Abstract
Background: Midterm comparative analyses of the latest iterations of the most used Evolut and SAPIEN platforms for transcatheter aortic valve implantation (TAVI) are lacking.
Aims: We aimed to compare 1-year clinical outcomes of TAVI patients receiving Evolut PRO/PRO+ (PRO) or SAPIEN 3 Ultra (ULTRA) devices in current real-world practice.
Methods: Among patients enrolled in the OPERA-TAVI registry, patients with complete 1-year follow-up were considered for the purpose of this analysis. One-to-one propensity score matching was used to compare TAVI patients receiving PRO or ULTRA devices. The primary endpoint was a composite of 1-year all-cause death, disabling stroke and rehospitalisation for heart failure. Five prespecified subgroups of patients were considered according to leaflet and left ventricular outflow tract calcifications, annulus dimensions and angulation, and leaflet morphology.
Results: Among a total of 1,897 patients, 587 matched pairs of patients with similar clinical and anatomical characteristics were compared. The primary composite endpoint did not differ between patients receiving PRO or ULTRA devices (Kaplan-Meier [KM] estimates 14.0% vs 11.9%; log-rank p=0.27). Patients receiving PRO devices had higher rates of 1-year disabling stroke (KM estimates 2.6% vs 0.4%; log-rank p=0.001), predominantly occurring within 30 days after TAVI (1.4% vs 0.0%; p=0.004). Outcomes were consistent across all the prespecified subsets of anatomical scenarios (all pinteraction>0.10).
Conclusions: One-year clinical outcomes of patients undergoing transfemoral TAVI and receiving PRO or ULTRA devices in the current clinical practice were similar, but PRO patients had higher rates of disabling stroke. Outcomes did not differ across the different anatomical subsets of the aortic root.
Introduction
Transcatheter aortic valve implantation (TAVI) has become the treatment of choice for elderly patients with severe symptomatic aortic stenosis, regardless of their surgical risk profile1. Different refinements have been brought to the most used self-expanding (SE) and balloon-expandable (BE) TAVI platforms over the past decade, leading to a marked improvement in patient outcomes2. The OPERA-TAVI registry compared the acute performances of the latest iterations of the Evolut PRO/PRO+ (PRO; Medtronic) and the SAPIEN 3 Ultra (ULTRA; Edwards Lifesciences) valves according to the Valve Academic Research Consortium (VARC)-3 criteria3. The aim of the present analysis was to assess 1-year clinical outcomes of patients enrolled in the OPERA-TAVI registry, investigating potential differences between the two platforms in different prespecified challenging anatomies.
Methods
REGISTRY DESIGN
The OPERA-TAVI (Comparative Analysis of Evolut PRO vs SAPIEN 3 Ultra Valves for Transfemoral Transcatheter Aortic Valve Implantation) is an investigator-initiated, multicentre registry which enrolled consecutive patients undergoing transfemoral TAVI with PRO or ULTRA devices at 15 centres in Europe and North America from September 2017 to January 2022. Details of the registry design have been previously published3.
STUDY OUTCOMES
The primary outcome of the analysis was a composite of all-cause death, disabling stroke and rehospitalisation for heart failure (HF) at 1 year. Secondary outcomes included 1-year all-cause death, disabling stroke and HF rehospitalisation, individually.
STATISTICAL ANALYSIS
Categorical variables are reported as counts and percentages. Continuous variables are reported as medians and interquartile ranges (IQRs). Continuous variables were compared with a t-test or Mann-Whitney U test, and categorical variables were compared with the chi-square statistics, Fisher's exact or McNemar tests as appropriate.
Propensity score matching (PSM) was used to account for possible confounding bias due to the non-randomised design of the study. The propensity score was estimated using a logistic regression model according to a non-parsimonious approach. The variables selected were sex, age, body mass index (BMI), diabetes, hypertension, peripheral artery disease (PAD), chronic obstructive pulmonary disease (COPD), renal failure (defined as estimated glomerular filtration rate <30 ml/min/1.73 m2), prior coronary artery bypass grafting (CABG), prior myocardial infarction (MI), prior stroke, prior pacemaker (PM) implantation, New York Heart Association (NYHA) Functional Class, atrial fibrillation (AF), baseline right bundle branch block (RBBB), Society of Thoracic Surgeons (STS) mortality score, left ventricular ejection fraction (LVEF), transaortic mean gradient, leaflet and left ventricular outflow tract (LVOT) calcification, bicuspid aortic valve, horizontal aorta, and area/perimeter-derived aortic annulus diameter <23 mm assessed at preprocedural computed tomography (CT) analysis.
