Abstract
Background: Transcatheter aortic valve implantation in an existing transcatheter valve (redo-TAVI) pins the index valve leaflets in the open position (neoskirt), which can cause coronary flow compromise and limit access. Whether anatomy may preclude redo-TAVI in self-expanding Evolut valves is unknown.
Aims: We aimed to evaluate the anatomical feasibility of redo-TAVI by simulating implantation of a balloon-expandable SAPIEN 3 (S3) within an Evolut or an Evolut within an Evolut.
Methods: A total of 204 post-TAVI computed tomography (CT) scans from the Evolut Low Risk CT substudy were analysed. Five redo-TAVI positions were evaluated: S3-in-Evolut inflow-to-inflow, S3 outflow at Evolut nodes 4, 5, and 6, and Evolut-in-Evolut inflow-to-inflow. Univariable modelling identified pre-TAVI clinical characteristics, CT anatomical parameters, and procedural variables associated with coronary flow compromise using the neoskirt height and post-TAVI aortic root dimensions.
Results: The risk of coronary flow compromise was lowest when the S3 outflow was at Evolut node 4 (20%) and highest when at Evolut node 6 (75%). The highest likelihood of preserving coronary accessibility occurred with the S3 outflow at Evolut node 4. Female sex and higher body mass index were associated with a higher risk of coronary flow compromise, as were a smaller annulus diameter, lower sinus of Valsalva height and width, shorter coronary height, smaller sinotubular junction diameter, and shallower Evolut implant depth.
Conclusions: The feasibility of redo-TAVI after Evolut failure is multifactorial and relates to the native annular anatomy, as well as the implantation depth of the index and second bioprostheses. Placement of an S3 at a lower Evolut position may reduce the risk of coronary flow compromise while preserving coronary access. ClinicalTrials.gov: NCT02701283.
Introduction
Transcatheter aortic valve implantation (TAVI) is now approved for patients with aortic stenosis (AS), regardless of surgical risk12. Per the Vizient Clinical Database, over 85% of AS patients are treated with TAVI, with the largest increase in TAVI use occurring in patients <65 years of age3. Post-TAVI structural valve deterioration and bioprosthetic valve failure are more likely to occur in patients with a longer life expectancy4. The feasibility of redo-TAVI has been reported5 with increasing frequency67. In the US, approval for redo-TAVI with the balloon-expandable SAPIEN 3 (S3; Edwards Lifesciences) transcatheter aortic valve (TAV) is limited to high- and extreme-risk patients.
The feasibility of redo-TAVI after the failure of a supra-annular, self-expanding Evolut TAV (Medtronic) is not well understood. The index TAV leaflets are pinned open, which creates a “tube graft” or neoskirt of tissue; this may potentially seal the aortic root (sinus sequestration) or directly obstruct the coronary ostia. Neoskirt interaction with the sinotubular junction (STJ) or coronary arteries can be predicted with preprocedural computed tomography (CT) analysis.
The Evolut Low Risk trial (ClinicalTrials.gov: NCT02701283), which evaluated the safety and efficacy of TAVI with Evolut versus surgery89, included a CT imaging substudy for the assessment of leaflet thickening or immobility10. In this work, we evaluate the feasibility of redo-TAVI with an S3 or an Evolut within an Evolut, based on a post-TAVI CT imaging analysis, and provide guidance for the placement of the second TAV in order to preserve future coronary access.
Methods
Study population
The Evolut Low Risk trial was a multicentre, prospective, randomised trial that assessed the safety and efficacy of TAVI and surgery in patients with an estimated surgical mortality risk <3%; the primary results, patient selection, and methodology have already been published89. The trial, including the Evolut Low Risk CT substudy, was conducted in compliance with the International Conference on Harmonization and the Declaration of Helsinki and approved by local institutional review boards or medical ethics committees. All patients provided informed signed consent.
Of 249 TAVI patients in the Evolut Low Risk CT substudy, 204 had analysable high-quality post-TAVI CT scans. If the 1-year CT scan was insufficient or unavailable, the 30-day CT scan was utilised. Patients included in this analysis were treated with either a 26 mm (n=43), 29 mm (n=89), or 34 mm (n=72) Evolut R or Evolut PRO as their index TAV. None of the patients who received a 23 mm Evolut had high-quality CT scans.
The post-TAVI imaging analysis was performed with 3Mensio software (version 10.2; Pie Medical Imaging). The virtual implantation of the S3 within the Evolut used the expanded Evolut diameters published in a recent in vitro redo-TAVI study11. For the Evolut-in-Evolut implantation, the dimensions of the index Evolut following redo-TAVI were based on the same in vitro analysis, indicating a negligible difference in diameters11. CT measurements were obtained and verified by core laboratory imaging analysts and confirmed by a sponsor-provided analyst. During the CT analysis, both the systolic and diastolic phases were evaluated. The phase with the least amount of motion artefact was used to minimise the risk of measurement error.
Second transcatheter aortic valve sizing and neoskirt dimensions
The index Evolut size determined the size of the second TAV. For the S3-in-Evolut implantation, the S3 size was downsized by one manufacturer size from the index Evolut. Sizing was confirmed by CT annular measurements of the index Evolut based on the published S3 indications for use. For the Evolut-in-Evolut implantation, the second Evolut was the same as the index Evolut.
The S3-in-Evolut inflow-to-inflow was the first redo-TAVI implantation position evaluated. This was done by approximating the alignment of the S3 inflow with the Evolut inflow (Central illustration). Due to variable S3 inflow foreshortening, analyses were also performed by aligning the S3 outflow relative to the nodes of the Evolut frame, as previously reported11. In total, four implant positions of the S3-in-Evolut were analysed: S3-in-Evolut inflow-to-inflow, and S3 outflow at Evolut nodes 4, 5, and 6 (Central illustration). The S3-in-Evolut inflow-to-inflow position was an estimation, and the node level was dependent on the size. This position correlates with the S3 outflow at node 4 for the 26 mm Evolut and at node 5 for the 29 mm and 34 mm Evolut valves.
