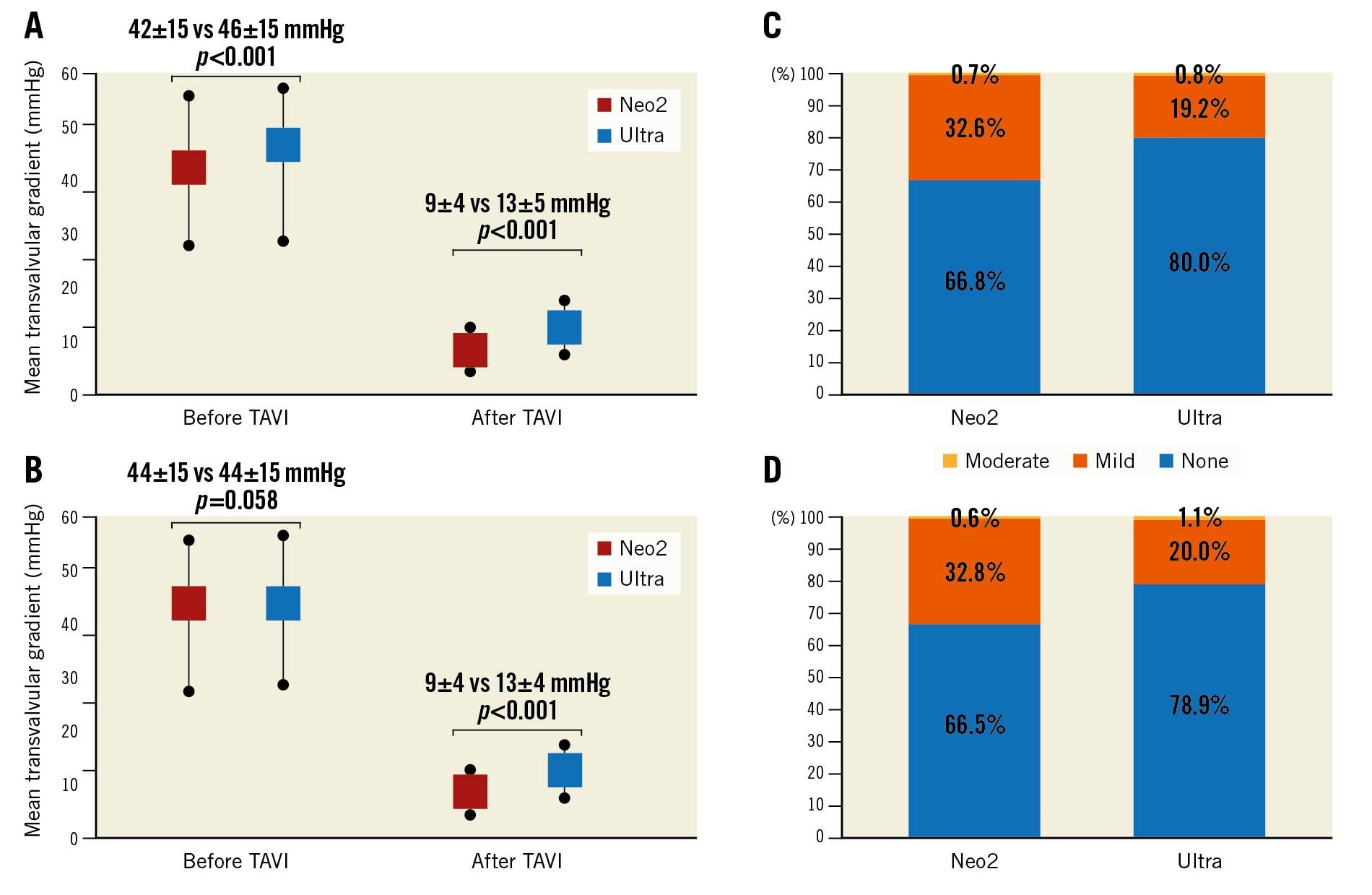
Figure 1. Mean transvalvular gradients before and after TAVI and rates of paravalvular leakage after transcatheter aortic valve implantation according to implanted THV for the entire (A, C) and the matched populations (B, D). Neo2: ACURATE neo2; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; Ultra: SAPIEN 3 Ultra
Discussion
The main results can be summarised as follows: 1) VARC-3 technical success was comparable in both THV, while device success was higher with the Neo2 THV than with the Ultra THV due to significantly lower transvalvular gradients after TAVI. 2) Rates of moderate or severe PVL were overall low and comparable with both THV, whereas mild PVL was significantly lower in Ultra THV compared to Neo2 THV recipients. 3) Event rates were overall low with both THV up to 30 days.
Growing evidence from randomised clinical trials and large registry studies with different TAVI platforms corroborate excellent results, promoting their fast expansion. Still, some procedural shortcomings including PVL and permanent pacemaker implantation (PPI) need to be addressed to safely move to a routine treatment of low-risk and younger patients. With this in mind, new iterations of THV need to overcome these drawbacks, focusing on novel designs and modified implantation techniques. In particular, for the SE Neo THV the previous clinical experience showed consistently higher rates of PVL compared to other available THV platforms, not least in the randomised SCOPE I and II trials312. To cope with this Achilles´ heel, the revised Neo2 THV exhibits a 60% larger sealing skirt. So far, promising early results were yielded in recent multicentric registries showing significantly lower rates of moderate to severe PVL with the Neo2 THV compared to its predecessor613. At the same time, the latest-generation Ultra THV with its adapted sealing properties, showed a further improvement in PVL rates, with already low rates of moderate to severe PVL ranging from 0.1% to 2.7%, and a further significant reduction of mild PVL rates414. In the current study, we present exceedingly low moderate to severe PVL rates for both platforms, reinforcing the positive evidence for these new THV. Compared with the Neo2, the Ultra THV further showed lower rates of mild PVL. While the presence of moderate to severe PVL is largely considered to negatively influence outcome15, the impact of mild PVL on outcome is still controversial. A recent meta-analysis suggested an association with increased mortality, especially in selected subsets of patients, which needs to be further investigated in larger analyses16.
