Abstract
Background: Given enough time, transcatheter heart valves (THVs) will degenerate and may require reintervention. Redo transcatheter aortic valve implantation (TAVI) is an attractive strategy but carries a risk of coronary obstruction.
Aims: We sought to predict how many TAVIs patients could undergo in their lifetime using computed tomography (CT) simulation.
Methods: We analysed paired CT scans (baseline and 30 days post-TAVI) from patients in the LRT trial and EPROMPT registry. We implanted virtual THVs on baseline CTs, comparing predicted valve-to-coronary (VTC) distances to 30-day CT VTC distances to evaluate the accuracy of CT simulation. We then simulated implantation of a second virtual THV within the first to estimate the risk of coronary obstruction due to sinus sequestration and the need for leaflet modification.
Results: We included 213 patients with evaluable paired CTs. There was good agreement between virtual (baseline) and actual (30 days) CT measurements. CT simulation of TAVI followed by redo TAVI predicted low coronary obstruction risk in 25.4% of patients and high risk, likely necessitating leaflet modification, in 27.7%, regardless of THV type. The remaining 46.9% could undergo redo TAVI so long as the first THV was balloon-expandable but would likely require leaflet modification if the first THV was self-expanding.
Conclusions: Using cardiac CT simulation, it is possible to predict whether a patient can undergo multiple TAVI procedures in their lifetime. Those who cannot may prefer to undergo surgery first. CT simulation could provide a personalised lifetime management strategy for younger patients with symptomatic severe aortic stenosis and inform decision-making. ClinicalTrials.gov: NCT02628899; ClinicalTrials.gov: NCT03557242; ClinicalTrials.gov: NCT03423459.
Introduction
Younger patients with symptomatic severe aortic stenosis (AS) who require (or request) a bioprosthetic valve will likely require more than one aortic valve replacement in their lifetime. Depending on their aortic root anatomy and the choice of transcatheter heart valve (THV), some patients should be able to undergo multiple transcatheter aortic valve implantation (TAVI) procedures in their lifetime. However, some patients may only be able to have one TAVI procedure because redo TAVI risks coronary obstruction. Knowing this in advance could inform patients’ decision-making in favour of surgical aortic valve replacement (SAVR) first, with the possibility of valve-in-valve TAVI later if needed. Previous computed tomography (CT) simulations have evaluated the risk of sinus sequestration and coronary obstruction in patients who have already undergone TAVI12345678. At-risk patients may require adjunctive leaflet modification prior to redo TAVI to preserve coronary flow91011. Herein, we sought to use patients’ baseline CT scans to predict how many TAVI procedures they could undergo in their lifetime using datasets from the LRT trial (Low-Risk Transcatheter Aortic Valve Replacement [TAVR]: NCT02628899, NCT03557242) and the EPROMPT registry (CoreValve Evolut Pro Prospective Registry: NCT03423459).
Methods
The LRT trial was the first trial in the United States to evaluate the safety and feasibility of TAVI in low-risk patients using commercially available balloon-expandable valves (BEVs) or self-expanding valves (SEVs). The LRT trial design and clinical results have been described previously12131415. The EPROMPT registry is a multicentre, prospective registry of patients with symptomatic severe tricuspid AS undergoing TAVI using a commercially available SEV16. THVs included in our analyses were the SAPIEN 3 (Edwards Lifesciences) and the CoreValve Evolut PRO/PRO+ (Medtronic). All patients in the LRT trial and those in the CT cohort of the EPROMPT registry underwent baseline and 30-day post-TAVI CT scans (Figure 1). The CT software used to perform our analyses was IntelliSpace Portal (Philips) and Synapse 3D (Fujifilm). Details of the CT acquisition protocol are described in Supplementary Appendix 1, Supplementary Appendix 2. The research protocols for the LRT trial and the EPROMPT registry were approved by the relevant Institutional Review Boards.