For S3-in-Evolut redo-TAVI, the neoskirt height was defined as the distance from the Evolut inflow to the pinned leaflet at the outflow of the S3. The Evolut leaflet heights were based on the manufacturer’s standard measurements, and neoskirt heights were measured directly in vitro11. (Supplementary Table 1). The Evolut leaflet tissue overhang was considered when measuring the neoskirt heights on the bench11. The degree of leaflet overhang following S3-in-Evolut and its impact on leaflet kinematics were previously reported by Akodad et al11. For Evolut-in-Evolut redo-TAVI, the neoskirt height was equal to the fully pinned leaflet height and was only analysed inflow-to-inflow (Supplementary Table 1).
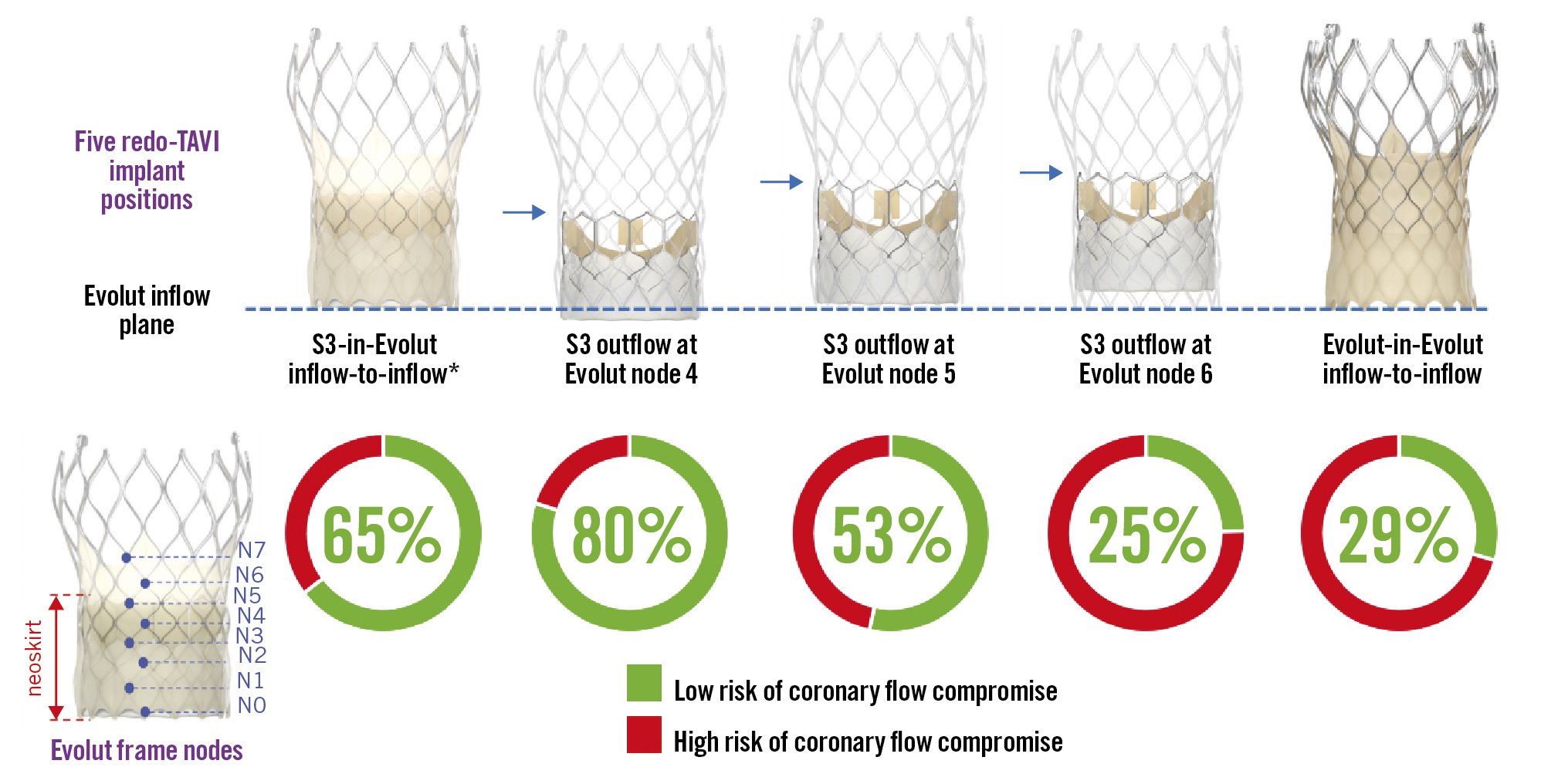
Central illustration. Feasibility of redo-TAVI in self-expanding Evolut valves: a CT analysis from the Evolut Low Risk trial substudy. The initial analysis was completed with the inflow of the S3 approximately aligned with the inflow of the Evolut, with additional analyses based on node levels 4, 5, and 6 of the Evolut frame. An Evolut-in-Evolut was analysed inflow-to-inflow. *S3-in-Evolut inflow-to-inflow is an approximation, and the node level was dependent on the size. It correlates with S3 outflow at node 4 for the 26 mm Evolut and S3 outflow at node 5 for the 29 mm and 34 mm Evolut valves. CT: computed tomography; S3: SAPIEN 3 valve; TAVI: transcathether aortic valve implantation
Determination of coronary flow compromise and access
Post-TAVI CT images were evaluated for the implantation of a virtual second valve using 3Mensio imaging software. The short axis in 3mensio’s stretched vessel view was used to measure the distance from the Evolut- or S3-in-Evolut frame to the coronary ostia midpoint: the valve-to-coronary distance (VTC; also considered the coronary risk plane). Coronary midpoint and sinus heights were measured from the inflow of the Evolut frame to allow for a direct comparison to the neoskirt height. Given the thickness of the Evolut frame and the blooming artefact on CT, measurements were taken at the middle of the Evolut frame. Figure 1 shows the redo-TAVI criteria used to determine coronary flow compromise and accessibility. An S3-in-Evolut implant was considered at low risk of coronary flow compromise if the neoskirt was 1) below the coronary ostium midpoint, OR 2) above the coronary ostium midpoint, but below the STJ, and the VTC distance ≥4 mm, OR 3) above the STJ, with the valve-to-STJ (VTSTJ) distance ≥2 mm, and the VTC distance ≥4 mm. For the Evolut-in-Evolut implant, the risk of coronary flow compromise was evaluated similarly, with one additional measurement, the valve-to-aorta (VTA) distance ≥2 mm at the neoskirt plane (Figure 1). Virtual implants that did not meet the defined criteria were considered at high risk of coronary flow compromise and coronary inaccessibility.