Five subgroups of patients were prespecified and tested for interaction for primary and secondary outcomes: moderate to severe aortic leaflet calcifications, moderate to severe LVOT calcifications, area/perimeter-derived annulus diameter <23 mm, horizontal aorta (defined as an angle between the horizontal plane and the aortic annulus ≥48°) and bicuspid aortic valve.
Time-to-event curves for the primary and co-primary outcomes were estimated using the Kaplan-Meier (KM) method. Landmark analyses were performed for each outcome of interest; 30 days after TAVI was considered as the cut-off date of interest. Cox regression analysis was performed for each outcome of interest. Results were reported as hazard ratio (HR) with 95% confidence interval (CI).
All statistical tests were performed two-tailed, and a p-value<0.05 was considered as the threshold for statistical significance (p-value<0.10 was the threshold for the interaction test). All statistical analyses were performed with R software, version 3.6.3 (R Foundation for Statistical Computing).
Results
BASELINE CHARACTERISTICS
A total of 3,518 consecutive patients undergoing transfemoral TAVI were enrolled in the OPERA-TAVI registry. Exclusion criteria for the analysis were as follows: patients who were not eligible for both PRO and ULTRA devices according to the manufacturers’ instruction for annular dimensions, and TAVI in pure aortic valve regurgitation and in degenerated surgical bioprosthetic valves. Patients without preprocedural CT and 1-year follow-up data were also excluded. Given a total of 1,897 patients in the prematched population, 1,098 patients received the PRO transcatheter aortic valve, whereas 799 patients received the ULTRA device. Baseline characteristics of the prematched population are reported in Supplementary Table 1
After adjustment for clinical and anatomical characteristics, 587 matched pairs treated with PRO or ULTRA devices were compared. Baseline characteristics were well balanced between the two study groups, with all standardised mean differences (SMDs) below 10%.
Baseline characteristics of the matched population are reported in Table 1.
The median age of the matched population was 82 years. Patients had low-to-intermediate surgical risk as predicted by the STS mortality score, with a median value of 3.2% (IQR 2.1-4.7%).
After analysis of the preprocedural CT characteristics, patients receiving SE devices had smaller sinotubular junctions (STJ; mean diameter 27.5 mm [IQR 25.4-29.9 mm] vs 28.5 mm [IQR 26.6-30.0 mm]; p<0.001), sinuses of Valsalva (SoV; mean diameter 30.5 mm [IQR 28.5-33.0 mm] vs 31.1 mm [IQR 29.0-33.0 mm]; p=0.010) and aortic annuli (perimeter 73.5 mm [IQR 69.0-77.1 mm] vs 74.2 [IQR 70.5-78.3 mm]; p<0.001).
Preprocedural CT characteristics are reported in Supplementary Table 2.
Table 1. Baseline characteristics of the matched population.
| Overall (n=1,174) | PRO (n=587) | ULTRA (n=587) | SMD | |
|---|---|---|---|---|
| Age, years | 82.0 [77.8, 86.1] | 82.0 [78.0, 86.0] | 82.0 [77.0, 86.3] | 0.054 |
| Female sex | 662 (56.4) | 338 (57.6) | 324 (55.2) | 0.048 |
| BMI, kg/m² | 26.4 [23.4, 30.0] | 26.3 [23.2, 30.0] | 26.4 [23.6, 29.8] | 0.006 |
| Hypertension | 1,004 (85.5) | 503 (85.7) | 501 (85.3) | 0.010 |
| Diabetes mellitus | 332 (28.3) | 168 (28.6) | 164 (27.9) | 0.015 |