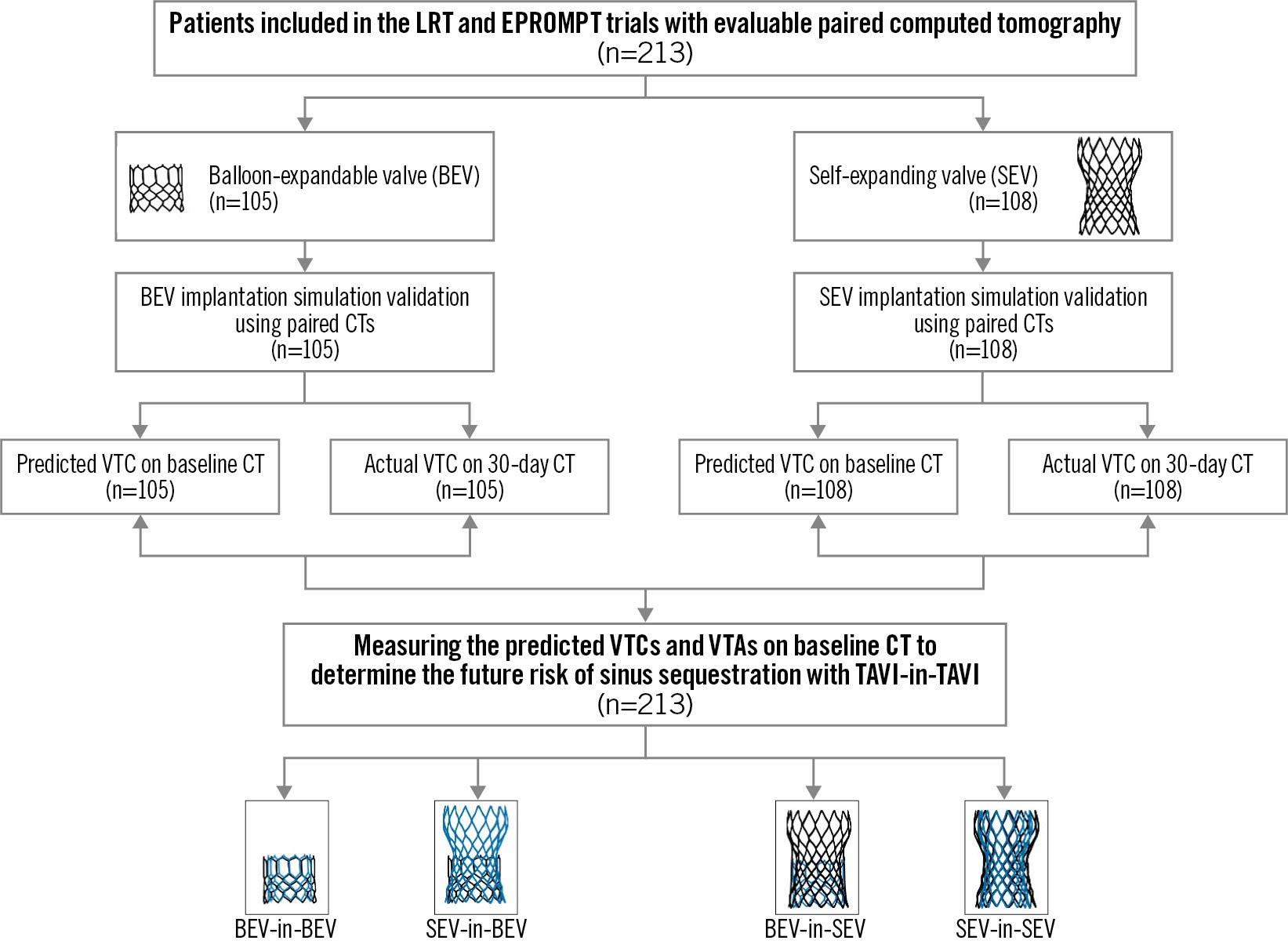
Figure 1. Study design. CT: computed tomography; TAVI: transcatheter aortic valve replacement; VTA: valve-to-aorta; VTC: valve-to-coronary
The purpose of this study was to simulate not one but two TAVI procedures (TAVI followed by redo TAVI) and to predict the risk of coronary obstruction. The predominant mechanism of coronary obstruction in this scenario is sinus sequestration, defined as complete isolation of a sinus of Valsalva from aortic blood flow. Sinus sequestration is a common mechanism of coronary obstruction following redo TAVI as the displaced THV leaflets are pinned outward in the open position by the second THV, creating a covered stent. For patients with unfavourable anatomy (low and narrow sinuses of Valsalva), this covered stent risks sequestering the sinus and obstructing blood flow to the coronary arteries that arise therein. We deliberately did not investigate challenges to selective coronary catheterisation following TAVI or redo TAVI in this study, as described in other studies17. Additionally, because current-generation THVs do not allow for precise commissural alignment, we elected not to factor this into the simulation.
Step 1: CT simulation of first TAVI using baseline CT scans
To determine the accuracy of CT simulation of first TAVI, we virtually implanted on baseline CT scans the THV each patient actually received (BEV or SEV) and compared predicted virtual valve-to-coronary (VTC) distances with actual VTC measurements on 30-day post-TAVI CT scans (Figure 1). The virtual THV implant was sized according to the native aortic annulus (area and perimeter were measured), and virtual implantation depth was selected according to contemporary TAVI techniques: 1) for BEV, an 80:20 implantation depth was used; and 2) for SEV, an implantation depth of 4 mm was used. Virtual VTC distances were then measured from the virtual valve to the ostia of both coronaries. We compared the degree of agreement between the predicted VTC distances on baseline CTs and actual VTC distances on 30-day CTs.
