
Abstract
Aims: Data on procedural and clinical outcomes after transcatheter aortic valve implantation (TAVI) with the new-generation self-expanding Medtronic Evolut R prosthesis in comparison with its predecessor, the Medtronic CoreValve, are scarce. The aim of this study was to assess the safety and efficacy of the Evolut R device compared with the former-generation CoreValve.
Methods and results: In a nationwide, prospective, multicentre cohort study (Swiss TAVI registry, NCT01368250), outcomes of consecutive transfemoral TAVI patients treated with the new-generation Medtronic Evolut R (September 2014 - February 2016) and the Medtronic CoreValve (February 2011 - February 2016) were investigated. Events were reported according to VARC-2 and adjudicated by a clinical events committee. During the study period, 317 and 678 consecutive patients underwent TAVI with the Evolut R and the CoreValve bioprosthesis, respectively. Baseline clinical characteristics between the groups were comparable, although Evolut R patients were lower risk according to the STS score (4.8±3.4% vs. 6.9±5.0%, p<0.001) and logistic EuroSCORE (17.3±13% vs. 20.1±13%, p=0.009). Implantation of the Evolut R was associated with a lower use of predilatation (48.1% vs. 72.4%, p<0.001), a shorter procedure time (67.9±36 min vs. 76.7±42 min, p=0.002), and less contrast dye use during the procedure (155.2±98 ml vs. 208.0±117 ml, p<0.001). Post-procedural mean gradient was comparable (7.4±4.7 mmHg vs. 7.5±5.0 mmHg), as were the 30-day rates of moderate to severe aortic regurgitation (8.5% vs. 10.5%), major vascular (9.8% vs. 10.3%) and life-threatening bleeding complications (5.4% vs. 5.3%), disabling stroke (1.9% vs. 1.6%), all-cause mortality (3.2% vs. 3.4%) as well as permanent pacemaker implantation (22.1% vs. 23.4%).
Conclusions: Thirty-day clinical outcomes were favourable and comparable between the Evolut R and the CoreValve bioprosthesis.
Abbreviations
CE: Conformité Européenne
Fr: French
MACCE: major adverse cardiac and cerebrovascular events
PCI: percutaneous coronary intervention
PVL: paravalvular leakage
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve implantation1 (TAVI) has developed from an experimental procedure into a routine intervention, especially suitable for elderly or high-risk patients. The recent availability of new-generation self-expanding transcatheter heart valves (THV) –with enhanced features, allowing recapture and repositioning of the prosthesis during valve deployment– may further improve outcomes after TAVI.
The latest CoreValve generation, the CoreValve® Evolut® R (referred to as Evolut R; Medtronic, Minneapolis, MN, USA), received CE mark approval in September 2014 for the 23 mm THV and in February 2015 for the 26 and 29 mm devices. As a consequence, the Evolut R has replaced its predecessor in Switzerland with the exception of the 31 mm device (used in aortic annulus dimensions between 26 mm and 29 mm), which remains the previous-generation CoreValve® (Medtronic).
Currently, periprocedural and clinical outcome data for the Evolut R are limited to the CE mark trial2 and a single-centre experience3. The CE mark trial included 60 selected patients, mainly treated by a transfemoral approach (98.3%), and showed favourable clinical outcomes without death or stroke events at 30-day follow-up2. In order to assess the safety and efficacy of the Evolut R device, periprocedural characteristics and clinical outcomes were investigated in a real-world patient population and compared with the former-generation CoreValve.
Methods
The Swiss TAVI registry (ClinicalTrials.gov NCT01368250) is an ongoing national, prospective cohort study aiming for consecutive patient enrolment (mandatory for participating centres), data monitoring and event adjudication by a clinical events committee according to the Valve Academic Research Consortium (VARC)-2 criteria4,5. Initiated in 2011, the registry was designed to evaluate thoroughly patient characteristics, procedural details as well as short- and long-term outcomes after TAVI with CE-approved devices6.