| Renal failure | 114 (9.7) | 62 (10.6) | 52 (8.9) | 0.081 |
| CAD | 457 (38.9) | 220 (37.5) | 237 (40.4) | 0.059 |
| Prior MI | 122 (10.4) | 61 (10.4) | 61 (10.4) | 0.034 |
| Prior CABG | 65 (5.5) | 32 (5.5) | 33 (5.6) | 0.007 |
| Prior PM | 92 (7.8) | 47 (8.0) | 45 (7.7) | 0.013 |
| PAD | 152 (12.9) | 75 (12.8) | 77 (13.1) | 0.010 |
| AF | 294 (25.0) | 147 (25.0) | 147 (25.0) | <0.001 |
| Prior stroke | 121 (10.3) | 57 (9.7) | 64 (10.9) | 0.039 |
| COPD | 134 (11.4) | 67 (11.4) | 67 (11.4) | 0.066 |
| NYHA Functional Class | 0.121 | |||
| I | 38 (3.2) | 15 (2.6) | 23 (3.9) | |
| II | 425 (36.2) | 202 (34.4) | 223 (38.0) | |
| III | 639 (54.4) | 331 (56.4) | 308 (52.5) | |
| IV | 64 (5.5) | 34 (5.8) | 30 (5.1) | |
| NA | 8 (0.7) | 5 (0.9) | 3 (0.5) | |
| NYHA Class >2 | 703 (59.9) | 365 (62.2) | 338 (57.6) | 0.100 |
| Prior RBBB | 88 (7.5) | 40 (6.8) | 48 (8.2) | 0.067 |
| STS mortality score | 3.2 [2.1, 4.7] | 3.3 [2.3, 4.6] | 3.2 [2.0, 4.7] | 0.053 |
| Echocardiographic characteristics | ||||
| LVEF, % | 60.0 [55.0, 65.0] | 60.0 [55.0, 65.0] | 60.0 [55.0, 65.0] | 0.002 |
| Aortic peak gradient, mmHg | 73.0 [58.8, 86.0] | 73.5 [59.3, 88.8] | 71.0 [58.0, 85.0] | 0.058 |
| Aortic mean gradient, mmHg | 44.0 [36.0, 53.0] | 44.0 [36.0, 54.0] | 44.0 [36.0, 52.0] | 0.018 |
| AVA, cm² | 0.7 [0.6, 0.8] | 0.7 [0.6, 0.8] | 0.7 [0.6, 0.8] | 0.042 |
| Data are presented as n (%) or median [IQR]. AF: atrial fibrillation; AVA: aortic valve area; BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; IQR: interquartile range; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NA: not available; NYHA: New York Heart Association; PAD: peripheral artery disease; PM: pacemaker; RBBB: right bundle branch block; SMD: standardised mean difference; STS: Society of Thoracic Surgeons | ||||
PROCEDURAL CHARACTERISTICS
Procedural details of the matched population are reported in Supplementary Table 3.
Patients treated with the PRO devices more frequently had pre- (42.9% vs 27.5%; p<0.001) and post-dilatation (26.5% vs 5.9%; p<0.001), compared to ULTRA patients.
Moreover, PRO recipients had greater valve oversizing (perimeter oversizing 18.4% vs 3.1%; p<0.001) and received a higher dose of contrast dye during the procedure (median 120 ml vs 100 ml; p<0.001).
STUDY OUTCOMES
In-hospital outcomes and the echocardiographic performance of the bioprostheses in the matched groups are reported in Supplementary Table 4 and Supplementary Table 5.
The primary composite endpoint of 1-year all-cause death, HF rehospitalisation or disabling stroke did not differ between PRO and ULTRA patients (KM estimates 14.0% vs 11.9%; log-rank p=0.27).
Rates of 1-year all-cause death (KM estimates 9.7% vs 10.6%; log-rank p=0.65) and HF rehospitalisation (KM estimates 3.1% vs 2.3%; log-rank p=0.46) were similar between the PRO and ULTRA recipients. Patients treated with PRO devices had higher rates of disabling stroke at 1 year (KM estimates 2.6% vs 0.4%; log-rank p=0.001).
One-year clinical outcomes are reported in the Central illustration and Table 2.
In the 30-day landmark analyses, a greater incidence of disabling stroke was observed, primarily within 30 days of the procedure (1.4% PRO vs 0.0% ULTRA; p=0.004). Subsequently, there was only a trend towards a higher rate of disabling stroke in ULTRA patients (1.3% PRO vs 0.4% ULTRA; p=0.091) (Figure 1).