For both the S3-in-Evolut and Evolut-in-Evolut redo-TAVI scenarios, the midpoint of the coronary ostia was selected as the coronary risk plane to assess direct coronary access with a standard diagnostic catheter. Three levels of coronary accessibility were defined: 1) low risk, the neoskirt was below the coronary risk plane; 2) challenging coronary access, the neoskirt was above the risk plane but met the redo-TAVI criteria; and 3) high risk, did not meet the redo-TAVI criteria (Figure 1). Additionally, an analysis was performed to quantify the risk of acute complete coronary occlusion, defined as a VTC, VTSTJ or VTA of 0 mm for the left or right coronary. This conservatively assumes if the VTC or VTA of one coronary is 0 mm, there is an acute coronary obstruction even if the other VTCs and VTAs are>0 mm.
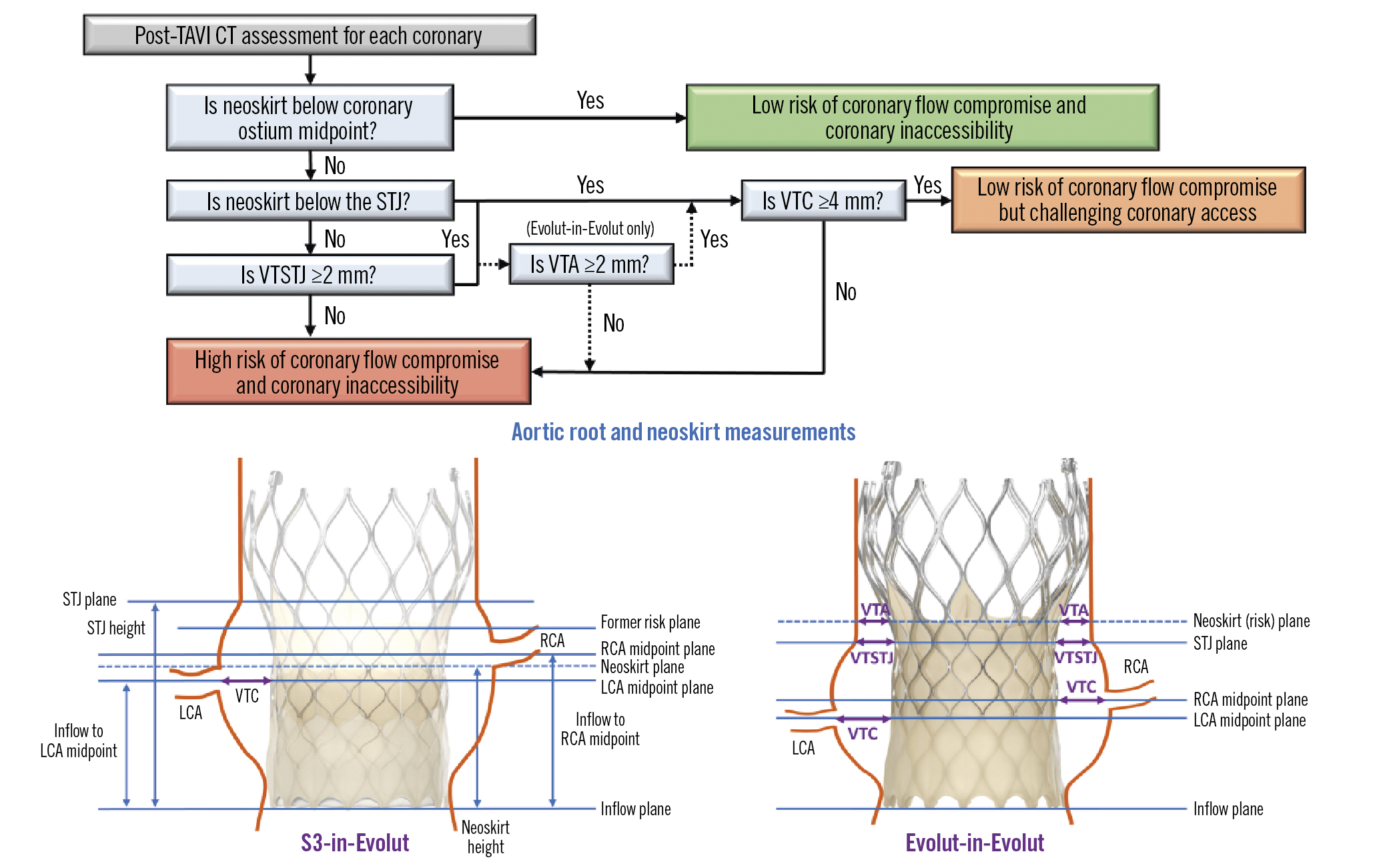
Figure 1. Redo-TAVI criteria to determine the risk of coronary flow compromise and accessibility. Measurements were determined based on the placement of a virtual second valve in an Evolut. CT: computed tomography; LCA: left coronary artery; RCA: right coronary artery; S3: SAPIEN 3 valve; STJ: sinotubular junction; VTA: valve-to-aortic wall distance at neoskirt plane; VTC: valve-to-coronary distance; VTSTJ: valve-to-sinotubular junction distance; TAVI: transcathether aortic valve implantation
Statistical analysis
Categorical variables are reported as counts and percentages. Continuous variables are presented as mean±standard deviation. Univariable logistic regression modelling identified initial pre-TAVI clinical characteristics, CT anatomical parameters, and procedural variables associated with a higher risk of coronary flow compromise following S3 outflow at Evolut node 4 implantations for the overall cohort, as well as by Evolut TAV size. Additionally, the optimal cut-off points for the predictors for the overall cohort were determined on a univariable level by receiver operating characteristic (ROC) curves and Youden’s J statistic. The odds ratios (OR) with associated 95% confidence intervals (CI) and Wald p-values for the continuous variables are reported.