For the purposes of this analysis, all patients from the Swiss TAVI registry undergoing TAVI with the CoreValve and the Evolut R using femoral access were considered eligible. Patients were treated with the CoreValve between February 2011 and February 2016, whereas the Evolut R was used from September 2014 (23 mm) and February 2015 (26 and 29 mm) until February 2016. In the absence of a larger than 29 mm Evolut R device, clinical outcomes of patients receiving the CoreValve 31 mm were excluded from the main analysis.
The Swiss TAVI registry protocol was approved by the local ethics committee at each participating centre. All patients provided written informed consent for prospective follow-up assessment. The registry was initiated and is performed under the lead of the Swiss Cardiovascular Center at Bern University Hospital, Bern, Switzerland. The Clinical Trials Unit Bern is responsible for data management and independent statistical analysis.
Statistical analysis
Continuous parameters are reported as mean±standard deviation (SD), and categorical variables are reported as numbers of patients (% of patients). Events are reported as counts of first occurrence per (sub-)type of event within 30 days of follow-up (% of all patients). Event rates at 30 days for patients treated with the Evolut R versus the CoreValve prosthesis were compared using Cox regressions, censoring patients at death or lost to follow-up. Reported are crude hazard ratios (HR with 95% confidence intervals) with p-values from Wald chi-square tests, or continuity corrected risk ratios with p-values from Fisher’s exact tests in case of zero events in one of the two prosthesis patient groups. Reported are adjusted HR (95% CI) using the following procedure: 1) 20 data sets were created imputing the missing values using chained equations (multiple imputation). 2) The estimates of the adjusted HR after multiple imputations were combined using Rubin’s rule and are presented with adjusted p-values. 3) Adjustment was for age (years), history of myocardial infarction, Canadian Cardiovascular Society angina class, STS risk score, procedure time (min), amount of contrast dye (ml), general anaesthesia and concomitant PCI (selected on the basis of an univariable p-value <0.2 comparing the two valves) and TAVI procedure date (i.e., potential general learning effect over time). No adjusted analyses were performed for outcomes with less than five events overall. Two-sided p-values <0.05 were considered statistically significant. All analyses were performed with Stata version 14 (StataCorp, College Station, TX, USA).
Results
PATIENT POPULATION
During the inclusion period, 317 consecutive patients were treated with the Evolut R and 678 patients with the 23 mm, 26 mm, or 29 mm CoreValve. Baseline clinical characteristics are detailed in Table 1: they were well balanced between both groups except for the STS and the logistic EuroSCORE, which were significantly lower in the Evolut R group (4.8±3.4% vs. 6.9±5.0%, p<0.001; and 17.3±12.9% vs. 20.1±13.2%, p=0.009, respectively). Specifically, no significant differences were found for age (82.1±6.4 vs. 82.8±6.3 years), gender (females 64.0% vs. 62.1%) and preprocedural aortic valve area (0.69±0.2 cm2 vs. 0.69±0.3 cm2) between Evolut R and CoreValve patients, respectively.
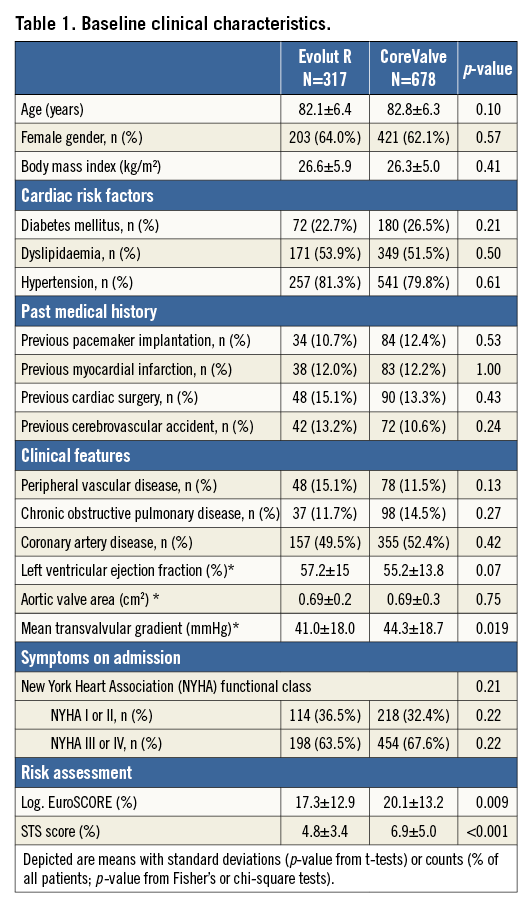
PROCEDURAL CHARACTERISTICS
Procedural characteristics are depicted in Table 2. Patients treated with the Evolut R had shorter procedural times (67.9±35.6 min vs. 76.7±41.8 min, p=0.002), received less contrast dye (152.4±90.0 ml vs. 208.1±118.9 ml, p<0.001), less frequently required general anaesthesia (26.8% vs. 40.7%, p<0.001), and were less frequently treated with predilatation (44.5% vs. 71.8%, p<0.001) compared with CoreValve patients. Post-procedural mean gradient, aortic valve area and PVL were comparable between the two groups.