Step 2: CT simulation of redo TAVI using baseline CT scans
To predict the feasibility of redo TAVI, we implanted a virtual covered stent on baseline CT scans. The diameter and height of the virtual covered stent was determined by the type of THV (BEV vs SEV), the combination of redo TAVI THV permutations (e.g., BEV-in-BEV, SEV-in-SEV, etc.), and the height at which the leaflets would be pinned in the open position (Supplementary Table 1). The minimum distances from this covered stent to the aorta wall above each coronary were defined as the virtual valve-to-aorta (VTA) distances. We classified the redo TAVI risk of sinus sequestration (Supplementary Figure 1) as follows:
a) Low risk: feasible without leaflet modification (VTC ≥4 mm and VTA ≥2 mm for both coronaries and/or coronary ostia that arise above the pinned leaflet plane);
b) Intermediate risk: feasible but may require leaflet modification (any other VTC/VTA combination);
c) High risk: feasible but very likely to require leaflet modification (VTC <4 mm and VTA <2 mm for both coronaries).
In two scenarios (BEV-in-BEV and SEV-in-BEV), the diameter and height of the virtual covered stent was predicted to be the same regardless of the type of the second THV. In SEV-in-SEV, the virtual covered stent was predicted to be the height of the pinned leaflet plane of the first THV. In the final scenario (BEV-in-SEV), we simulated the BEV aligned with the inflow of the SEV, which theoretically maximises leaflet overhang, which we estimated may lower the pinned leaflet plane and, thus, the virtual covered stent by approximately 5 mm compared to SEV-in-SEV. However, in most patients, BEV-in-SEV was also predicted to expand the original SEV waist, which we accounted for by widening the diameter of the virtual covered stent.
Step 3: CT simulation of redo TAVI using 30-day CT scans
To validate the redo TAVI simulation, we implanted virtual BEV-in-BEV for patients who actually received a BEV as their first TAVI (n=105) and virtual SEV-in-SEV for patients who actually received an SEV as their first TAVI (n=108), using their actual 30-day CT scans with THV in situ.
Statistical analysis
Continuous variables are presented as mean±standard deviation. Categorical variables are presented as n/N (%). Bland-Altman plots were used to evaluate agreement between predicted and actual CT measurements as well as to evaluate interobserver variability.
Results
Study population
A total of 213 patients with evaluable paired CT scans (baseline and 30 days post-TAVI) were included in the analysis. Table 1 summarises their baseline characteristics. Supplementary Table 2 and Supplementary Table 3 summarise native aortic root CT measurements. Of the 213 included patients, 105 actually received a BEV and 108 actually received an SEV (Figure 1). No patient actually underwent redo TAVI within 30 days.
Step 1: CT simulation of first TAVI
The implantation depth goal of ≤20% ventricular with BEV was achieved in 74.3% (n=78/105) of patients and the goal of 3-5 mm with SEV was achieved in 68.5% (n=74/108) of patients on 30-day post-TAVI CT. Despite this, predicted VTC using baseline CT demonstrated excellent agreement to actual VTC using 30-day post-TAVI CT in patients who received a BEV (n=105; left VTC bias: -0.53 mm and right VTC bias: 1.45 mm) and patients who received an SEV (n=108; left VTC bias: 0.51 mm and right VTC bias: 0.88 mm) (Figure 2, Supplementary Table 2, Supplementary Table 3). Additionally, we demonstrated acceptable interobserver variability for predicted VTC distances in a random subset (Supplementary Figure 2).