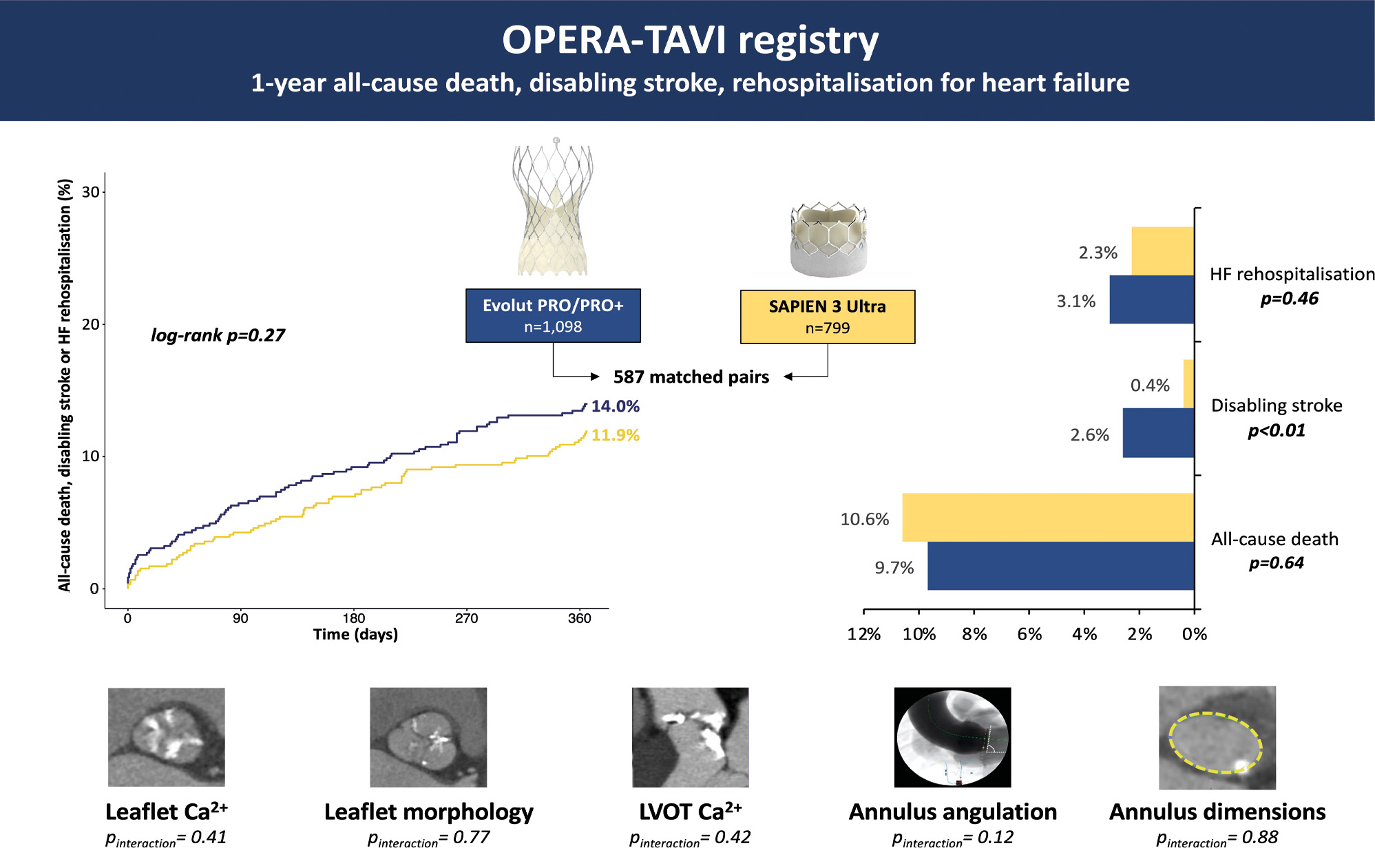
Central illustration. OPERA-TAVI registry: 1-year all-cause death, disabling stroke, rehospitalisation for heart failure. Ca²+: calcium; HF: heart failure; LVOT: left ventricular outflow tract
Table 2. One-year clinical outcomes of the matched population.