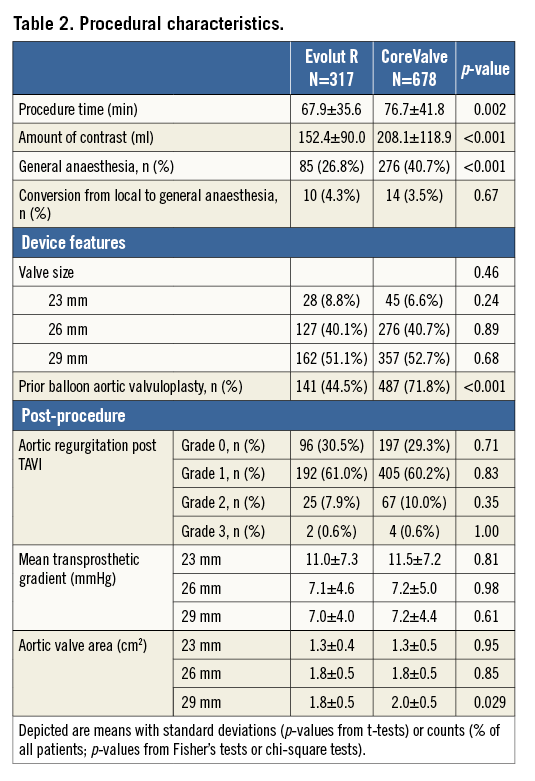
CLINICAL OUTCOMES
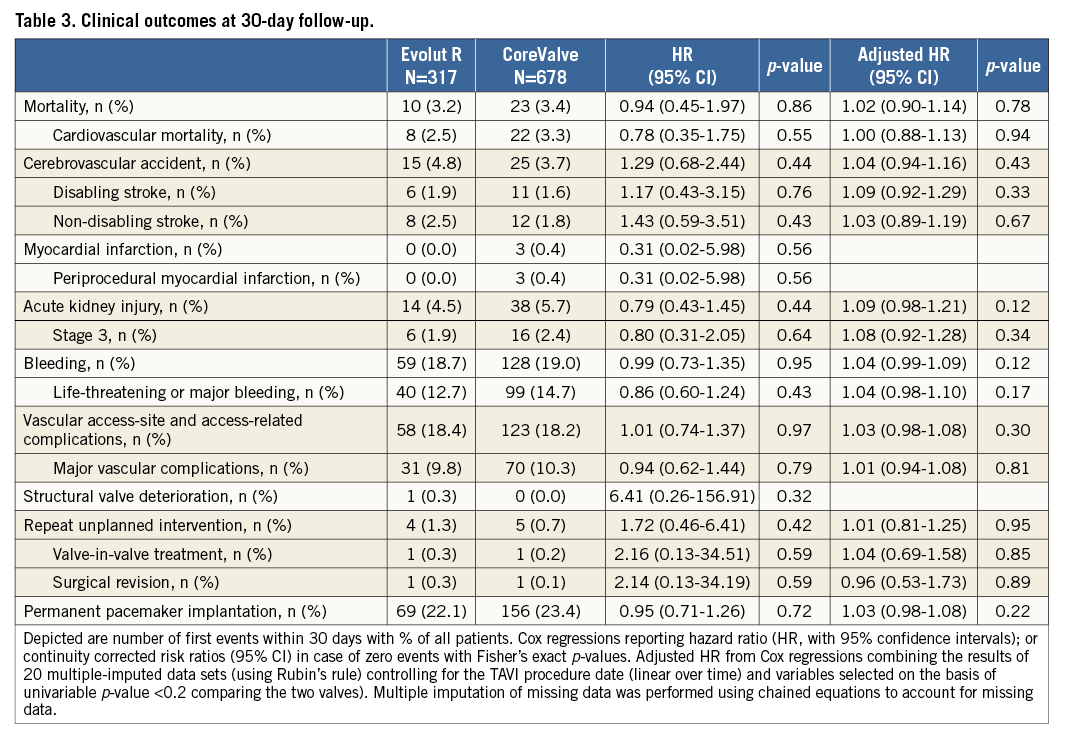
Periprocedural clinical outcomes at 30 days are detailed in Table 3. No significant differences were found for death (3.2% vs. 3.4%, HRadj 1.02, 95% CI: 0.90-1.14), disabling stroke (1.9% vs. 1.6%, HRadj 1.09, 95% CI: 0.92-1.29), major vascular (9.8% vs. 10.3%, HRadj 1.01, 95% CI: 0.94-1.08) and bleeding complications (18.7% vs. 19.0%, HRadj 1.04, 95% CI: 0.99-1.09). Moreover, VARC-2 stage 3 kidney injury (1.9% vs. 2.4%, HRadj 1.08, 95% CI: 0.92-1.28) and rates of permanent pacemaker (PPM) implantation after TAVI were comparable (22.1% vs. 23.4%, HRadj 1.03, 95% CI: 0.98-1.08) between the Evolut R and CoreValve patients.
Discussion
Our study is the largest report on periprocedural and 30-day outcomes comparing the new-generation Evolut R device and the previous CoreValve generation in a nationwide prospective cohort study of consecutive patients undergoing TAVI. The main findings can be summarised as follows:
1. Clinical outcomes at 30 days were favourable with the Evolut R, with low morbidity and mortality rates in a consecutive, real-world patient population.
2. Clinical outcomes of the Evolut R were comparable with the previous-generation CoreValve.
3. The procedural duration with the Evolut R was significantly shorter, less contrast dye was required and there were lower rates of predilatation when compared with the CoreValve.
Valve-related outcomes were similar between the two devices with overall low PVL rates and comparable transvalvular gradients within the respective device sizes.
Despite the lower STS score and the logistic EuroSCORE of the Evolut R population, we could not demonstrate a reduction in mortality with the new device. However, the observed 30-day mortality rates (3.2% for Evolut R and 3.4% for CoreValve) were lower than expected according to the STS score. Our results compare well with other national TAVI registries reporting periprocedural mortality rates between 4.2% and 9.7%7-11. Also, our mortality rates are comparable to the 30-day results of the new-generation balloon-expandable Edwards SAPIEN 3 prosthesis (Edwards Lifesciences Inc., Irvine, CA, USA) (all-cause mortality 3.3%) in a similar patient population in Switzerland12.