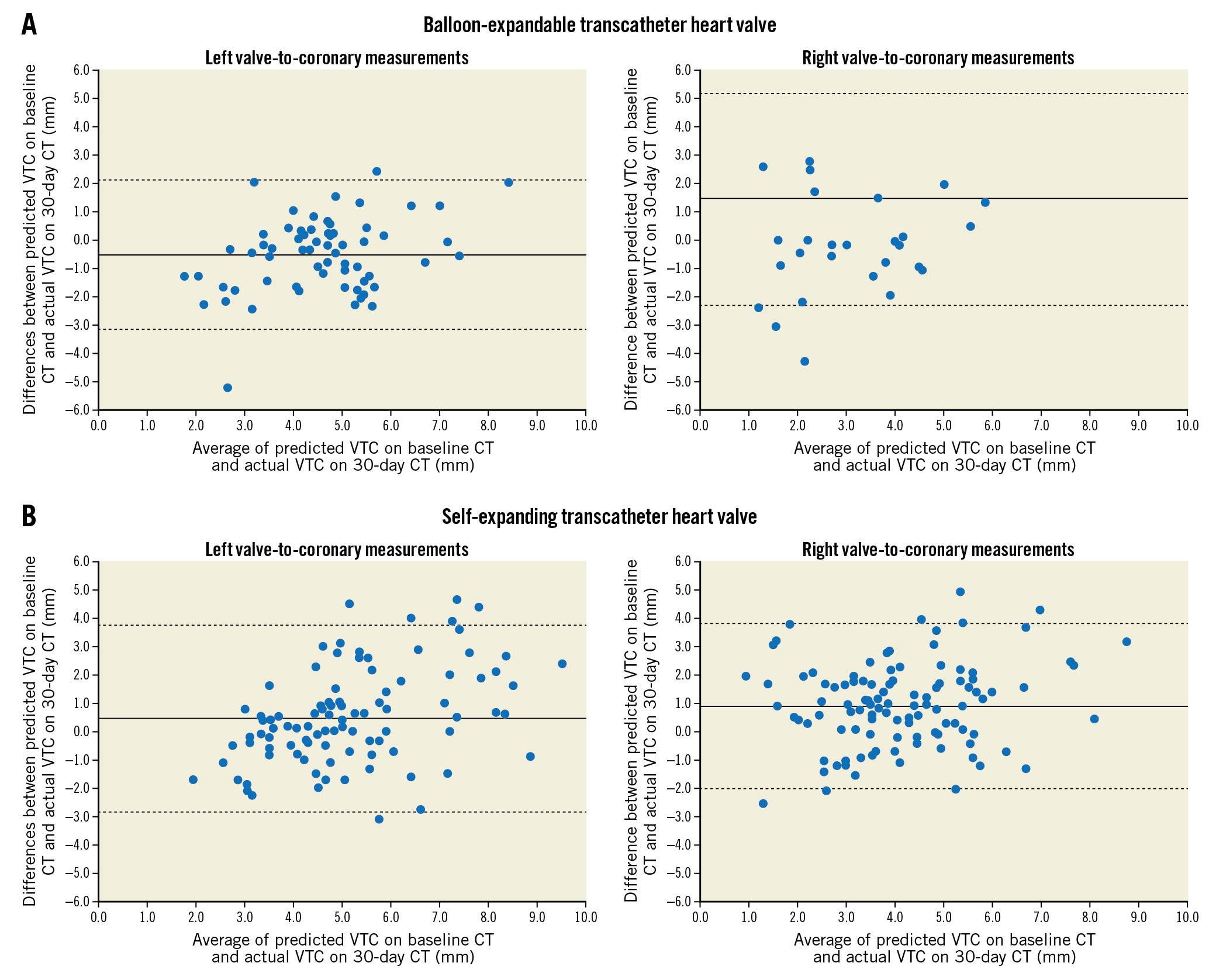
Figure 2. Bland-Altman plots of predicted valve-to-coronary (VTC) on baseline computed tomography (CT) compared to actual VTC on 30-day CT in patients who received A) the balloon-expandable transcatheter heart valve (THV; left VTC bias: -0.53 mm and right VTC bias: 1.45 mm) or B) the self-expanding THV (left VTC bias: 0.51 mm and right VTC bias: 0.88 mm). Only patients whose coronary ostia arose below the pinned leaflet plane were included in this analysis.
Step 2: CT simulation of redo TAVI using baseline CT scans
If the first THV implanted was a BEV, we predicted that redo TAVI should be feasible, regardless of the type of the second THV (BEV or SEV), without the need for leaflet modification in 72.3% of patients (154/213) and would likely require leaflet modification in 27.7% of patients (59/213) (Figure 3). If both the first and second THVs were SEVs, we predicted that redo TAVI should be feasible without the need for leaflet modification in 25.4% of patients (54/213) but would likely require leaflet modification in 74.6% of patients (159/213) (Figure 3). If the first THV was an SEV and the second was a BEV, we predicted that redo TAVI should be feasible without the need for leaflet modification in 8.9% of patients (19/213) and would likely require leaflet modification in 91.1% of patients (194/213). Overall, 25.4% of patients (54/213) had anatomy that should allow for TAVI followed by BEV-in-BEV, SEV-in-BEV or SEV-in-SEV with a low risk for sinus sequestration and no need for leaflet modification. Additionally, 46.9% of patients (100/213) had anatomy that should allow for TAVI followed by BEV-in-BEV or SEV-in-BEV with a low risk for sinus sequestration and no need for leaflet modification but would likely require leaflet modification prior to SEV-in-SEV. Finally, the remaining 27.7% of patients would be high risk for sinus sequestration and would likely require leaflet modification prior to the second procedure regardless of the combination of THVs (Figure 3, Central illustration). Representative CT scans can be found in Figure 4.
