
Abstract
Aims: The aim of this study was to investigate the risk of impaired coronary access and coronary obstruction after redo TAVI.
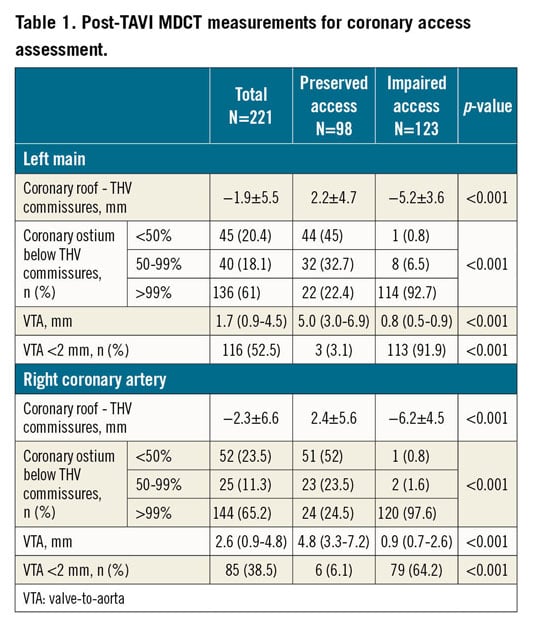
Methods and results: Post-procedure multidetector computed tomography (MDCT) scans of 221 TAVI recipients were analysed. Increased risk of impaired coronary access was defined as a coronary ostium below the TAVI commissures with a valve-to-aorta distance <2 mm at this level. Increased risk was found in 123 (55.6%) cases: the left main was involved in 109 (49.3%), the right coronary in 79 (35.7%), and both were involved in 65 (29.4%) patients. A small sinotubular junction (STJ width OR 0.68, CI: 0.56-0.81, p<0.001; STJ height OR 0.81, CI: 0.69-0.95, p<0.011) and supra-annular devices (OR 19.8, CI: 6.6-58.8, p<0.001) predicted increased risk. Increased risk of coronary obstruction, defined as a coronary ostium below the TAVI commissures with a valve-to-coronary distance <2 mm, was observed in 14.9% of patients; in 17.2% of cases complete sealing of the STJ would occur.
Conclusions: Post-TAVI MDCT suggested an increased potential risk of impaired coronary access in more than half of the patients should redo TAVI be required, predicted by a small STJ and supra-annular device design. Furthermore, 10-20% of patients presented an increased risk of coronary obstruction. While this theoretical study is hypothesis-generating, it raises concerns that need to be further investigated and addressed before TAVI is extended to patients with longer life expectancy.
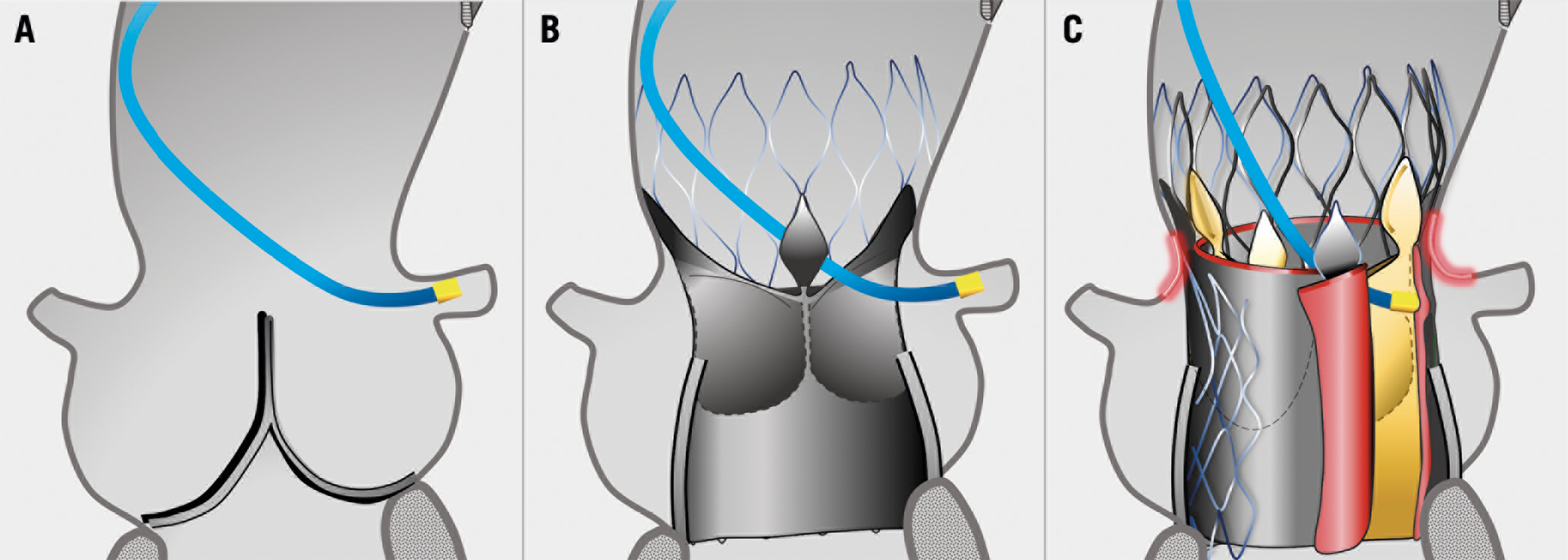
Visual summary. Aortic root in native anatomy (A), after TAVI (B) and after redo TAVI (C): small sinotubular junction and high leaflets of the transcatheter heart valve, pushed up and outwards by the second device, are associated with impaired coronary access and perfusion after redo TAVI.