The Evolut R is currently delivered with the EnVeo-R catheter and the integrated InLine sheath (both Medtronic) –with a 14 Fr equivalent profile corresponding to a true 18 Fr outer diameter– allowing sheathless implantation of the Evolut R device. With these specifications, the Evolut R is currently considered the device with the lowest delivery profile on the market, a factor potentially reducing the rate of vascular access-related complications. While Barbanti et al were able to prove this association and showed that lower profile sheaths (14 Fr-18 Fr) were linked to significantly fewer major vascular complications compared to larger vascular access sheath dimensions (19 Fr-24 Fr)13, we were not able to show significant differences between the previous-generation CoreValve device (18 Fr delivery sheath) and the Evolut R (14 Fr delivery profile). Indeed, major vascular and access-related complications were observed in 10.3% of patients receiving the CoreValve and 9.8% of patients receiving the Evolut R (HRadj 1.01, 95% CI: 0.94-1.08) in the present analysis. However, the rate of major vascular complications in our Evolut R patient series (9.8%) is comparable with the rate of vascular complications reported in the Evolut R CE mark trial (8.7%)2 as well as in a recent publication from the Swiss TAVI registry in which the rate of major vascular complications was 9.3% for the Edwards SAPIEN 3 (815 cases) and 7.2% for the Lotus™ valve (140 cases) (Boston Scientific, Marlborough, MA, USA)14. A detailed and thorough comparison of vascular access-site complication rates would demand analyses adjusting for anatomical factors such as calcification and tortuosity of the vasculature, the type of access to the vessel and the use of different closure devices. Furthermore, a specific selection bias cannot be excluded, as the lower profile of the Evolut R system might have led to its more frequent use for small femoral vasculature and difficult access sites. As a consequence, the benefit of the lower profile may have been counterbalanced by challenging anatomical circumstances.