No adjustments were made for multiple comparisons. Results were considered statistically significant if p<0.05. All statistical analyses were performed using the SAS software, version 9.4 (SAS Institute).
Results
For this redo-TAVI CT-based analysis, 204 post-TAVI CT scans were analysed for implantation of a virtual second TAV in 5 different positions. A detailed description of the specific anatomical measurements is provided in Supplementary Table 2.
Predicted coronary flow compromise and coronary accessibility
The predicted percentages of coronary flow compromise and accessibility are shown in Table 1 and Figure 2. The CT-identified risk of coronary flow compromise was higher for Evolut-in-Evolut implantations compared to S3-in-Evolut implantations (except for cases where the S3 outflow was at Evolut node 6) (Figure 2A). When the second TAV was placed approximately inflow-to-inflow, the S3-in-Evolut was below the coronary risk plane and considered at low risk for coronary flow compromise for both coronaries in 65% of cases and in 29% of cases for Evolut-in-Evolut implantation. Overall, for S3-in-Evolut redo-TAVI, the predicted percentages of unaffected coronary flow were highest with the S3 outflow positioned at Evolut node 4 (80% suitable), and lowest at node 6 (25% suitable). The risk of coronary flow compromise was variable based on the implant depth of the S3. Table 1 summarises all redo-TAVI scenarios and provides additional information for each coronary.
Coronary accessibility was similarly demonstrated when an S3 was placed in an Evolut at node 4; in 68% of cases, the neoskirt was below the midpoint of the coronary ostia for both coronaries, allowing direct coronary access. In an additional 12%, coronary access was deemed challenging but feasible (Table 1, Figure 2B). Notably, with an implant depth at node 4, 83% of right coronaries and 73% of left coronaries were deemed to have suitable coronary access. Difficult coronary access was most frequently found with the Evolut-in-Evolut, with patients having challenging access (29%) or a high risk (71%) of coronary inaccessibility. The predicted risk of coronary inaccessibility appeared to be highest for the left coronary artery in all the redo-TAVI scenarios (Table 1).
The predicted percentage of acute complete coronary occlusion was lowest (2%) with the S3 outflow at Evolut node 4; it was 11.8% and 17.6% for node 5 and node 6, respectively. For Evolut-in-Evolut inflow-to-inflow, 2.5% of cases were predicted to lead to acute complete coronary occlusion. The predicted rates for coronary flow compromise and accessibility according to the different combinations of index Evolut and second TAV sizes are listed in Supplementary Table 3 and Supplementary Table 4.
Table 1. CT-predicted risk of compromised coronary flow and access in patients undergoing S3-in-Evolut and Evolut-in-Evolut redo-TAVI.
| Second TAV position | Low risk of coronary flow compromise (%) | Low risk of coronary inaccessibility (%) | |
|---|---|---|---|
| S3-in-Evolut | |||
| Overall | Inflow-to-inflow* | ||
| Left coronary | 76 | 55 | |
| Right coronary | 72 | 67 | |
| Both | 65 | 49 | |
| Overall | Node 4 | ||
| Left coronary | 86 | 73 | |
| Right coronary | 87 | 83 | |
| Both | 80 | 68 | |
| Overall | Node 5 | ||
| Left coronary | 64 | 44 | |
| Right coronary | 61 | 55 | |
| Both | 53 | 39 | |
| Overall | Node 6 | ||
| Left coronary | 36 | 8 | |
| Right coronary | 37 | 18 | |
| Both | 25 | 4 | |
| Evolut-in-Evolut | |||
| Overall | Inflow-to-inflow | ||
| Left coronary | 39 | 1 | |
| Right coronary | 53 | 6 | |
| Both | 29 | 0 | |
| Data are presented as percentages. *S3-in-Evolut inflow-to-inflow is an approximation and the node level is dependent on the size. It correlates with S3 outflow at node 4 for the 26 mm Evolut, and S3 outflow at node 5 for the 29 mm and 34 mm Evolut valves. CT: computed tomography; S3: SAPIEN 3 valve; TAV: transcatheter aortic valve; TAVI: transcatheter aortic valve implantation | |||
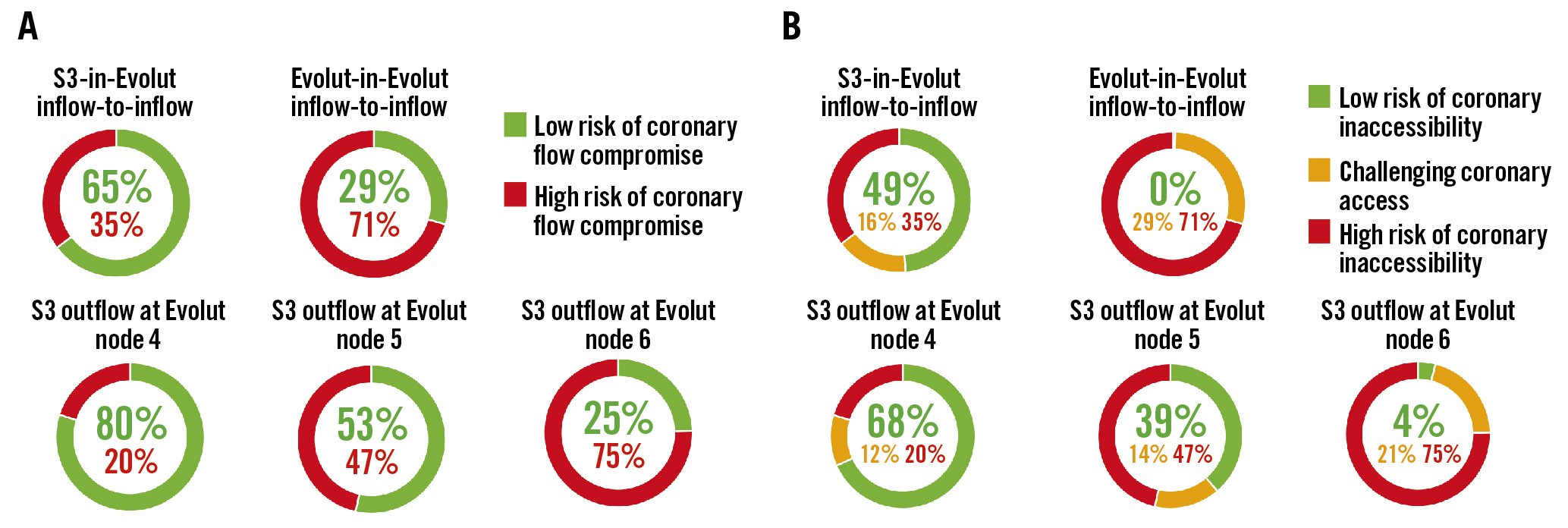