Introduction
Over the last decade, transcatheter aortic valve implantation (TAVI) has become an invaluable treatment for patients with aortic stenosis. Most transcatheter heart valves (THV) are designed to have free open cells that maintain blood flow and allow coronary cannulation. However, if TAVI is performed within a previous THV, the new device will push the leaflets of the previous THV open upwards, converting it into a tubular “covered” stent up to the edge of the leaflets and creating a barrier impeding catheters and blood flow to the coronaries1. Moreover, depending on THV design, superimposition of the two stent frames may further impair catheter manoeuvrability, even above the leaflets. Such concepts have recently been rapidly emerging but real-world clinical evidence is still lacking1,2,3,4,5.
The number of redo TAVI procedures has been limited until now6 due to the advanced age and morbidity profile of treated cases; thus, the durability of THV has exceeded the life expectancy of the patient. However, indications for TAVI are now extending towards patients with a longer life expectancy and, while the durability of THV appears favourable in this early stage7, the number of redo TAVI procedures is destined to increase in the future. At the same time, these patients are also more likely to require coronary catheterisation over the course of their lives8.
The aim of this study was to use post-TAVI MDCT analysis to investigate the potential risk of impaired coronary access and coronary obstruction if redo TAVI is required.
Methods
STUDY RATIONALE
After redo TAVI, coronary access and perfusion will depend on three major THV aspects. First, the index THV leaflets pushed up and outwards are the most important determinant of the “neo-barrier” that will impede catheters and blood flow to the coronaries. The height reached by the edge of the leaflets is known to be different according to each THV design3. This has been confirmed in bench and MDCT observations (Supplementary Figure 1, Supplementary Figure 2). Second, index THV leaflet disease over time, which may vary in location, extension and kind (retraction, tear, thickening, calcification, etc.), can lead to tissue “growth” or “reduction”, as well as to changes in leaflet movement and folding. Third, the design of the redo TAVI THV, its implantation height and expansion can differentially impact on the final height of the index leaflet barrier as well as on stent cell frame superimposition above the leaflets.
Leaflet disease and the second THV are not predictable and are beyond the scope of the present study, which is to perform a theoretical analysis of the risk of impaired coronary access and perfusion based on the index THV design.
STUDY POPULATION
MDCT acquired after TAVI in three high-volume European centres (San Raffaele Scientific Institute, Milan, Italy; Rigshospitalet University Hospital, Copenhagen, Denmark; and Heart and Vascular Center, Semmelweis University, Budapest, Hungary) between 2007 and May 2018 was retrospectively reviewed. Patients with non-ECG-synchronised and poor-quality MDCT, a previous aortic prosthesis and pure aortic regurgitation were excluded.
The following THV were included in the analysis: SAPIEN (Edwards Lifesciences, Irvine, CA, USA), CoreValve® (Medtronic, Minneapolis, MN, USA), Portico™ (Abbott Vascular, Santa Clara, CA, USA), ACURATE neo™ (Boston Scientific, Marlborough, MA, USA), CENTERA (Edwards Lifesciences). The Direct Flow Medical® (Direct Flow Medical, Santa Rosa, CA, USA) and Lotus™ (Boston Scientific) valves were excluded because their innate dense stent design impedes catheters and blood flow through the valve frame, even after the first procedure.
The study complies with the Declaration of Helsinki and was approved by the locally appointed ethics committees.
MDCT ANALYSIS
Each centre provided MDCT scans by means of Digital Imaging and Communications in Medicine (DICOM) files. MDCT acquisitions were performed according to site-specific institutional protocols, comprising contrast-enhanced, multiphasic, electrocardiography (ECG)-synchronised imaging.
San Raffaele Scientific Institute served as the core lab for all analyses. Measurements were determined on multi-planar reconstruction by consensus of two investigators with >10 years of experience in MDCT analysis in the setting of TAVI. Dubious cases were agreed by consensus with a third senior TAVI operator. Images were analysed with the OsiriX DICOM Viewer MD 5.8 (Pixmeo SARL, Bernex, Switzerland).
THV oversizing was calculated as: [(prosthesis measurement/patient measurement)−1]*100. Perimeter was used for self-expanding devices while area was used for balloon-expandable devices.
To assess the risks of impaired coronary access and perfusion after redo TAVI, the following parameters were used:
– Level of THV commissures: the plane perpendicular to the prosthesis long axis passing through the THV leaflet commissures, as shown in Figure 1. This level was based on each THV design as confirmed by bench (Supplementary Figure 1) and MDCT direct leaflet visualisation (Supplementary Figure 2).