| PRO (n=587) | ULTRA (n=587) | HR (95% CI) | p-value | |
|---|---|---|---|---|
| Composite endpoint | 14.0 | 11.9 | 0.84 (0.61-1.15) | 0.274 |
| All-cause death | 9.7 | 10.6 | 1.09 (0.76.1.56) | 0.645 |
| Disabling stroke | 2.6 | 0.4 | 0.13 (0.03-0.58) | 0.007 |
| Rehospitalisation for HF | 3.1 | 2.3 | 0.76 (0.37-1.57) | 0.457 |
| Data are presented as %. CI: confidence interval; HF: heart failure; HR: hazard ratio | ||||
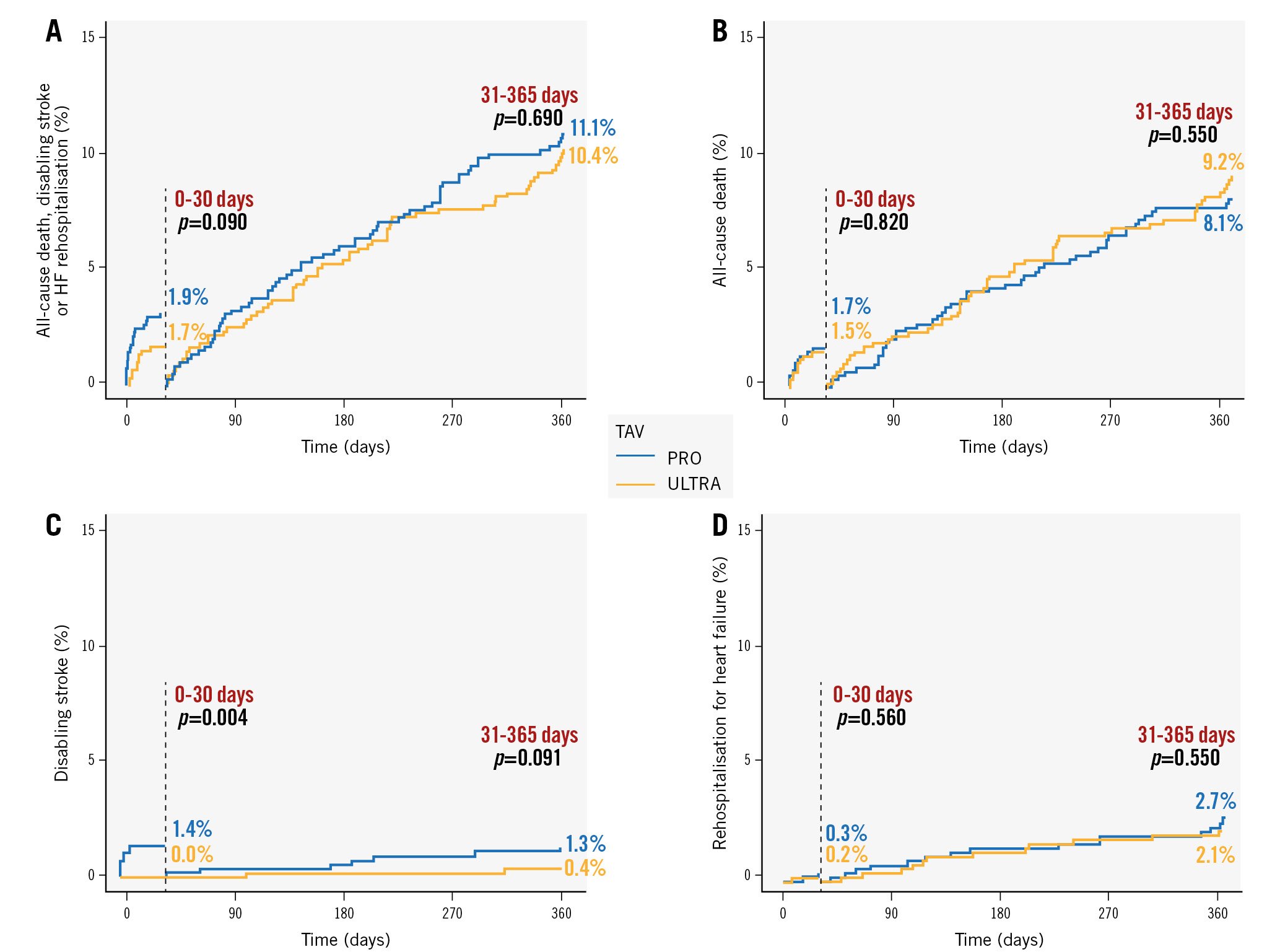
Figure 1. Thirty-day landmark analyses for the composite primary endpoint and its individual components. A) Composite primary endpoint, B) all-cause death, C) disabling stroke and D) rehospitalisation for heart failure. HF: heart failure; TAV: transcatheter aortic valve
SUBGROUP ANALYSIS
Primary and secondary outcomes were analysed separately according to patients’ native annulus dimensions and angulation, leaflet morphology, LVOT and leaflet calcification grades.
Outcomes in each subgroup of patients were consistent with those reported in the whole study population (all pinteraction>0.10) (Figure 2, Supplementary Figures 1-5).