Figure 2. CT-predicted risk of compromised coronary flow and access after redo-TAVI. A) Based on the redo-TAVI criteria, the lowest risk for coronary flow compromise occurs when an S3 is placed at node 4 of the Evolut frame, and redo-TAVI is feasible 80% of the time. The risk of coronary flow compromise is variable based on the implantation depth of the S3. For Evolut-in-Evolut placed inflow-to-inflow, 29% of cases are at low risk for coronary flow compromise. B) Based on the redo-TAVI criteria, the lowest risk for coronary inaccessibility occurs when an S3 is placed at node 4 of the Evolut frame. S3-in-Evolut inflow-to-inflow is an approximation, and the node level is dependent on the size. It correlates with S3 outflow at node 4 for the 26 mm Evolut and S3 outflow at node 5 for the 29 mm and 34 mm Evolut valves. CT: computed tomography; S3: SAPIEN 3 valve; TAVI: transcathether aortic valve implantation
Univariable predictors and optimal cut-off points for high risk of coronary flow compromise after S3 outflow at Evolut node 4
Pre-TAVI clinical characteristics, native CT parameters, and initial procedural characteristics associated with a higher risk of coronary flow compromise for the S3 outflow at Evolut node 4 implant scenario are presented in Table 2, Table 3 and Table 4. Female sex and higher body mass index (BMI) were predictors associated with a higher risk of coronary flow compromise (Table 2). CT parameters associated with a higher risk were a smaller aortic annulus, a shorter coronary ostium height, a shorter sinus of Valsalva (SoV) height, a narrower SoV width, and a smaller STJ diameter (Table 3). In the procedural characteristics, a shallower implantation depth of the index Evolut at the non-coronary cusp (NCC) was associated with a higher risk (Table 4).
The optimal cut-off points for the significant predictors associated with a higher risk of coronary flow compromise, averaged across all valve sizes, included the following: aortic annulus perimeter <78.0 mm, annulus perimeter-derived diameter <24.8 mm, average SoV height <21.0 mm, average coronary ostium height <15.6 mm, average SoV width <32.0 mm, average STJ diameter <29.4 mm, and an index Evolut depth of implant at the NCC <2.3 mm (Table 3, Table 4). The predictors associated with a higher risk of coronary flow compromise following S3 outflow at Evolut node 4 redo-TAVI according to the different index Evolut TAV sizes are listed in Supplementary Table 5.
Table 2. Pre-TAVI clinical characteristics and univariable predictors of a high risk of coronary flow compromise for S3 outflow at Evolut node 4 redo-TAVI.
| Univariable model | ||||
|---|---|---|---|---|
| High risk of coronary flow compromise (N=41) | Low risk of coronary flow compromise (N=163) | Odds ratio (95% CI) | p-value1 | |
| Age, years | 73.1±5.0 (41) | 74.1±5.4 (163) | 0.97 (0.91-1.03) | 0.323 |
| Female | 58.5 (24/41) | 30.1 (49/163) | 3.28 (1.62-6.65) | <0.001 |
| NYHA III/IV | 29.3 (12/41) | 23.3 (38/163) | 1.36 (0.63-2.92) | 0.429 |
| STS-PROM score, % | 2.01±0.61 (41) | 1.89±0.60 (163) | 1.38 (0.79-2.43) | 0.261 |
| BMI, kg/m2 | 33.1±6.5 (41) | 30.1±5.0 (163) | 1.10 (1.03-1.17) | 0.003 |
| BSA*, m2 | 1.99±0.20 (41) | 2.01±0.20 (163) | 0.94 (0.67-1.32) | 0.704 |
| Peripheral artery disease | 4.9 (2/41) | 7.4 (12/162) | 0.64 (0.14-2.98) | 0.571 |
| Cerebrovascular disease | 7.3 (3/41) | 9.8 (16/163) | 0.73 (0.20-2.62) | 0.624 |
| Chronic lung disease/COPD | 23.1 (9/39) | 14.2 (22/155) | 1.81 (0.76-4.33) | 0.180 |
| Serum creatinine >2 mg/dl | 0.0 (0/41) | 0.6 (1/163) | 1.32 (0.01-122.06) | 0.905 |
| Previous CABG | 2.4 (1/41) | 1.8 (3/163) | 1.33 (0.14-13.16) | 0.805 |
| Atrial fibrillation/atrial flutter | 4.9 (2/41) | 9.8 (16/163) | 0.47 (0.10-2.14) | 0.329 |
| Data are presented as mean±standard deviation (n) or percentage (n/N) 1Wald p-value. *OR per 0.2 m2 increase in BSA. BMI: body mass index; BSA: body surface area; CABG: coronary artery bypass grafting; CI: confidence interval; COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; OR: odds ratio; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TAVI: transcatheter aortic valve implantation | ||||
Table 3. Pre-TAVI CT parameters and univariable predictors of a high risk of coronary flow compromise for S3 outflow at Evolut node 4 redo-TAVI.