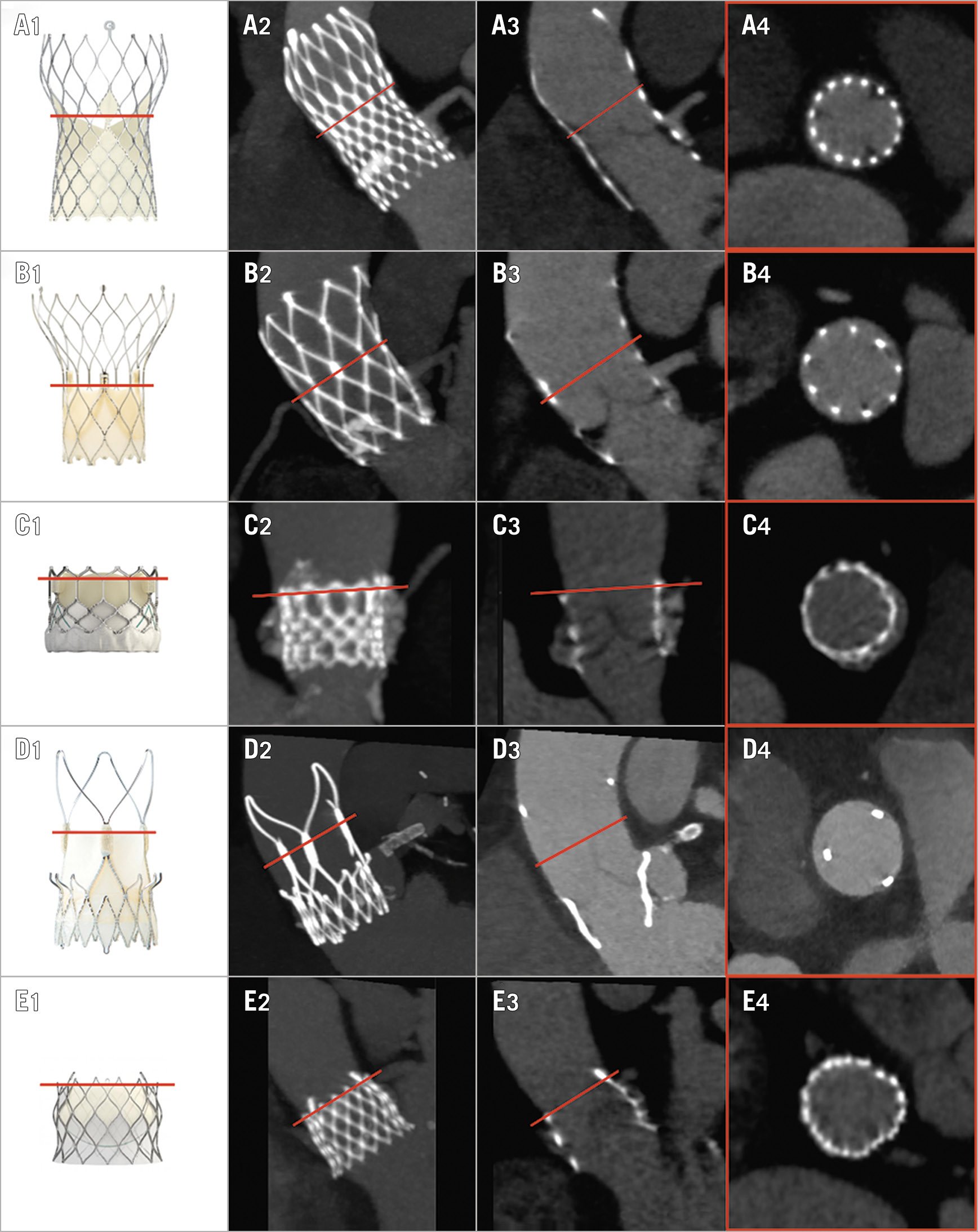
Figure 1. MDCT multiplanar analysis after TAVI. Each THV (column 1) has a specific stent and leaflet design. Identification of the level of the THV commissures is undertaken using a combination of maximum intensity projection (MIP, column 2) and 2D multiplanar reconstructions (columns 3 and 4). Each row depicts a specific THV class in order of decreasing prevalence in the study, top to bottom: A) CoreValve; B) Portico; C) SAPIEN; D) ACURATE neo; E) CENTERA. The precise level reached by the THV leaflets was confirmed at bench observation (Supplementary Figure 1) and direct leaflet MDCT measurement (Supplementary Figure 2).
– Valve-to-aorta distance (VTA): the shortest distance between the THV stent at or below the level of the THV commissures and the aortic wall at or above the roof of the coronary ostium (Figure 2).
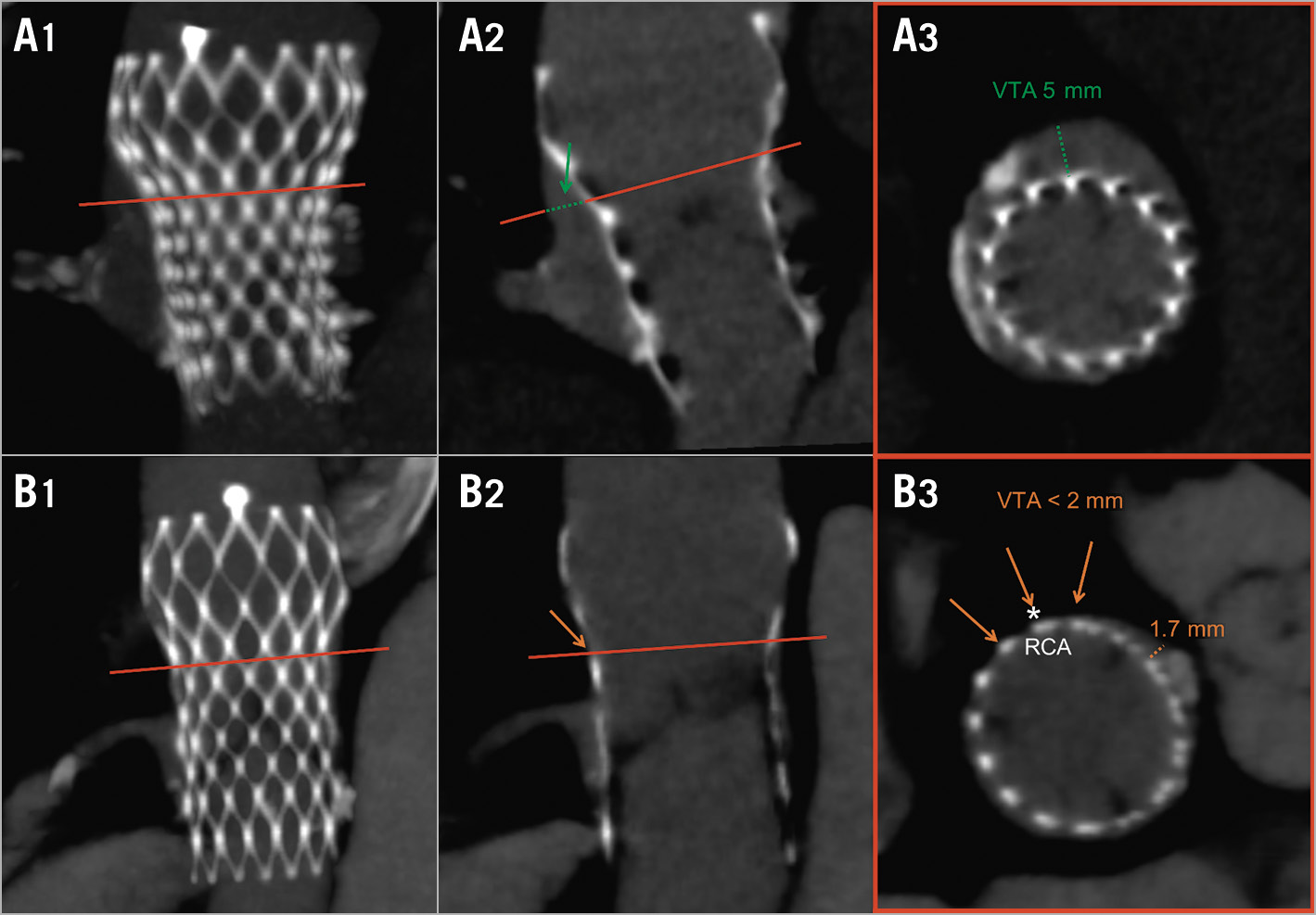
Figure 2. Valve-to-aorta free distance: the shortest distance, at/below the level of the THV commissures, between the THV stent and the aortic wall. Row A: wide residual VTA. Row B: insufficient residual VTA prohibiting catheter access to the RCA (*). Column 1: MIP; column 2: longitudinal THV view; column 3: axial THV view.
– Valve-to-coronary distance (VTC): distance between the THV and each coronary ostium5,9.
– THV commissure-coronary overlap: angle between each coronary ostium and the closest THV commissure (severe overlap defined as <20%)10.
Increased risk of impaired coronary access was defined as THV commissures above 99% of the coronary ostium and VTA <2 mm for either coronary artery (Figure 2). A 2 mm cut-off was chosen based on a standard 6 Fr PCI catheter size3.
Increased risk of coronary obstruction was defined as THV commissures above 99% of the coronary ostium and VTC <2 mm for either coronary artery. Since the present analysis is only theoretical without real cases of obstruction documented, the 2 mm cut-off was chosen in agreement with other series in the field4,5. Moreover, to provide additional information, the number of patients with VTC <4 mm (according to the VIVID registry9) was reported.