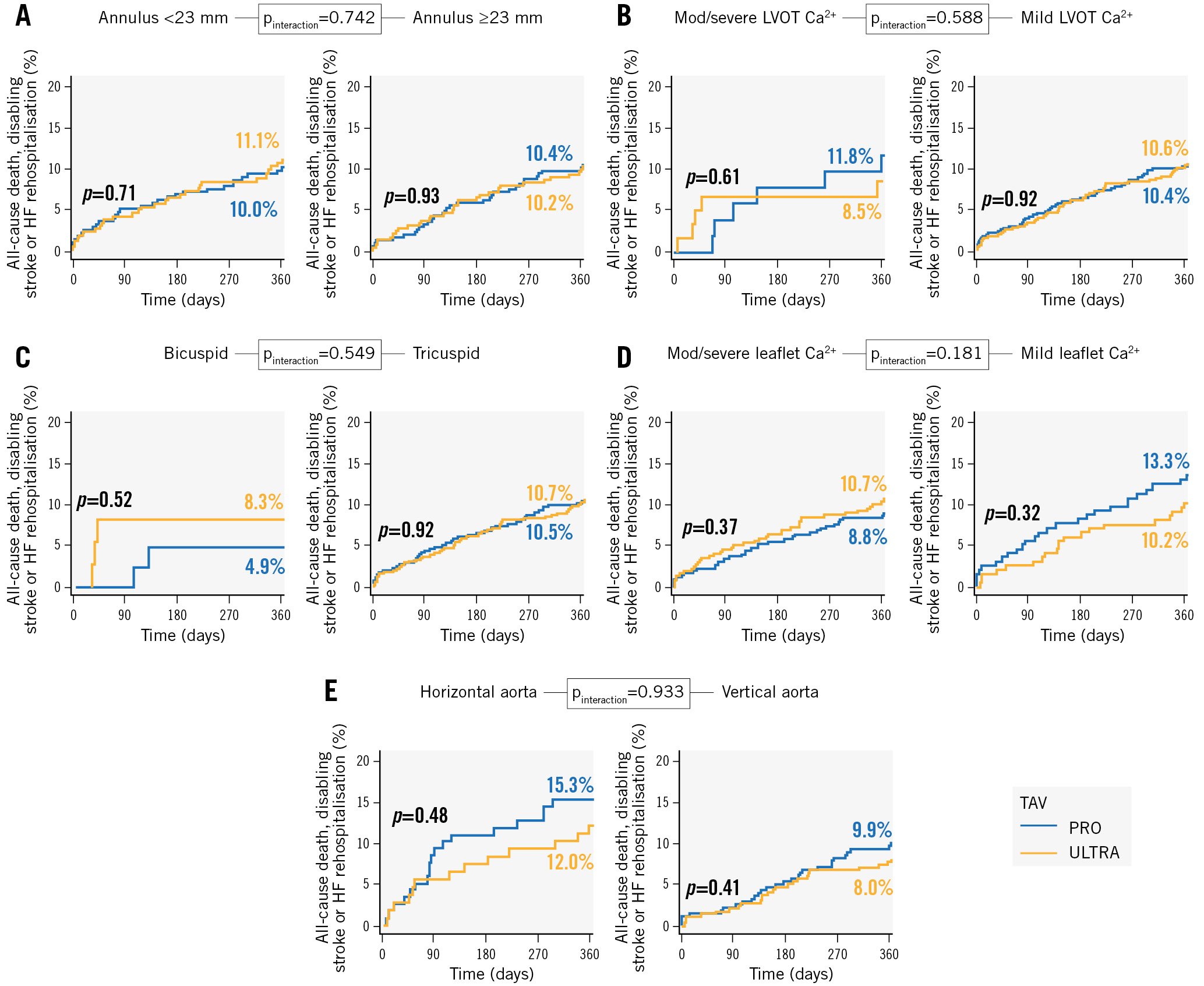
Figure 2. Subgroup analysis of the primary composite endpoint in the five prespecified anatomical subsets. A) Annulus dimension. B) LVOT calcifications. C) Leaflet morphology. D) Leaflet calcifications. E) Annulus angulation. Ca²: calcium; HF: heart failure; LVOT: left ventricular outflow tract; TAV: transcatheter aortic valve
Discussion
During the last fifteen years, several studies have compared clinical outcomes and device performance in patients undergoing TAVI with different device iterations456789. OPERA-TAVI was the first registry to report outcomes of patients undergoing TAVI who received the latest PRO or ULTRA TAVI platforms3. In the present analysis, we aimed to compare the midterm clinical outcomes of patients receiving these two platforms. Additionally, we sought to investigate potential differences in specific anatomical subsets that present challenges, for which one platform has been hypothesised to potentially outperform the other, and vice versa.