| Univariable model | |||||
|---|---|---|---|---|---|
| High risk of coronary flow compromise (N=41) | Low risk of coronary flow compromise (N=163) | Odds ratio (95% CI) | p-value1 | Optimal cut-off point | |
| Aortic annulus perimeter, mm | 76.2±6.3 (41) | 79.4±6.9 (163) | 0.93 (0.88-0.98) | 0.010 | 78.0 |
| Aortic annulus perimeter-derived diameter, mm | 24.3±2.0 (41) | 25.3±2.2 (163) | 0.80 (0.67-0.95) | 0.009 | 24.8 |
| Aortic annulus perimeter-derived diameter ≤23mm | 34.1 (14/41) | 20.9 (34/163) | 1.97 (0.93-4.16) | 0.076 | NA |
| Valve oversizing, % | 21.68±6.01 (41) | 19.85±6.14 (163) | 1.05 (0.99-1.11) | 0.089 | 25.1 |
| SoV height at right coronary cusp, mm | 21.5±2.8 (41) | 23.9±2.9 (163) | 0.74 (0.65-0.85) | <0.001 | 23.6 |
| SoV height at left coronary cusp, mm | 21.3±2.6 (41) | 23.5±2.9 (163) | 0.75 (0.65-0.86) | <0.001 | 22.8 |
| SoV height at non-coronary cusp, mm | 20.4±3.1 (41) | 22.7±2.9 (163) | 0.76 (0.67-0.87) | <0.001 | 20.6 |
| Average SoV height, mm | 21.1±2.3 (41) | 23.4±2.5 (163) | 0.67 (0.57-0.79) | <0.001 | 21.0 |
| Right coronary ostium height, mm | 15.3±2.5 (41) | 17.5±2.8 (163) | 0.73 (0.63-0.85) | <0.001 | 15.9 |
| Left coronary ostium height, mm | 13.8±2.1 (41) | 15.5±2.9 (163) | 0.80 (0.70-0.92) | 0.001 | 15.2 |
| Average coronary ostium height, mm | 14.6±1.9 (41) | 16.5±2.3 (163) | 0.66 (0.55-0.80) | <0.001 | 15.6 |
| SoV width at right coronary cusp, mm | 29.6±2.4 (41) | 32.1±3.4 (163) | 0.76 (0.67-0.87) | <0.001 | 31.6 |
| SoV width at left coronary cusp, mm | 31.0±2.3 (41) | 33.3±3.4 (163) | 0.78 (0.68-0.89) | <0.001 | 33.5 |
| SoV width at non-coronary cusp, mm | 30.2±2.3 (41) | 33.0±3.3 (163) | 0.71 (0.62-0.82) | <0.001 | 30.7 |
| Average SoV width, mm | 30.3±2.2 (41) | 32.8±3.2 (163) | 0.73 (0.64-0.85) | <0.001 | 32.0 |
| Ascending aorta minor diameter, mm | 31.8±2.8 (41) | 32.1±3.1 (163) | 0.96 (0.86-1.08) | 0.514 | 32.0 |
| STJ max diameter, mm | 28.0±3.0 (41) | 29.6±3.2 (163) | 0.85 (0.75-0.96) | 0.007 | 27.7 |
| STJ min diameter, mm | 26.8±2.9 (41) | 28.3±3.2 (163) | 0.84 (0.74-0.95) | 0.006 | 28.8 |
| Average STJ diameter, mm | 27.4±2.9 (41) | 29.0±3.2 (163) | 0.84 (0.74-0.95) | 0.006 | 29.4 |
| Aortic valve calcium volume*, mm3 | 685.6±517.0 (41) | 795.8±500.9 (163) | 1.00 (0.99-1.00) | 0.213 | 764.4 |
| Data are presented as mean±standard deviation (n) or percentage (n/N).1Wald p-value.*OR per 10 mm3 increase in calcium volume. CI: confidence interval; CT: computed tomography; max: maximum; min: minimum; NA: not applicable; SoV: sinus of Valsalva; STJ: sinotubular junction; TAVI: transcatheter aortic valve implantation | |||||
Table 4. Initial procedural characteristics and univariable predictors of a high risk of coronary flow compromise for S3 outflow at Evolut node 4 redo-TAVI.
| Univariable model | ||||||
|---|---|---|---|---|---|---|
| High risk of coronary flow compromise (N=41) | Low risk of coronary flow compromise (N=163) | Odds ratio (95% CI) | p-value1 | Optimal cut-off point | ||
| Valve type | Evolut R | 53.7 (22/41) | 62.0 (101/163) | Reference | NA | |
| Evolut PRO | 46.3 (19/41) | 38.0 (62/163) | 1.41 (0.71-2.81) | 0.333 | ||
| Valve size | 0.385 | NA | ||||
| 26 mm | 26.8 (11/41) | 19.6 (32/163) | Reference | |||
| 29 mm | 46.3 (19/41) | 42.9 (70/163) | 0.79 (0.34-1.85) | 0.806 | ||
| 34 mm | 26.8 (11/41) | 37.4 (61/163) | 0.52 (0.21-1.34) | 0.180 | ||
| Valve size ≤26 mm | 26.8 (11/41) | 19.6 (32/163) | 1.50 (0.68-3.31) | 0.314 | NA | |
| Index Evolut depth of implant at NCC*, mm | 1.9±2.5 (41) | 4.9±2.3 (161) | 0.57 (0.46-0.69) | <0.001 | 2.3 | |
| Periprocedural MI | 2.4 (1/41) | 0.6 (1/163) | 4.01 (0.25-65.50) | 0.330 | NA | |
| Coronary obstruction | 2.4 (1/41) | 0.0 (0/163) | 12.30 (0.13-1,171.74) | 0.280 | NA | |
| Data are presented as mean±standard deviation (n) or percentage (n/N).1Wald p-value.*CT measured if available, otherwise fluoroscopy. CI: confidence interval; CT: computed tomography; MI: myocardial infarction; NA: not applicable; NCC: non-coronary cusp; TAVI: transcatheter aortic valve implantation | ||||||
Discussion
In this first-of-a-kind study analysing post-TAVI CT scans among low surgical risk patients who underwent Evolut TAVI to determine the anatomical feasibility of redo-TAVI, we report the following key findings: 1) coronary access was found to be the most favourable, with the least risk of coronary flow compromise when the S3 outflow was positioned at node 4 of the Evolut frame (Central illustration); 2) the predictors associated with a higher risk of coronary flow compromise with the S3 outflow at Evolut node 4 included female sex, higher BMI, a smaller aortic annulus diameter, shorter SoV height, narrower SoV width, shorter coronary ostium height, smaller STJ diameter, and shallower implant depth of the index Evolut; and 3) the feasibility of redo-TAVI after a failed Evolut is multifactorial and relates to native annular anatomy and the implant depth of the index Evolut, as well as the implant position of the second TAV.