Importantly, coronary access and perfusion are very different entities, since blood reaches coronary ostia sideways more easily than catheters.
STATISTICAL ANALYSIS
Normally distributed variables are presented as mean±standard deviation and were compared using the Student’s t-test. Non-normally distributed data are presented as median (interquartile range) and compared with the Mann-Whitney test for independent samples. Categorical variables are presented as number (percentage) and compared by the chi-square/Fisher’s exact test, as appropriate. Stepwise multivariate logistic regression was implemented to detect predictors of impaired access. The goodness-of-fit of the model was assessed with the Hosmer-Lemeshow test. A p-value <0.05 was considered significant. Statistical analyses were performed using Stata 13 (StataCorp LLC, College Station, TX, USA).
Results
A total of 221 patients with baseline and post-TAVI MDCT scans were evaluated. The reasons for MDCT being carried out were: participation in clinical studies (89.6%), patient re-assessment prior to reintervention (6.3%), and index procedural complications (4.1%).
The median time of MDCT acquisition from index TAVI was 10 (6-21) months; at this time no significant leaflet degeneration was observed in the present series.
The median depth of index THV implantation was 7.0 mm (4.8-9.4 mm). Implantation depth was <4 mm in 34 (15.4%) patients and >6 mm in 138 (62.4%) patients. The median distance between the THV commissure level and aortic annulus was 18.8±5.1 mm. Detailed post-TAVI MDCT measurements for each coronary are reported in Table 1.
Increased risk of impaired coronary access following redo TAVI was found in 123 (55.7%) cases. The left main (LM) was involved in 109 (49.3%) patients, the right coronary artery (RCA) in 79 (35.7%) patients, and both coronaries in 65 (29.4%) patients.
Baseline and procedural patient characteristics are shown in Table 2 and Table 3. Patients at high risk of impaired coronary access had smaller aortic anatomy and more frequently received supra-annular THV (Figure 3, Figure 4).