The need for PPM was comparable between devices and amounted to 22.1% after the Evolut R and 23.4% after the CoreValve in our study. This observation was surprising and unexpectedly high, given the low PPM rate in the Evolut R CE mark trial (11.7%)2. However, in a recent single-centre report from Heidelberg, the PPM rate amounted to 23.3% in 100 Evolut R cases3, which is comparable to ours. Previous studies have extensively studied independent predictors of PPM after TAVI with different devices: in addition to individual pre-existing conduction disturbances, the device implantation depth has been shown to be independently associated with a higher rate of conduction abnormalities and the need for PPM after TAVI15. Similarly, the rate of PPM after the CoreValve was found to be lowest (10.6%) in cases where the implantation depth was between 0 and 6 mm within the left ventricular outflow tract16. The association between implantation depth and PPM is similar for the Evolut R. An analysis of patients in the CE mark trial2 showed no PPM in patients with an implantation depth of 3.3±2.5 mm, and a new PPM in patients with an implantation depth of 8.1±3.5 mm. While the Evolut R is supposed to be more stable during the implantation phase, thereby enhancing the operator positioning accuracy and ensuring an optimal placement in the desired annular position, this did not translate into a lower rate of PPM when compared to the previous-generation CoreValve in our study of unselected, real-world patients. All efforts should be made to reduce the rate of conduction disturbances and the need for PPM, as conflicting evidence is currently available on the benign nature of PPM implantation in this setting17,18.
In our study, we observed a significantly lower rate of predilatation among patients treated with the Evolut R compared to the CoreValve (44.5% vs. 71.8%), which contributed to the shorter procedure duration. In order to minimise the interaction with the calcified native aortic valve and avoid the risk of embolism of calcified debris, there is a general trend of limiting predilatation. However, there is conflicting evidence in the literature on the safety of direct valve implantation. While some small series report similar outcomes after valve implantation without predilatation19,20, some others provide evidence of a higher rate of major adverse cardiac and cerebrovascular events (MACCE) mainly driven by an increased risk of stroke during the periprocedural period among patients treated without predilatation21. The effect of predilatation on clinical outcome is being assessed in the randomised “Transcatheter Aortic Valve Implantation Without Predilatation (SIMPLIFy-TAVI) study” (NCT01539746) and the “Balloon Expandable Transcatheter Aortic Valve Implantation Without Predilatation of Aortic Valve (EASE-IT) study” (NCT02127580) which will shed further light on this issue.
Rates of significant PVL (i.e., ≥2) were comparable and without significant differences between the Evolut R and the CoreValve in this study (8.5% vs. 10.6%). While the rate with the Evolut R was found to be higher compared with the rate in the CE mark trial (8.5% vs. 3.4%)2, our results with the CoreValve are in line with the rate of PVL ≥2 reported in the US pivotal trials (10.7% for extreme risk, 9.0% for high-risk patients)22,23. In comparison, the rate of PVL ≥2 with the SAPIEN 3 was 3.4% in the largest series (>1,600 patients)24 and 3.5% in the first multicentre trial including 150 patients25, whereas, in the previous report of the Swiss TAVI registry, the rate of PVL ≥2 was 1.3%12. The Lotus valve has also shown a low rate of PVL ≥2 at 1.9% in the REPRISE-II study26.