Optimal implant position of the S3 in a failed Evolut to optimise coronary flow and accessibility
Analysing post-TAVI CTs from the Evolut Low Risk trial allowed for a real-world assessment of valve positioning. Based on this CT-based simulation work, with Evolut valves placed by trial site operators, 80% of patients are predicted to be at low risk of coronary flow compromise after redo-TAVI with an S3 positioned at Evolut node 4. Placement at node 4 also demonstrated the highest likelihood of coronary access; there was a low risk of coronary inaccessibility in 68% of cases with the S3 below the coronary risk plane at the midpoint of the coronary ostia, and an additional 12% had challenging but feasible access. When a second Evolut valve was used, there was a low risk of coronary flow compromise in 29% of cases, but all cases were considered challenging or at high risk of coronary inaccessibility. Thus, Evolut-in-Evolut redo-TAVI appears feasible in specific patients with favourable anatomies; however, future coronary access is likely to be more challenging. These findings are aligned with a previously published CT simulation study of repeat TAVI after Evolut PRO implantation in 81 patients from the CoreValve Evolut PRO Prospective Registry (EPROMPT; ClinicalTrials.gov: NCT03423459). The authors used less stringent anatomical criteria (the superior edge of the coronary ostia defined the complication risk) but demonstrated 23% SoV sequestration12. The neoskirt height in their simulation predicted 78% of patients would have coronary access issues after redo-TAVI with a second Evolut TAV. To address the growing concern of lifetime management of aortic valve disease in young patients, other investigators have analysed redo-TAVI feasibility based on the initial CT scan, simulating the initial implant and subsequent TAV. A high risk of coronary compromise was reported in 27.7% of redo-TAVIs, regardless of initial valve type13.
Importantly, when our redo-TAVI analysis was performed to identify patients at risk for acute complete coronary occlusion at the time of redo-TAVI, among those with VTC, VTSTJ, or VTA distances of 0 mm, very few patients were at risk: 2% with the S3 at Evolut node 4 and 2.5% with Evolut-in-Evolut. This is reassuring; however, a true assessment of redo-TAVI feasibility will not be available until enough valves have failed and clinical data are systematically captured and analysed to validate the assumptions in the models. These findings, however, provide some indication that redo-TAVI with an S3 in an Evolut can be done in most patients and that a preprocedural CT assessment of the failed TAV is necessary in every patient.
Predictors of feasibility of redo-TAVI with S3 outflow at Evolut node 4
When the S3 outflow was placed at node 4 of the Evolut, univariable logistic regression modelling found that female sex and a higher BMI were associated with a higher risk of coronary flow compromise. This was not surprising as one would assume women, having a smaller aortic root anatomy, would be at higher risk. Indeed, when evaluating native CT anatomical measurements, smaller aortic annulus dimensions were significant predictors associated with a higher risk of coronary flow compromise. The only initial procedural characteristic associated with a higher risk of coronary flow compromise was the implantation depth of the index Evolut. This would seem intuitive, as the higher the valve is placed in the annulus, the further the risk plane extends above the coronary ostia.
The optimal cut-off points for these significant predictors were evaluated to facilitate their clinical interpretation but may not be generalisable outside this population. The optimal cut-off point for the implantation depth of the index Evolut was 2.3 mm at the NCC. To better understand the parameters by index Evolut size, optimal cut-off points were evaluated for each index Evolut size. However, due to the small sample size of patients at higher risk, this analysis did not have adequate statistical power. A preprocedural CT planning approach of the index TAVI is necessary for every patient to facilitate future coronary access after redo-TAVI. Understanding the native anatomy and implanting an appropriately sized index Evolut valve at an optimised implantation depth will facilitate future coronary access after redo-TAVI.
Optimising index Evolut implant depth to facilitate redo-TAVI
Some may question the present-day utility of an analysis of Evolut valves from the Evolut Low Risk CT substudy (index TAVI implantation from 2016 to 2018), as the target implantation depth was 3-5 mm at the NCC, and the average implantation depth by fluoroscopy was 3.9 mm in the Low Risk trial8. However, as the cusp-overlap technique was popularised concurrently and experiences were published in 201814, there is reason to believe that a higher Evolut implantation − to avoid the conduction tissue − may have been employed during the Evolut Low Risk CT substudy enrolment. Moreover, in the Optimize PRO study (ClinicalTrials.gov: NCT04091048), the target implantation depth is 3 mm at the NCC, with a range of 1-5 mm based on patient anatomy. At the second interim analysis including 400 patients in North America, the average implantation depth was 3.1±3.0 mm at the NCC, thus deeper than the optimal anatomical cut-off point of 2.3 mm identified in this study15. This provides additional support for the feasibility of redo-TAVI even when striving for a higher initial implantation.
In some patients, a deeper index Evolut implantation may be needed to assure future redo-TAVI, a benefit which should be balanced against the potential increased risk of conduction abnormalities, particularly in younger patients. The next-generation Evolut FX system (also Medtronic), with a redesigned delivery catheter and commissure alignment markers located at 3 mm on the valve frame, may provide better control and facilitate more accurate implantation. These changes potentially translate into a lower risk of interaction with the conduction tissue and better coronary access after redo-TAVI16.