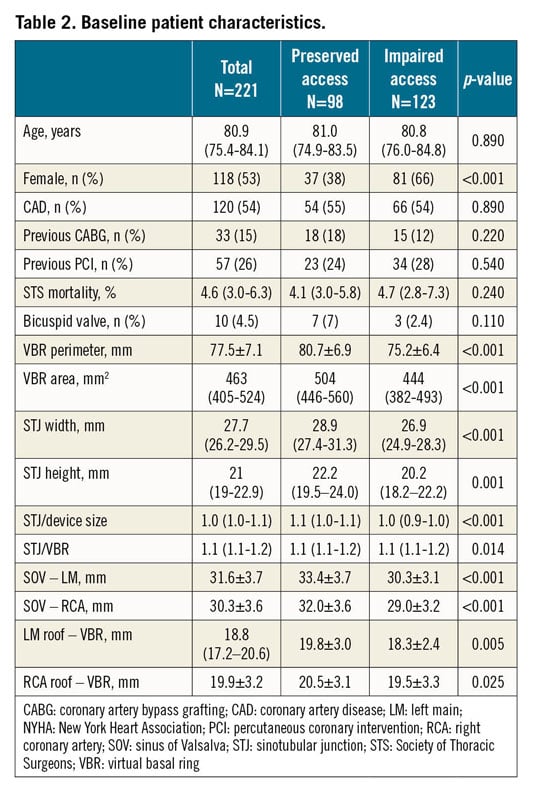
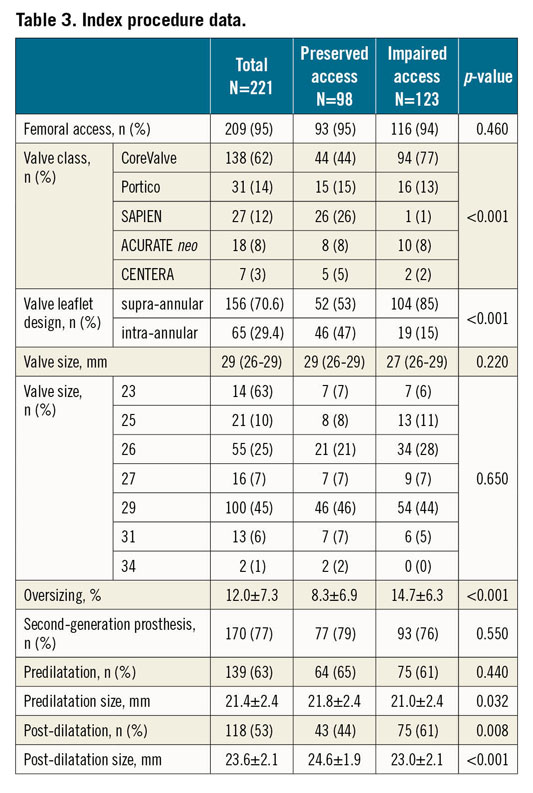
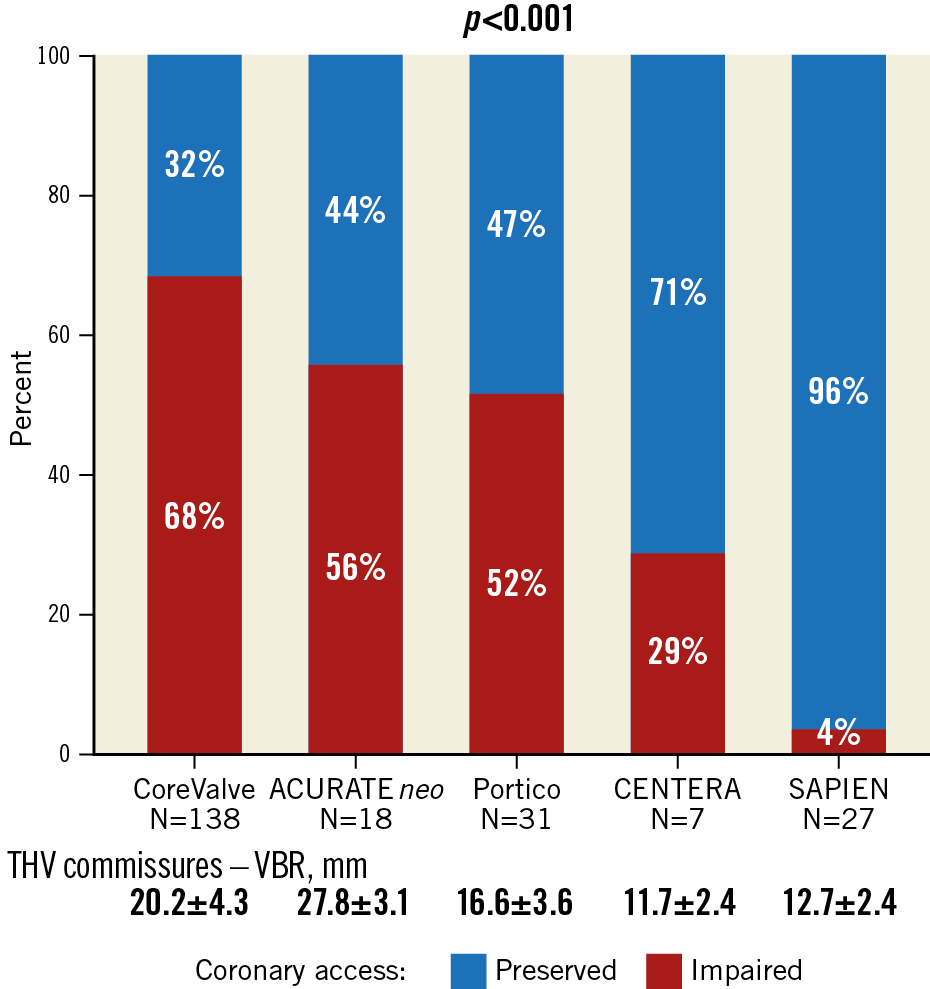
Figure 3. Proportion of patients at increased risk of impaired coronary access according to THV class.