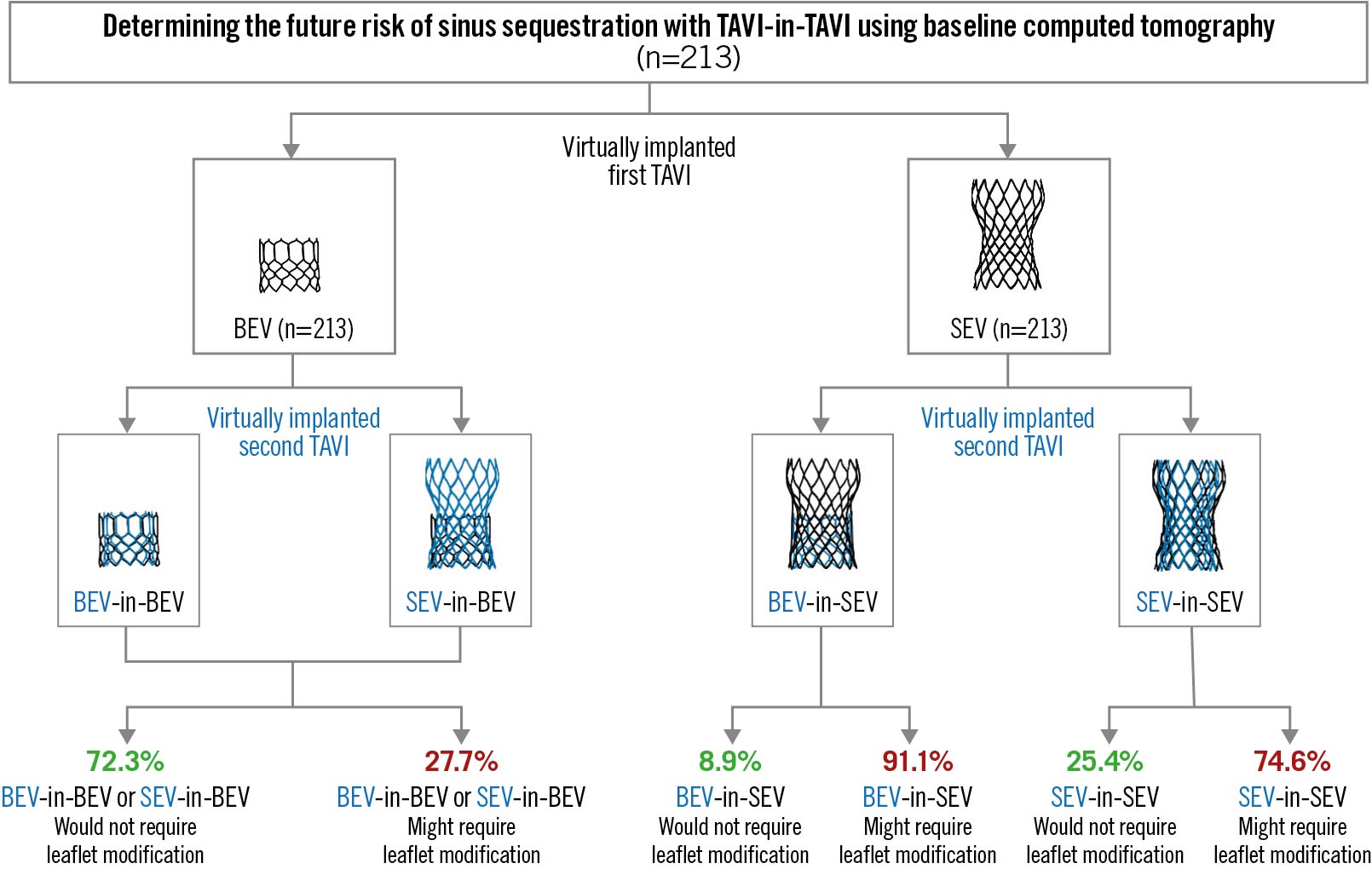
Figure 3. CT simulation of serial TAVI procedures with different combinations of THV. BEV: balloon-expandable valve; CT: computed tomography; SEV: self-expanding valve; TAVI: transcatheter aortic valve replacement; THV: transcatheter heart valve
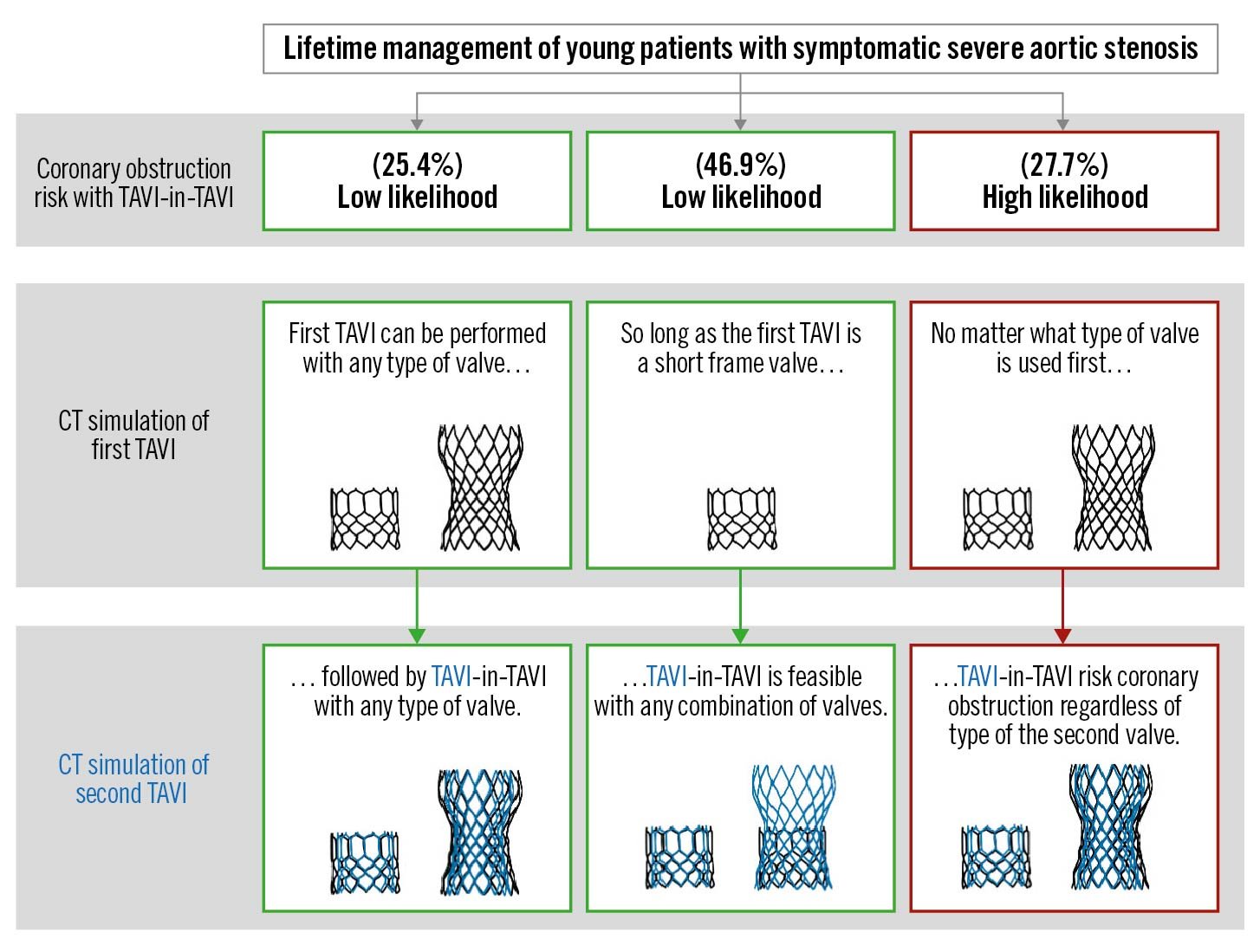
Central illustration. Coronary obstruction risk with serial aortic valve interventions. CT: computed tomography; TAVI: transcatheter aortic valve implantation
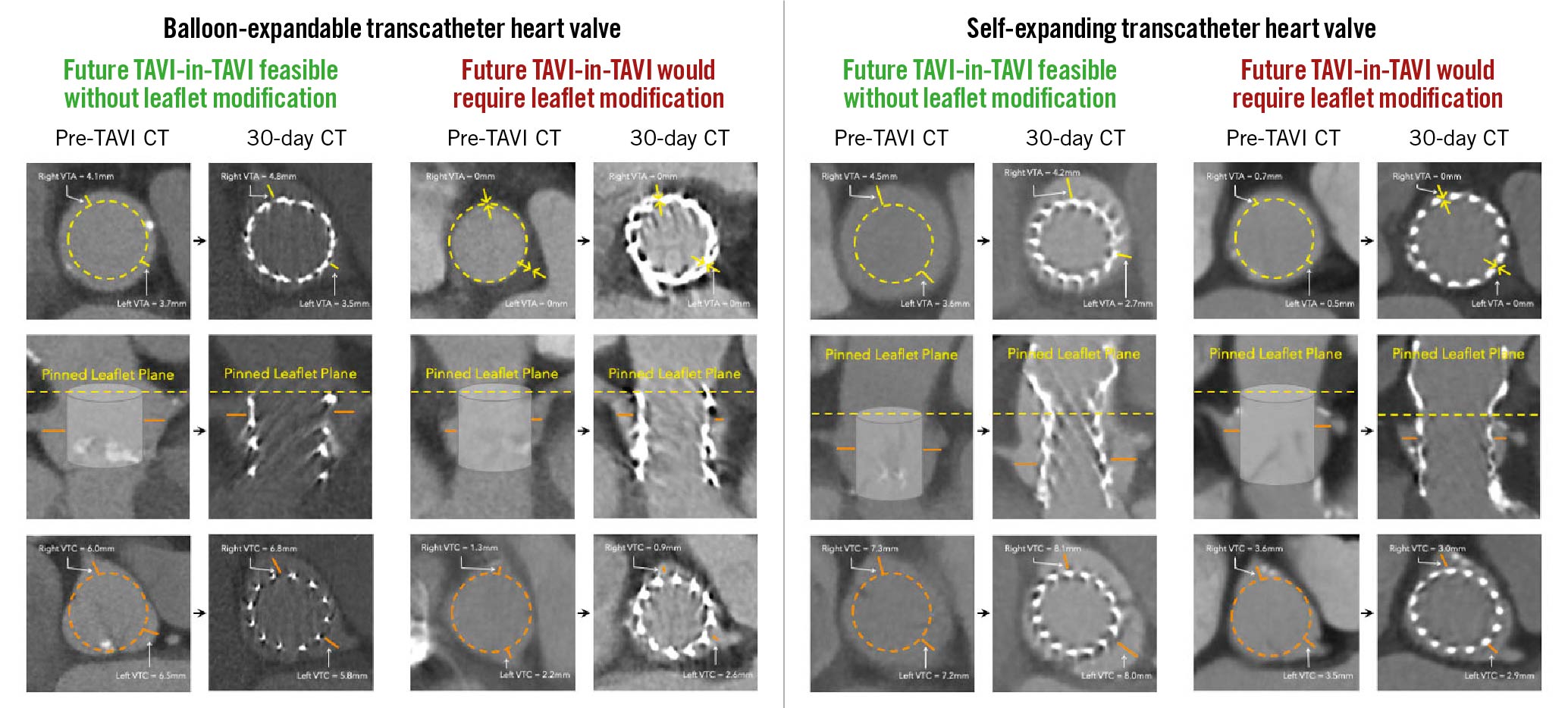
Figure 4. Aortic root anatomies and measurements on baseline and 30-day CT using both the balloon-expandable (left) and self-expanding (right) transcatheter heart valve. CT: computed tomography; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; VTA: valve-to-aorta; VTC: valve-to-coronary
Step 3: CT simulation of redo TAVI using 30-day CT scans
For patients who actually received a BEV, we predicted that redo TAVI should be feasible regardless of the type of the second THV (BEV or SEV) without need for leaflet modification in 70.5% of patients (74/105) and would likely require leaflet modification in 29.5% of patients (31/105) (Supplementary Figure 3). If both the first and second THVs were SEVs, we predicted that redo TAVI should be feasible without the need for leaflet modification in 21.3% of patients (23/108) but would likely require leaflet modification in 78.7% of patients (85/108) (Supplementary Figure 3). If the first THV