The main findings of the study were as follows:
1) At 1 year, PRO and ULTRA patients exhibited comparable rates of the composite outcome, which included all-cause mortality, disabling stroke, and rehospitalisation due to HF.
2) Patients receiving PRO devices had higher rates of disabling stroke, with the higher incidence predominantly confined to the first 30 days after TAVI.
3) Across all prespecified anatomical subgroups of patients, clinical outcomes did not differ between the two study groups.
4) Differences in bioprosthetic haemodynamics did not have an impact on clinical outcomes.
A total of 1,174 consecutive patients at low-to-intermediate surgical risk undergoing transfemoral TAVI in real-world practice with PRO or ULTRA devices were compared in the present analysis. At 1 year, the primary composite endpoint of all-cause death, disabling stroke or HF rehospitalisation did not differ between patients receiving PRO or ULTRA TAVI devices (14.0% vs 11.9%; log-rank p=0.27). The rate of all-cause death was not statistically different between the study devices at 1 year, nor the rate of HF rehospitalisation. Nevertheless, patients receiving the PRO devices showed higher rates of disabling stroke (2.6% vs 0.4%). This datum was in contrast with that previously reported in the SOLVE-TAVI (CompariSon of secOnd-generation seLf-expandable vs. balloon-expandable Valves and gEneral vs. local anesthesia in Transcatheter Aortic Valve Implantation) randomised clinical trial, which reported significantly higher stroke rates in patients receiving previous-generation BE valves (BE: 6.1% vs SE: 0.8%, HR 6.63; p=0.013)6. The landmark analysis showed that the increased risk of stroke was mostly confined to the first 30 days after TAVI (1.4 % vs 0.0%; p=0.004), with no significant difference after 30 days (p=0.091). As previously discussed in the analysis of the OPERA-TAVI registry3, the higher rates of pre- and post-dilatation observed in the PRO groups could have affected this finding in our analysis1011. Besides, one can speculate that this difference might be related to the difference in flexibility of the two delivery systems. Indeed, the PRO delivery system is more rigid than that of the ULTRA device, which also has the possibility to mechanically flex its distal part to facilitate the crossing of the aortic arch. It is also possible that the PRO system scratches the aortic arch during crossing manoeuvres, displacing calcium particles and debris that may embolise in the cerebral vessels. Similarly, the PRO system might displace calcium particles during the crossing of the native aortic valve, as this device is more difficult to centre and to place coaxially compared to the ULTRA TAVI platform. The next-generation Evolut FX (Medtronic) TAVI system promises to significantly improve this key aspect and, therefore, improve patient outcomes.
We assessed clinical outcomes in five prespecified subgroups of patients with different anatomical characteristics that might have lead to intrinsic procedural challenges and, therefore, suboptimal results1213141516. At 1 year, clinical outcomes of each subgroup were similar to those of the whole study population. No significant interactions in annuli dimensions and angulation, leaflet and LVOT calcification grades, and leaflet morphologies with valve-specific outcomes were detected. Based on the results of our analysis, it can be assumed that both the PRO and ULTRA devices were equally safe and effective, even in challenging anatomies, when TAVI is performed by expert operators. Along with the technical improvements brought by TAVI platform iterations, the increasing expertise in pre-TAVI computed tomography angiography assessment and procedural planning may play an important role in the optimisation of TAVI procedures in real-world practice.
Despite comparable midterm outcomes, residual transprosthetic gradients were significantly lower in patients treated with the PRO devices; these patients exhibited larger indexed effective orifice areas. This evidence confirmed the benefit of the supra-annular design of the PRO devices, in line with previous studies comparing the two TAVI platforms17. Remarkably, the ULTRA device had higher rates of mean residual transprosthetic gradients greater than 20 mmHg. This datum is of particular interest, as it was shown to be associated with higher rates of long-term mortality18. However, despite higher rates of patient-prosthesis mismatch (PPM) after TAVI in patients receiving the ULTRA device, no difference in patients with severe grade of PPM was encountered between PRO and ULTRA recipients19.
Contrarily, the device performances in terms of paravalvular regurgitation (PVR) were favourable to the ULTRA device.