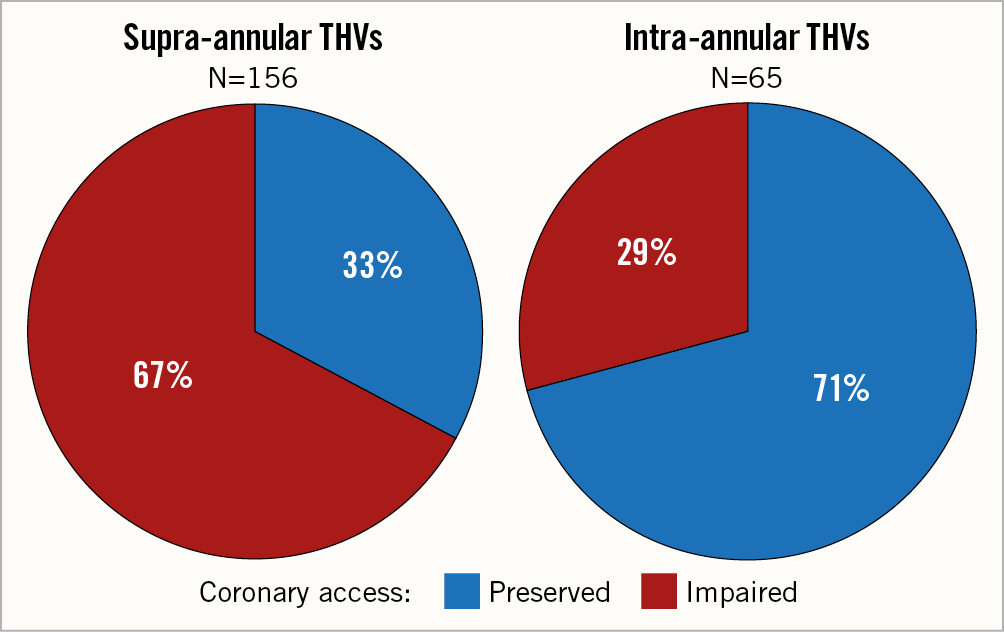
Figure 4. Proportion of patients at increased risk of impaired coronary access according to THV leaflet design.
At multivariable analysis, a larger STJ, a higher STJ and a lower implantation depth were protective factors, whereas device oversizing and supra-annular prosthesis design independently predicted a high risk of impaired coronary access (Table 4).
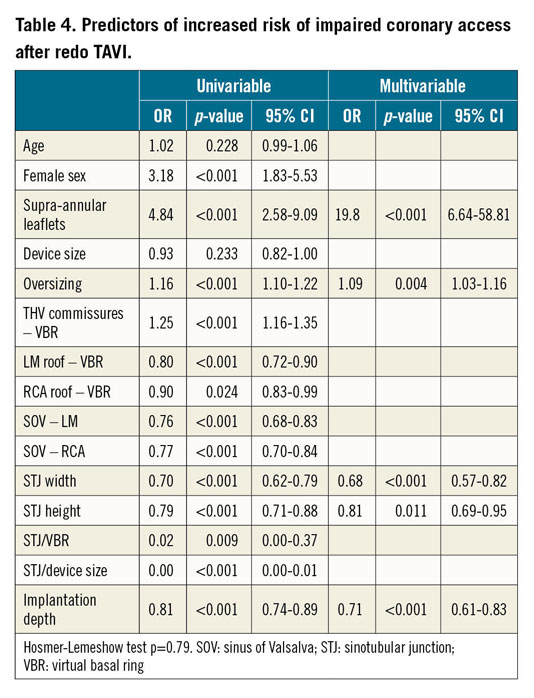
In the supra-annular THV subgroup, STJ width, implantation depth and oversizing were all confirmed independent predictors (Supplementary Table 1). For these three parameters, receiver operating characteristic (ROC) curve analysis identified threshold values as follows: STJ width 28 mm (area under the curve [AUC] 0.82), implantation depth 8.4 mm (AUC 0.73), and oversizing 12.5% (AUC 0.76) (Supplementary Figure 3).
In the intra-annular THV subgroup, STJ height remained a strong protective factor of impaired access (Supplementary Table 1), with a ROC curve suggested threshold of 20.3 mm (AUC 0.77).
Separate analyses for impaired access to the LM and RCA are reported in Supplementary Table 2; STJ width and supra-annular device design were confirmed to be strong predictors for both coronaries. A higher coronary ostium resulted in being a protective factor for the LM but not for the RCA.
Increased risk of coronary obstruction was observed in 33 (14.9%) patients: the LM was involved in 17 (7.7%), the RCA in 24 (10.9%), and both coronaries in 8 (3.6%). The same numbers increased if a <4 mm VTC cut-off was used: 93 (42.1%) overall, the LM being involved in 62 (28.1%), the RCA in 72 (32.6%), and both in 41 (18.6%) (Figure 5A). Of note, in 38 (17.2%) patients, the STJ would be completely sealed by the index THV leaflets but 9 of these patients presented a VTC distance >4 mm at the level of the sinuses of Valsalva (Figure 5B).