Limitations
The results of the present study need to be interpreted with the following limitations. First, the number of patients is limited in the Evolut R group and, for the purposes of this analysis, only periprocedural events have been used to focus on potential differences with the previous-generation CoreValve device. Second, periprocedural results are self-reported and, in the absence of a central core laboratory for the evaluation of echocardiographic results, there might be some heterogeneity in the assessment of PVL. However, PVL was assessed by experienced echocardiographers for both devices, which reduces centre-specific assessment as a confounder. Third, serious adverse events were self-reported; however, all subjects underwent central data monitoring and events were systematically adjudicated by a dedicated clinical events committee using VARC-2 criteria. Fourth, no technical or specific details on the procedure itself are available, including rates of valvular re-sheathing and numbers of valve placement attempts for the Evolut R as well as the number of predilatations or post-dilatations, which might all have an impact on procedural results. Finally, no detailed information on vascular access-site anatomy was collected, precluding a sophisticated and thorough analysis investigating the benefit of the lower vascular sheath dimensions of the Evolut R, and no detailed information on the device implantation depth was collected in order to correlate the PPM rate and implantation depth.
Conclusions
The use of the new-generation Evolut R self-expanding prosthesis is associated with low morbidity and mortality rates at 30-day follow-up. Overall, the results of the Evolut R appear comparable to the Medtronic CoreValve THV. The use of the Evolut R device appears to facilitate a faster and simpler TAVI procedure as the duration is shorter, more commonly performed under local anaesthesia and with less contrast dye. Longer-term follow-up is needed to compare the two devices further with respect to PVL evolution.
| Impact on daily practice In a consecutive, real-world patient population, 30-day clinical outcomes of the new-generation Evolut R self-expanding prosthesis were comparable with the previous-generation CoreValve. Valve-related outcomes were also similar between the two devices, with overall low PVL rates and comparable transvalvular gradients within the respective device sizes. The use of the Evolut R device appears to facilitate a faster and simpler TAVI procedure as the duration is shorter, more commonly performed under local anaesthesia and with less contrast dye. |
Funding
The Swiss TAVI registry is supported (unrestricted funding) by: Swiss Working Group of Interventional Cardiology, Swiss Heart Foundation, Boston Scientific, Edwards Lifesciences, Medtronic, St. Jude Medical.
Conflict of interest statement
S. Noble serves as a consultant to Medtronic. M. Roffi has received institutional research grants from Abbott Vascular, Boston Scientific, Biotronik, Biosensors and Medtronic. R. Jeger serves as a consultant to St. Jude Medical and has received reimbursement for travel expenses from Medtronic, Boston Scientific and Edwards Lifesciences. S. Toggweiler has received speaker fees from Edwards Lifesciences, Symetis and Medtronic, and is a Symetis proctor and consultant for NVT. E. Ferrari is an Edwards Lifesciences proctor. P. Jüni is an unpaid steering committee or statistical executive committee member of trials funded by Abbott Vascular, Biosensors, Medtronic and Johnson & Johnson. F. Nietlispach serves as a consultant to Edwards Lifesciences, Medtronic, St. Jude Medical, 4Tech and has received speaker’s fees from Biotronik. M. Taramasso serves as a consultant for St. Jude Medical. C. Huber is an Edwards Lifesciences proctor and consultant for Medtronic. S. Windecker has received institutional research contracts from Abbott, Boston Scientific, Biosensors, Cordis, Medtronic, Biotronik, Edwards Lifesciences and St. Jude. P. Wenaweser serves as proctor for Medtronic, Edwards Lifesciences and Boston Scientific, and has received an unrestricted institutional grant from Medtronic (University of Bern). CTU Bern (part of Bern University) has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in the design, conduct or analysis of clinical studies funded by the industry. D. Tueller received speaker fees from Edwards Lifesciences and travel expenses from Medtronic. F. Maisano is a consultant for Medtronic, Edwards Lifesciences, St. Jude, Abbott Vascular and Valtech Cardio and he receives royalties from Edwards. The other authors have no conflicts of interest to declare.

