Not so long ago, towards the end of the last century, surgical aortic valve replacement (SAVR) with a mechanical prosthesis combined with lifelong anticoagulation using vitamin K antagonists (requiring regular haematological monitoring and with an attendant risk of bleeding) was the only treatment option for patients with severe aortic stenosis. The mainstream application of bioprosthetic surgical valves in the 1980s and 1990s presented a major breakthrough, allowing older patients at higher risk of bleeding the possibility of surgery without the need for lifelong anticoagulation. These patients were initially deemed unlikely to outlive their valve and the prospect of a second intervention was seldom considered.
Fast forward three decades and bioprosthetic valves are now used in the vast majority of patients undergoing aortic valve surgery while transcatheter aortic valve implantation (TAVI) has established its safety and efficacy across the spectrum of surgical risk such that clinical and anatomical factors (including age and life expectancy) now drive the choice between TAVI and SAVR12. In 2022, no one wants a mechanical valve and bioprosthetic valves are used in the majority of patients aged greater than 50 years undergoing surgery (driven by their reduction in bleeding complications, lower risk of valve thrombosis, and patient preference), often without robust evidence to support their durability. Meanwhile, TAVI is rapidly supplanting SAVR as the gold standard for first-time intervention for aortic stenosis, and valve-in-valve TAVI is a commonplace intervention for patients with failing surgical bioprostheses.
These seismic developments have transformed the management of patients with aortic stenosis and generated a new conversation concerning their lifetime journey, entailing a need for appropriate planning and optimal sequencing in the context of repeated valve interventions. The notion that successive TAVI (so-called TAV-in-TAV or revalving) procedures3 can be undertaken every ten years or so throughout a patient’s lifetime is appealing but overly simplistic for several reasons. First, the long-term durability of current TAVI devices remains unknown. Although data from the randomised trials and international registries are reassuring out to eight years, it will be some time before ten- to fifteen-year data become available to match those of contemporary surgical bioprosthetic valves4. Second, many younger patients with aortic stenosis have bicuspid valves – a population that was excluded from the pivotal randomised TAVI trials. Although registry data have demonstrated positive outcomes in carefully selected patients with bicuspid valves undergoing TAVI in highly experienced centres5, there is much to learn with regard to optimal device sizing and procedural technique6. Third, successive device implants may be the cause of progressive patient-prosthesis mismatch, which is associated with increased mortality and morbidity in both SAVR and TAVI recipients7. Fourth, multiple overlapping transcatheter heart valve frames in the aortic root (particularly those of supra-annular design) may present technical challenges at the time of diagnostic coronary angiography or percutaneous coronary intervention in individuals with pre-existing or de novo coronary artery disease (and could also be associated with a heightened risk of valve or coronary thrombosis over prolonged follow-up). And finally, if needed, surgical excision of TAVI devices is frequently difficult, with an increased incidence of adverse outcomes and high likelihood of aortic wall injury8.
A more sophisticated approach involving careful forethought, consideration of different interventional sequences and meticulous procedural planning is therefore essential when mapping out the lifetime journey of patients with aortic stenosis. In this issue of EuroIntervention, Medranda et al9 present a novel study utilising computed tomography (CT) simulation techniques to determine the risks of coronary obstruction (a potentially fatal complication of TAVI) in subjects undergoing a virtual TAV-in-TAV procedure. Amongst 213 participants enrolled in the low-risk TAVI (LRT) trial and EPROMPT registry, the accuracy of CT modelling was confirmed by a high level of concordance between predicted valve-to-coronary distance at baseline and 30-day follow-up. Subsequent CT simulation of TAVI followed by redo TAV-in-TAV predicted a low risk of coronary obstruction in 25% of subjects and a high risk in 28% (owing to sinus sequestration with need for leaflet modification or TAVI explant), regardless of the type of implanted valve. Redo TAV-in-TAV in the remaining 47% was associated with a low risk of coronary obstruction when using a balloon-expandable valve but a substantially higher risk when using a self-expanding valve. Within the constraints of CT modelling algorithms (and notwithstanding the numerous other clinical and anatomical factors outlined above), these findings demonstrate considerable interindividual variation in the feasibility of repeated TAV-in-TAV procedures, the potential importance of modified implantation techniques (such as commissural alignment) in at-risk subjects (particularly when using self-expanding valves), a need for continued bioengineering advances in this field, the importance of detailed imaging analysis in determining the appropriate choice of transcatheter heart valve and sequence of interventional procedures, and the potential role of CT simulation in guiding the lifetime journey for patients with aortic stenosis.