As we look for solutions for the lifetime management of low-risk patients, understanding the current situation will allow for pre-emptive development of techniques and technology to address further concerns. Leaflet height and risk of coronary flow compromise are particularly important with Evolut TAVI. Leaflet modification techniques like BASILICA (Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction)17 are potentially less useful in redo-TAVI after implantation of an Evolut, as leaflet splay is minimal and coronary flow remains compromised18. Leaflet removal has only been demonstrated in a case report and is experimental at best19. However, leaflet modification can only be achieved if the TAV commissures are relatively aligned with the native commissures; the Evolut Low Risk CT substudy showed an incidence of commissural alignment in <60% of cases20. The newer-generation Evolut FX has improved alignment to >90%, which should facilitate leaflet modification and better enable redo-TAVI20. With present-day TAVI, there is a gap in available treatment options for previously implanted valves, and leaflet modification techniques or removal tools accessible to the average structuralist are needed. Future valve innovations may focus on next-generation valve design. Planning for redo-TAVI gives younger patients a strategy for lifetime management at the time of index TAVI.
Limitations
There are several limitations to the current study. Patients did not undergo an actual redo-TAVI procedure; thus, the CT-predictive nature of this study may not reflect physiological conditions in clinical practice, and the evaluation criteria need to be validated. The S3-in-Evolut assessment assumed the Evolut leaflets would overhang the S3 when the S3 outflow was placed at nodes 4 and 5, based on in vitro work11, and was recently expanded upon in a consensus document21. Further studies are warranted to better understand overhang of calcified or thickened Evolut leaflets and their implications following redo-TAVI. The Evolut-in-Evolut assessment may overestimate the risk of coronary flow compromise, as the neoskirt was defined as the fully pinned leaflet height. In vitro measurements for Evolut expansion by the S3 may not accurately predict in vivo redo-TAVI frame expansion, as the Evolut frame occupies space in the sinus, and there is blooming artefact in the CT images. Thus, all measurements were conservative and measured at half of the frame width. This study did not include any size 23 mm valves, as only 1.2% of patients in the Evolut Low Risk trial were implanted with a 23 mm Evolut8, and none of these patients had analysable CT scans. The feasibility of redo-TAVI in very small annuli requires further study, but this patient cohort is expected to be at higher risk. The Evolut Low Risk trial excluded patients with bicuspid aortic valves, thus our findings may not apply to bicuspid valve anatomy. The predictor analysis results only apply to the S3 outflow at Evolut node 4 implant position and are limited by a small sample size especially when dividing by index Evolut valve size. Thus, these findings should not be extrapolated to direct clinical practice without considering individual patient factors. Finally, 35% of the patients evaluated in this study were implanted with a 34 mm index Evolut. This valve has an extended sealing skirt compared to the other Evolut TAVs. This should be considered when interpreting the findings of this analysis.
Conclusions
For failed Evolut TAVs, the feasibility of redo-TAVI depends on the native annular anatomy and on the implantation depth of the index and second TAVs. Placement of the S3 outflow at node 4 of the Evolut frame appears to provide the lowest risk for coronary flow compromise and coronary inaccessibility. Individualised preprocedural planning of the initial Evolut valve and precise placement of the S3 can increase the feasibility of future coronary access following redo-TAVI.
Impact on daily practice
The feasibility of future coronary access following redo-TAVI after Evolut failure is poorly understood. Using the Evolut Low Risk trial post-TAVI CT database, placement of a SAPIEN 3 outflow at the Evolut’s node 4 predicted the lowest risk of coronary flow compromise and coronary inaccessibility. This CT-based simulation study suggests a high implant position of the index Evolut in the presence of a small aortic root anatomy may compromise coronary flow and lead to challenging coronary access after redo-TAVI. Redo-TAVI in an Evolut can be done in most patients, and individualised preprocedural planning of the implantation depth of the index Evolut and precise placement of the S3 can increase the feasibility of redo-TAVI. A clear lifetime strategy and initial valve choice must be discussed by the Heart Team, particularly in younger, low-risk patients with longer life expectancies.
Acknowledgements
Andres Caballero, PhD, an employee of Medtronic, assisted in the preparation of the manuscript, including drafting the methods and results sections, creating tables and figures, ensured the accuracy of the data presented, and provided editorial support under the direction of the lead author. Sarah Verdoliva Boatman, MSc, an employee of Medtronic, provided statistical analysis support.
Funding
This work was supported by Medtronic, MN, USA.
Conflict of interest statement
K.J.Grubb is aproctor, principal investigator and serves on the advisory board for Medtronic; and also serves on the advisory board or is aconsultant for Ancora Heart, Boston Scientific, Abbott, 4C Medical, and Edwards Lifesciences. J.Spencer is an employee and shareholder of Medtronic. G.H.L.Tang is aphysician proctor and consultant for Medtronic, aconsultant and physician advisory board member for Abbott Structural Heart, and aphysician advisory board member for JenaValve. J.Zhingre Sanchez is an employee and shareholder of Medtronic. J.Sathananthan is aconsultant to Edwards Lifesciences and Medtronic; and has received speaker fees from Edwards Lifesciences and NVT.T.Rogers is aconsultant and physician proctor to Edwards Lifesciences, Medtronic, and Boston Scientific; is an advisory board member to Medtronic; has equity in Transmural Systems; and is aco-inventor on patents, assigned to NIH, for transcatheter electrosurgery devices. M.Deeb serves on an advisory board for Medtronic; and has received institutional grant support from Boston Scientific, Edwards Lifesciences, and Medtronic; he receives no personal remunerations. S.Fukuhara is aconsultant for Terumo Aortic, Medtronic, and Artivion. P.Blanke holds institutional research core lab agreements with Medtronic, Edwards Lifesciences, and Abbott, with no personal compensation. J.A.Leipsic holds institutional research core lab agreements with Medtronic, Edwards Lifesciences, Abbott, Boston Scientific, and Pi-Cardia, with no personal compensation. J.K.Forrest has received grant support/research contracts and consultant fees/honoraria/speakers’ bureau fees from Edwards Lifesciences and Medtronic. M.J.Reardon has received fees to his institution from Medtronic for consulting and providing educational services. P.Gleason’s employer receives institutional grants and educational funding from Edwards Lifesciences and Medtronic; he has no personal financial disclosures. The other authors have no conflicts of interest relevant to this paper to declare.
Supplementary data
To read the full content of this article, please download the PDF.