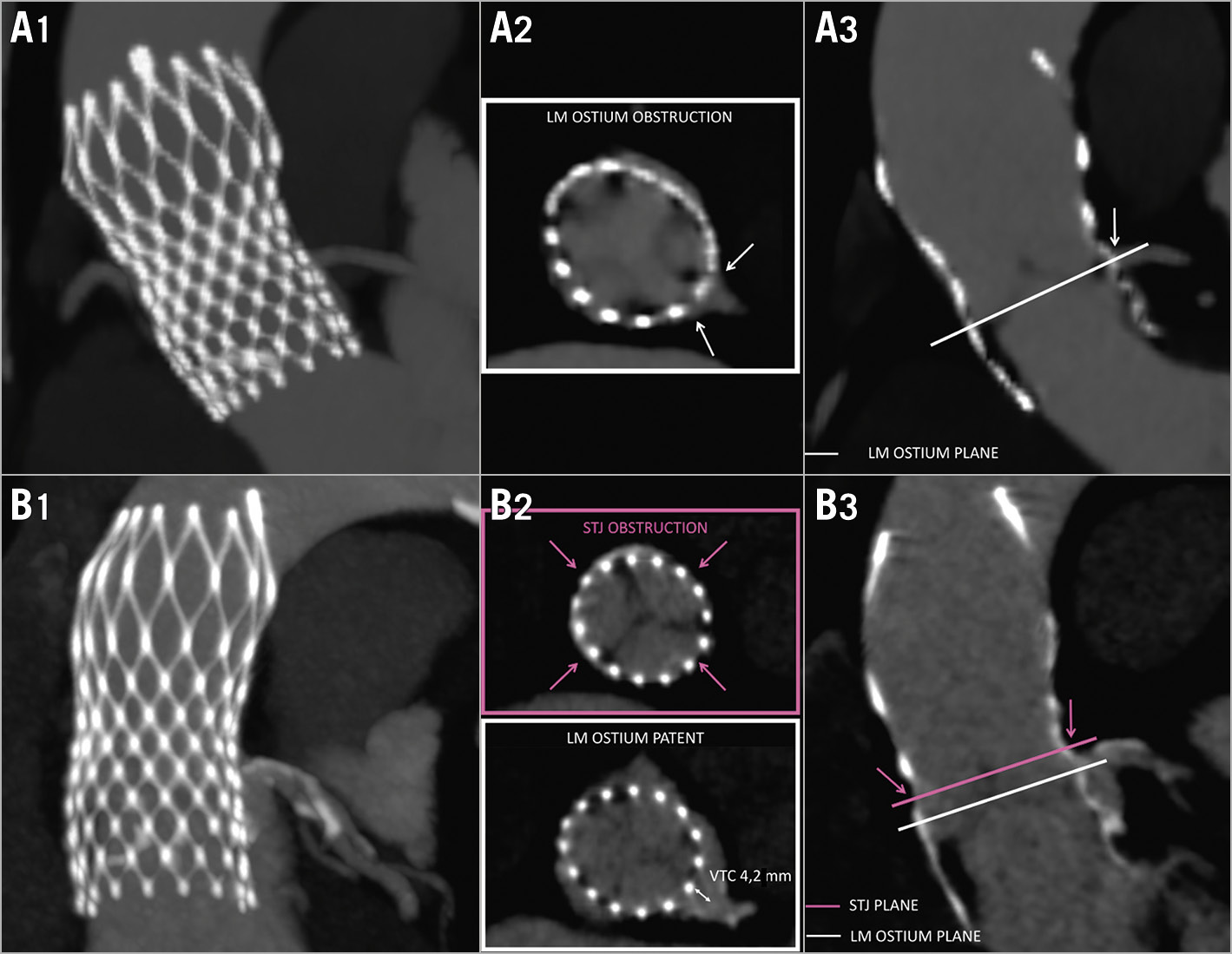
Figure 5. Predicted risk of coronary obstruction after redo TAVI. A) Risk of coronary obstruction after redo TAVI due to direct LM ostium occlusion. B) Risk of coronary obstruction after redo TAVI due to complete STJ closure; note misleading VTC >4 mm at the LM ostium in the sinuses of Valsalva. Column 1: MIPs; column 2: axial views; column 3: longitudinal THV views.
The mean angle of THV commissure-coronary ostium overlap was 31°±16° (≤20° in 27.5% of cases) for the LM, and 32°±16° (≤20° in 28.3% of cases) for the RCA.
Among the 14 patients who underwent MDCT prior to aortic reintervention, none presented a VTC <4 mm but one patient with a CoreValve 29 presented an increased risk of impaired access to the LM. Nine patients underwent redo TAVI, there was 1 valvuloplasty and 1 leak closure, and 3 did not undergo any reintervention. Among the 9 redo TAVI cases (including the one with increased risk of impaired LM access), no coronary obstruction was observed and no attempt at coronary cannulation was performed after the procedure.
Discussion
The present analysis of post-TAVI MDCT revealed that in case of redo TAVI: 1) more than half of all patients potentially have an increased risk of impaired coronary access; and 2) 10-20% of patients present an increased risk of coronary obstruction.
We previously highlighted the issues of coronary access and obstruction following redo TAVI, reporting MDCT images on the subject1. Such concepts are now spreading, but clinical data clarifying these risks are still limited.
In line with our findings, other studies based on angiography after TAVI suggested that redo TAVI would not be feasible in >20% of patients due to the risk of LM obstruction4 and in >30% of patients due to impaired coronary access3. Based on MDCT after TAVI, Rogers et al also suggested that redo TAVI would not be feasible due to the risk of coronary obstruction in 13.1% of 137 SAPIEN 3 patients5.
The present experience is currently the largest series to assess the risk of coronary access and obstruction after redo TAVI specifically through post-TAVI MDCT analysis. Importantly, this is a wide real-world “all-comer” TAVI population, treated with all of the different currently available devices. The images observed are quite provocative and the proportion of patients at risk is alarming.
Our results suggest that the most important baseline anatomical feature associated with impaired coronary access after redo TAVI is a small aortic STJ. Irrespective of the size of the sinuses of Valsalva, the STJ merits serious consideration because it provides the true bottleneck regulating access to the aortic root and coronary ostia.
On the other hand, the most important procedural predictor of impaired access was the height of index THV leaflets. While caution should be used when interpreting the exact numbers due to the limited cases in some subgroups, a trend is clear. Indeed, 85% of all cases of impaired access occurred in supra-annular THVs, the incidence in this group being two thirds of treated patients and being more than double compared to intra-annular THVs.
