Description - technical specifications
The Medtronic CoreValve Evolut RTM (Medtronic, Minneapolis, MN, USA) provides several refinements to improve anatomical fit, annular sealing, and durability. In particular, the device is designed to enable recapturability and repositionability. With the introduction of the full Evolut R product family (23, 26, 29, and 31 mm), annulus sizes from 18 mm to 29 mm can be treated.
The Evolut frame is tailored to reduce the overall height, approximately 10% shorter than the original CoreValve frame, while preserving the height of the pericardial skirt (12 mm) with an extended skirt of the inflow tract to provide a seal against paravalvular leakage (Figure 1). The shortened valve height is designed to optimise fit, especially in angulated anatomy. The Evolut R maintains the cell geometry of the recent CoreValve prosthesis to optimise frame conformance to the native aortic annulus, especially in patients with non-circular or asymmetrically calcified annulus.
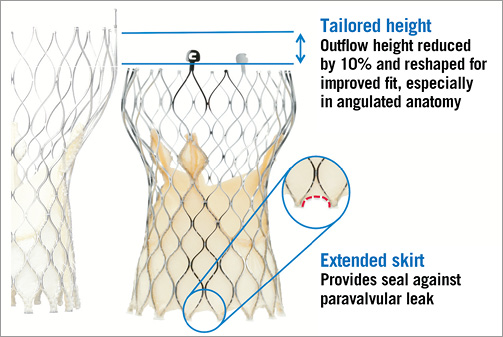
Figure 1. Design modifications of the new Medtronic CoreValve Evolut R.
Implantation procedure
The Evolut R will be delivered with the EnVeo R delivery system (Medtronic). This next-generation delivery system is designed to have a stable and predictable deployment with proper implantation depth. However, in some circumstances, if repositioning is necessary, the ability to resheath, reposition, and redeploy the valve is an advantage over the recent AccuTrak system (Medtronic) (Figure 2). The deployment of the Evolut R –especially when the valve is “flowering”– will be more predictable through the EnVeo handle with 1:1 response and an improved valve release mechanism (Figure 3).
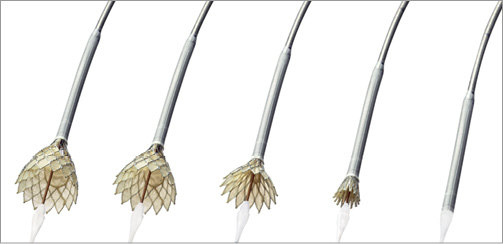
Figure 2. Recapturing the new CoreValve Evolut R.
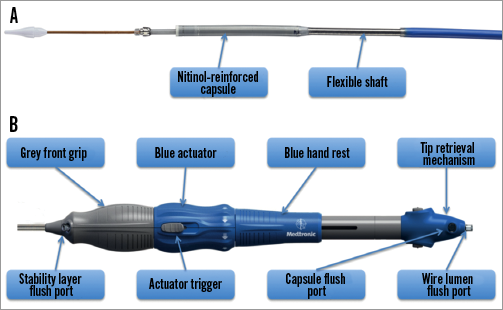
Figure 3. Features of the new EnVeo delivery catheter.
To prevent vascular complications, the Evolut R system will use the new, catheter-mounted InLine sheath, which eliminates the need for an external sheath. This sheath has a lower profile with an 18 Fr outer diameter - compared to 22 Fr outer diameter sheaths of the recent CoreValve system - and is equivalent to 14 Fr inner diameter systems of other transcatheter heart valve (THV) manufacturers. Once fully inserted, the InLine sheath maintains haemostasis while it remains in place, and the valve is advanced into final position for the deployment (Figure 4). However, post-dilation of the prosthesis in patients with suboptimal frame expansion and consecutive paravalvular aortic regurgitation would require a sheath exchange.
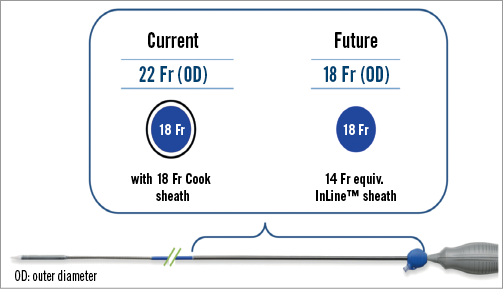
Figure 4. Size of the EnVeo system compared to a recent transfemoral sheath with 18 Fr (inner diameter).
Clinical data
The first-in-man study for the Evolut R delivered with the EnVeo delivery system will start in early autumn 2013 including four centres in Germany, United Kingdom, and Australia. In this study it is planned to enrol approximately 60 patients.
Discussion
The next generation of transcatheter heart valves promises to simplify transcatheter aortic valve implantation (TAVI) and to address TAVI-related shortcomings such as paravalvular aortic regurgitation and conduction disturbances. The design of the recent Medtronic CoreValve prosthesis is characterised by the self-expanding nitinol frame design with a porcine pericardial tissue valve in supra-annular position. Today, four valve sizes are available (23, 26, 29, and 31 mm) and cover aortic annuli with a diameter from 18 to 29 mm. All valve sizes are delivered with the 18 Fr AccuTrak delivery system (Medtronic). The ADVANCE study with 1,015 patients treated at 44 experienced TAVI centres in 12 countries in Western Europe, Asia and South America has demonstrated high success (97.5%) and a low 30-day mortality rate (4.5%) for TAVI with the CoreValve prosthesis1.
With the new prosthesis frame design and extended pericardial skirt, the Evolut R provides more seal against paravalvular aortic regurgitation, while the maintained cell design and the supra-annular position of the valve will serve a broad patient population, regardless of whether the aortic annulus is non-circular and/or calcified. In patients in whom a proper positioning of the THV is not achieved the first time, the new recapture and repositioning technology will help to ensure deployment of the prosthesis at the desired implantation depth and thus make the procedure more predictable. These features might further reduce the rate of clinically significant paravalvular aortic regurgitation and the new onset of conduction disturbances requiring pacemaker implantation. In addition, the reduced sheath diameter with the use of the InLine sheath will help to prevent vascular complications.
Future studies have to elucidate whether design modifications of the upcoming second-generation THVs can be translated into a survival benefit for patients undergoing TAVI with these new devices.
Conflict of interest statement
J.M. Sinning, N. Werner, and G. Nickenig receive speaker honoraria and research grants from Medtronic and Edwards Lifesciences. E. Grube is a proctor for Medtronic.