The main driver leading to the marked difference found in device success rates was the presence of elevated transvalvular gradients ≥20 mmHg after TAVI, which were significantly higher in Ultra recipients. These findings are in line with previous data3171819 and may likely be explained by the supra-annular design of the Neo2 THV. The clinical relevance of higher transvalvular gradients and PPM with potential less symptomatic benefit and faster THV deterioration is still a matter of debate. Yet, a recent analysis from the FRANCE-2 Registry showed an increased mortality among patients with persistently elevated gradients at 1 year20. Thus, further knowledge and evidence is needed to fully comprehend this subject.
The need for PPI remains a considerable downside even in contemporary TAVI practice with rates ranging from 6.7% to 39.2%21. The lowest pacemaker rates were found with the Neo THV, with rates ranging from 2%-10%222324. Of note, pacemaker rates with the SAPIEN 3 valve showed a decrease after the initial experience from 16% to 5.5% due to a higher device positioning approach2526. While no difference in pacemaker rates was found in the SCOPE I trial, registry data showed consistently lower rates when using the Neo THV1727. Recent analyses of the Neo2 THV showed similarly low PPI rates ranging from 8% to 11%, despite its revised annular sealing properties613. Similarly, recent experience with the Ultra THV showed excellent pacemaker rates of 4.5% to 6.4%414. Consistently, in this direct comparison we found overall low and comparable rates of PPI for both valves. Addressing the issue of pacemaker implantation is of the utmost importance to reduce potential adverse long-term effects, especially when moving towards the treatment of younger patients.
Despite the significantly higher use of pre- and post-dilatation in Neo2 recipients, this did not translate into longer procedural times or alleged higher complication rates, such as stroke, annulus rupture or the need for PPI. Evidence from randomised clinical trials comparing the BE SAPIEN 3 THV (DIRECT TAVI trial; NCT02729519) and the SE CoreValve Evolut R/Pro THV (Medtronic) (DIRECT trial; NCT02448927), with or without predilatation, showed no difference in clinical outcome2829, suggesting the feasibility of both implantation techniques and thereby leaving the decision to the discretion of the operators, who should then take into consideration important anatomical characteristics, particularly valvular calcification. For the Neo/Neo2 THV, which exhibit less radial force compared to BE and other SE THV, predilatation is mandatory. A recent analysis focusing on dilatation strategy from the NeoPro registry showed a comparable outcome when predilatation was omitted30. However, randomised data with the novel Neo2 THV are warranted to fully assess the optimal implantation technique for this THV.
The significant difference concerning the amount of contrast agent used for TAVI found in this analysis can be attributed to a minimalistic approach used in 1 participating centre and should be interpreted with care7.
Limitations
Besides the inherent limitations of a retrospective, non-randomised study setting, we would like to address some limitations. Firstly, the sample size is rather modest. However, this is the first ever comparative analysis of the 2 valve platforms. PSM may not rule out immeasurable confounders. After PSM, severely calcified anatomies were excluded; thus, these findings are limited to mildly and moderately calcified aortic valves. Although clinical events were categorised according to standardised definitions, there was no adjudication by an independent committee. Echocardiography was performed according to current recommendations; however, there was no core laboratory for echocardiographic analyses831.
Conclusions
In this multicentre registry, outcomes after TAVI using the Neo2 and the Ultra THV were excellent and, overall, comparable. However, transvalvular gradients were lower for the Neo2 platform and translated into a higher rate of device success. Rates of moderate to severe PVL were low with both THV; however, the Ultra THV showed significantly lower rates of mild PVL.
Impact on daily practice
No direct comparisons between latest-generation self-expanding and balloon-expandable transcatheter prostheses are available so far. In this multicentre, propensity-matched comparison of the self-expanding ACURATE neo2 and the balloon-expandable SAPIEN 3 Ultra prostheses, we found comparable short-term outcomes with both valves. However, transprosthetic gradients were lower with the ACURATE neo2 platform, which translated into a higher rate of device success. Rates of mild PVL were significantly lower with the SAPIEN 3 Ultra prosthesis.
Conflict of interest statement
A. Wolf received proctor fees from Edwards Lifesciences and Boston Scientific. E.I. Charitos has received proctor fees from Boston Scientific and holds stock or stock options in Edwards Lifesciences. M. Joner reports lecture fees and research grants from Edwards Lifesciences, Cardiac Dimensions, Infraredx, and Boston Scientific; is a consultant for Biotronik, Boston Scientific, Cardiac Dimensions, and Shockwave Medical; has received honoraria from Abbott, AstraZeneca, Biotronik, Boston Scientific, Edwards Lifesciences, ReCor, Shockwave Medical, and Orbus Neich; and is a Member of the Board of Biotronik and Shockwave Medical. W-K. Kim received proctor and/or speaker and/or advisory honoraria from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, Meril Life Sciences, and Shockwave Medical. C. Pellegrini received a personal research grant from Else Kröner Fresenius Stiftung. T. Rheude received lecture fees from SIS Medical AG, and Astra Zeneca; and travel support from SIS Medical AG.O. Dörr received honoraria for lectures from Edwards Lifesciences. E. Xhepa reports lecture fees and honoraria from AstraZeneca, Boston Scientific, and SIS Medical, not related to the current work; he reports proctor fees from Abbott Vascular, and financial support for attending meetings and/or travel expenses from Abbott Vascular.The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