was an SEV and the second was a BEV, we predicted that redo TAVI should be feasible without the need for leaflet modification in 9.3% of patients (10/108) and would likely require leaflet modification in 90.7% of patients (98/108). The prediction of coronary obstruction using 30-day CTs showed good agreement with the prediction of coronary obstruction using baseline CTs (agreement in 93.0% [198/213] of patients).
Discussion
This is the first study to attempt to predict how many TAVI procedures a patient can undergo in their lifetime. The objective is to provide patients with a personalised lifetime management strategy assuming they will need more than one bioprosthesis. The principal findings of this CT simulation study are as follows: 1) approximately 1/4 of patients had aortic root anatomy that would allow for TAVI followed by redo TAVI with any combination of THVs with a low risk for coronary obstruction and no need for leaflet modification; 2) just over 1/4 of patients had aortic root anatomy that would likely require leaflet modification prior to redo TAVI regardless of the type of the first THV; 3) just under 1/2 of patients had aortic root anatomy that would allow for TAVI followed by redo TAVI without leaflet modification if the first valve was a BEV but would require leaflet modification if the first valve was an SEV; 4) and finally, the BEV-in-SEV leaflet overhang strategy did not appear to substantially alter the predicted risk of sinus sequestration and need for leaflet modification.
Coronary obstruction is a rare but devastating complication of TAVI that occurs more frequently in patients undergoing valve-in-valve TAVI for failing surgical bioprostheses181920. During TAVI, native or bioprosthetic aortic valve leaflets are displaced by the new THV and pinned in the open position. In patients with a narrow sinotubular junction, low coronaries and/or effaced sinuses of Valsalva, these displaced leaflets can occlude the coronary ostia or sequester an entire sinus of Valsalva, leading to coronary obstruction. Given its relatively recent emergence and because most early TAVI patients were older with multiple comorbidities, the collective experience with failing THV remains limited. Surgical explantation may require ascending aorta repair and/or root reconstruction and is associated with increased morbidity and mortality2122. Redo TAVI is an attractive alternative to surgery and, in carefully selected patients, appears to be a safe procedure23. However, because the pinned leaflet plane of a failing THV is often above the coronary ostia, coronary obstruction from sinus sequestration is predicted to be more common123. Case series of successful redo TAVI procedures23 potentially belie the true risk because CT is routinely performed preprocedure, and patients at risk are screened out to undergo surgery or adjunctive leaflet modification procedures (e.g., BASILICA9) or to be managed conservatively.