Surgical and transcatheter interventions are complementary options for patients with valve disease, and the pros and cons of different procedural sequences over the lifetime journey of patients with aortic stenosis is now a hot topic (Figure 1)10. Whilst the appropriate treatment algorithm may be self-evident for patients in their seventh or eighth decades, careful consideration is essential in younger patients who may face the prospect of three or four interventions – particularly those with grown-up congenital heart disease (who may have already undergone surgery or transcatheter intervention in infancy or childhood) and women of childbearing age who may wish to avoid the hazards of mechanical valves and mandated anticoagulation during pregnancy.
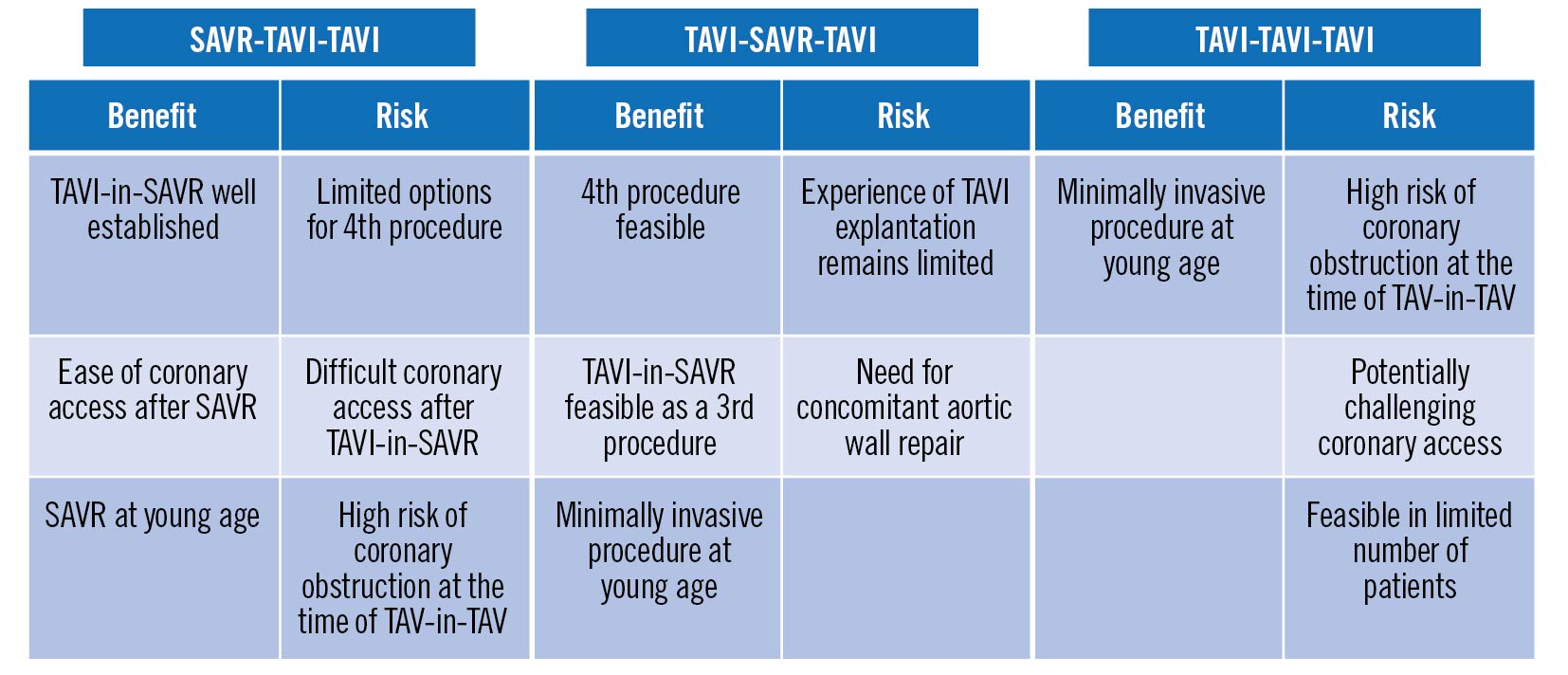
Figure 1. Risks and benefits of three potential interventional sequences in the lifetime management of severe aortic stenosis. Adapted with permission from reference 10. SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation
The present data reaffirm that the first intervention paves the way for lifetime management of patients with aortic stenosis. As in all aspects of TAVI, careful CT-facilitated preprocedural planning and a multidisciplinary Heart Team discussion concerning specific clinical and anatomical factors are essential to achieve the optimal lifelong outcome for individual patients.
Conflict of interest statement
B. Prendergast reports research and education grants to the institution from Edwards Lifesciences; consultancy fees from Microport and Anteris; speaker fees from Abbott, Medtronic and Edwards Lifesciences; and participation on the Colibri Data Safety Monitoring Board. T. Patterson has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