Of note, the rates of moderate-to-severe PVR were similar between the two devices, with lower 1-year rates for PRO recipients when compared to those reported for its predecessor in the SOLVE-TAVI trial6. Nevertheless, the overall PVR rate was significantly lower in ULTRA recipients, attributable to the lower number of patients with mild PVR.
Although the role of moderate to severe PVR after TAVR on midterm outcomes has been largely investigated15202122232425, the clinical impact of residual mild PVR after TAVI is a matter of ongoing debate. In the PARTNER-1 trial26, mild PVR was associated with higher mortality at 5 years after TAVI in a high-risk population. On the contrary, the results of the PARTNER-2 trial, which enrolled intermediate-risk patients, did not show an association between mild PVR and long-term clinical outcomes27. A recent meta-analysis showed that mild PVR was associated with a higher risk of mortality and rehospitalisation in the long term, regardless of the type of transcatheter aortic valve implanted, and that the impact of mild PVR on clinical outcomes increases over the years28.
Longer-term, robust follow-up data from prospective, randomised studies are awaited to analyse the real impact of devices’ haemodynamic differences on clinical outcomes.
Limitations
This was an observational study without independent adjudication of events or independent core laboratory imaging analysis. Although PSM adjustment resulted in 2 groups for comparison with homogeneous baseline characteristics, unmeasured confounders might have remained and could have potentially affected the results because of the non-randomised nature of the study. Finally, the registry did not collect data regarding specific procedural challenges (i.e., aortic arch angulation and stretchability), which could have influenced clinical outcomes.
Conclusions
In the real-world OPERA-TAVI registry, patients undergoing TAVI using PRO and ULTRA devices exhibited comparable rates of the composite endpoint of all-cause mortality, rehospitalisation for heart failure, or disabling stroke at 1 year. However, those who received the PRO devices had higher rates of disabling stroke, particularly within the initial 30 days following the procedure. These results remained uniform across various anatomical subsets of the aortic root. In spite of these similar clinical outcomes, the PRO devices demonstrated higher rates of PVR, while exhibiting lower transprosthetic gradients after the TAVI procedure. Ad hoc randomised clinical trials are required to validate the findings of this study and to specifically compare the two devices in peculiar anatomical subsets.
Impact on daily practice
Different refinements in TAVI platforms have contributed to the improvement of patient outcomes seen in randomised clinical trials over the past decade. OPERA-TAVI was the first registry to compare the latest PRO and ULTRA devices in consecutive patients undergoing transfemoral TAVI in current real-world practice. In this analysis, the two TAVI iterations showed similar 1-year clinical outcomes in terms of all-cause death, disabling stroke or rehospitalisation for heart failure, but the PRO devices yielded higher rates of disabling stroke, with the increased risk confined to the first 30 days after the procedure. Outcomes were consistent across different subsets of aortic root anatomies. Longer-term follow-up data from prospective studies are eagerly awaited to analyse the impact of devices’ haemodynamic differences on clinical outcomes.
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Conflict of interest statement
T. Pilgrim reports research, travel or educational grants to the institution, without personal remuneration, from Biotronik, Boston Scientific, Edwards Lifesciences, and ATsens; speaker fees and/or consultancy fees to the institution from Biotronik, Boston Scientific, Edwards Lifesciences, Abbott, Medtronic, Biosensors, and HighLife. M. Abdel-Wahab has reported that his institution received speaker honoraria and/or consultancy fees on his behalf from Boston Scientific and Medtronic. P. Garot has reported serving as medical director and being a shareholder of CERC, a CRO dedicated to cardiovascular diseases; and has received speaker/consultancy fees from Abbott, Biosensors, Boston Scientific, Edwards Lifesciences, and GE HealthCare. A. Latib has reported serving on advisory boards or as a consultant for Medtronic, Boston Scientific, Philips, Edwards Lifesciences, and Abbott. D. Mylotte is a consultant for Medtronic, Boston Scientific, and MicroPort. O. De Backer has received institutional research grants and/or consulting fees from Abbott and Boston Scientific. M. Akodad has reported receiving research funding from Medtronic, Biotronik, MUSE-Explore, Villa M, and La Federation Française de Cardiologie. D. Meier is supported by the Swiss National Science Foundation (grant P2LAP3_199561). L. Sondergaard has received consultancy fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic, and SMT. C. Tamburino is a consultant for Medtronic. M. Barbanti has reported that he is a consultant for Boston Scientific, Edwards Lifesciences, and Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.