Among supra-annular THVs, three parameters mitigated the risk of impaired access in the present series: STJ width ≥28 mm, oversizing ≤12% and low implantation ≥8 mm. Unfortunately, such low implantation is not recommended since it carries a higher risk of paravalvular leak, conduction disturbances and device embolisation.
In intra-annular THVs, the overall numbers were limited, and the observed risk of impaired access was heterogeneous among different devices, due to the different heights of the leaflet commissures. A lower STJ was associated with increased risk in intra-annular devices, as well as female sex, probably because of the smaller anatomy frequently present in females.
While the present study was conducted in typical elderly TAVI patients, we believe that its findings will be largely applicable to a younger low-risk population. Bicuspid anatomy, however, which is more frequent in younger patients, may represent a notable exception, since it could not be reliably evaluated in our series due to small numbers. While our findings need validation, preliminary observations suggest that, to maintain an easier long-term coronary access, short intra-annular THVs should be favoured when treating patients with long life expectancy who may require redo TAVI, unless a large STJ is present.
Given the small number and the strict selection of procedures performed, little evidence is currently available about the actual risk of coronary obstruction after redo TAVI. The current largest real-world experience reported a 1% rate of coronary obstruction after redo TAVI6. In the present series, similarly to others4,5, a worrisomely higher 14.9% of cases were estimated to be at increased risk of coronary obstruction. It must be noted that procedures performed so far6 represent just a selected minority of TAVI patients, while our analysis includes a wider “all-comer” population. Furthermore, a number of redo TAVI procedures were performed due to index THV low malposition, which protects from the risk of coronary obstruction. Finally, as anticipated, actual THV interaction may sometimes reduce the risk of predicted coronary obstruction: short THVs inside tall THVs may not always completely jail the index leaflets, allowing some leaflet movement and resulting in a lower leaflet barrier.
Of note, using the VIVID 4 mm VTC cut-off, 42% of patients resulted in being at increased risk of coronary obstruction. The relevance of this finding has still to be determined. In contrast with surgical prostheses, THV leaflets are mounted inside the stent, which can prevent material protrusion towards the coronaries. However, THV are more oversized, can expand further after a second device implantation and frequently have higher leaflets. Furthermore, encumbrance of native valve debris and multiple THV in a small aortic root may cause slow blood flow, leading not only to acute but also to delayed coronary occlusion11.
Finally, we found that the STJ will become completely sealed by the index THV leaflets in 17% of patients, with nine of them showing a misleadingly reassuring VTC >4 mm. Once again, the STJ plays a major role, not just for coronary access but also in the development of coronary obstruction following redo TAVI, and will require major consideration.
The management of coronaries after TAVI is already demanding and will become even more challenging following redo TAVI, especially in peripheral centres undertaking emergency primary PCI. While the Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (“BASILICA”) may be helpful in this regard12, it requires alignment of THV commissures to coronary ostia, which is not the case in many TAVI patients, as confirmed in our study. The ability to control THV alignment recently described could potentially reduce the risk of commissure-coronary overlap and have a favourable impact on redo TAVI9.
Given such complex management, preventive measures are critical. While the medical device industry is working on the development of shorter prostheses, operators should start thinking ahead to the potential risks of redo TAVI when faced with a patient who has a long life expectancy. Indeed, the present data suggest that the coronaries can be preserved with careful assessment of patient anatomy and selection of device design.
Study limitations
This study must be considered preliminary and hypothesis-generating. The topic of coronary access and obstruction after redo TAVI is new; a validated body of knowledge, both MDCT and clinical, has not yet been established. Most importantly, like other studies in the field, the present analysis is theoretical, without documented control cases to validate the findings. Our data focused on prediction of the behaviour of the index TAVI device based upon its design. Structural leaflet disease over time could not be taken into account, as well as the possible different interactions between index and second THV, both at the level of the leaflets and above at the level of stent cell superimposition. Finally, this study was retrospective and observational with the inherent problems associated with such a design. The number of patients was also limited, especially when assessing subgroups. Further studies will be needed to validate our findings and investigate current gaps in knowledge.
Conclusions
Post-TAVI MDCT revealed that more than half of treated patients potentially have an increased risk of impaired coronary access if they were to undergo redo TAVI in the future. A small STJ and supra-annular leaflet design were associated with increased risk. Moreover, approximately 10-20% of patients presented an increased risk of coronary obstruction should redo TAVI be required. While this study is theoretical and hypothesis-generating, its implications raise an important concern that needs to be further investigated and addressed as TAVI is extended to younger low-risk patients with a longer life expectancy.
|
Impact on daily practice With the expansion of TAVI, the need for redo TAVI and maintaining coronary patency thereafter is destined to increase. The present MDCT analysis suggests that after redo TAVI coronary access and perfusion may be jeopardised in a significant proportion of patients. The development of shorter devices may help, and careful anatomy assessment and prosthesis selection should be carried out when considering TAVI in patients with a long life expectancy. |
Acknowledgements
Stefania Ruggeri, MS, conducted all statistical analyses.
Conflict of interest statement
O. De Backer has received consultant fees from Abbott and Boston Scientific. L. Søndergaard has received consultant fees and institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic and Symetis. B. Prendergast has received institutional research grants from Edwards Lifesciences. M. De Bonis has received consultant fees from Abbott Vascular and Medtronic. M. Montorfano has received consultant fees from Abbott Vascular, Boston Scientific and Edwards Lifesciences. A. Latib has received consultant fees from Abbott, Edwards Lifesciences and Medtronic and institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