BASILICA (bioprosthetic or native aortic scallop intentional laceration to prevent coronary artery obstruction) is an electrosurgical technique to lacerate the leaflet of a failing native, surgical bioprosthesis or THV, so that once a new THV is implanted inside, the laceration splays the two halves of the leaflet to either side creating a pathway for blood to reach the sequestered sinus of Valsalva and the coronary that arises therein92425. Initial experience in the prospective multicentre BASILICA trial demonstrated feasibility and effectiveness in preventing sinus sequestration and subsequent coronary obstruction with both native aortic and surgical bioprosthetic aortic valves9. BASILICA has since been shown to be safe and effective in a multicentre, real-world registry of over 200 procedures, with high procedural success and low complication rates11. However, redo TAVI presents additional complexity due to arbitrary commissural alignment of the first THV: indeed, simply creating a splay in a leaflet may not be effective if the commissures of the first THV are misaligned. Furthermore, long THV leaflets26 may require modification of “conventional” BASILICA to include angioplasty at the leaflet base before laceration to maximise the splay (so-called balloon-assisted BASILICA10). In both these scenarios, partial or full leaflet excision may be needed. This technology is in development.
Previous studies predicted risk of sinus sequestration and coronary obstruction during redo TAVI using CT or fluoroscopy analysis of patients who had already undergone TAVI12345678. Ideally, lifetime management strategies should be determined before the first TAVI, while patients can still consider alternatives including surgery first. In these studies, as many as 25.0% of patients with a previous BEV, and as many as 88.9% of patients with a previous SEV, were predicted to be at risk for coronary obstruction during redo TAVI using the same THV (BEV-in-BEV or SEV-in-SEV). The present study resulted in similar predictions using the baseline CT scan rather than the post-TAVI CT scan, affirming the accuracy of our CT simulation model. However, our prediction model was contingent on THV sizing and deployment according to the instructions for use and does not account for extremely shallow or deep implantations or under- or over-expansion. This is important because sinus sequestration appears to be rare in the setting of ad hoc redo TAVI during an index procedure. This is likely because the indication for the second THV is commonly severe paravalvular leak related to deep implantation of the first THV26. Deep implantation of the first THV lowers the pinned leaflet plane, which reduces the risk of sinus sequestration when the second THV is implanted. With regard to commissural alignment, current-generation THVs do not allow for precise commissural alignment, but we acknowledge that fortuitous commissural alignment may tip the scales to decrease – or conversely increase – the risk of sinus sequestration in real-world experience. Additionally, certain implantation techniques during SEV delivery and deployment may improve commissural alignment as well.
The key difference between BEVs and SEVs is the supra-annular design of the SEV, which maximises the valve effective orifice area to optimise haemodynamics and durability27. This also elevates the pinned leaflet plane above the coronary ostia in nearly every patient, hence the predicted higher risk of sinus sequestration with redo TAVI in SEV. One possible strategy to mitigate this is to implant a BEV deep inside an SEV to allow for “leaflet overhang” and effectively lower the pinned leaflet plane28. Implanting a BEV inside an SEV typically expands the SEV frame at the waist, transforming its hourglass shape into a cylinder, which reduces VTC distances. This strategy also assumes the leaflets of the failing SEV are pliable and able to hang over the top of the BEV stent frame in diastole to permit blood flow down to the sinuses of Valsalva. Degenerated, calcified and stiff leaflets are less likely to behave predictably in this way and may instead pin open if the hinge point interacts with the BEV stent frame, regardless of its implantation depth.
Counselling younger patients with symptomatic severe AS should include consideration of lifetime management strategies; specifically, what treatment options would be available in the future if they opt for a transcatheter procedure and their bioprosthesis later fails. The CT simulation described in this study brings a more analytical approach to future lifetime management considerations and should inform patient and Heart Team shared decision-making. For example, if CT simulation predicts that a young patient can only have one TAVI procedure, then the patient may prefer to undergo surgery with implantation of a mechanical valve or a bioprosthetic surgical valve that is amenable to future valve-in-valve TAVI. If CT simulation predicts that a patient can have two TAVI procedures, regardless of the type of THV, then this should reassure the patient that surgery can be avoided, and the THV can be selected based on other factors (e.g., haemodynamics, presence of left ventricular outflow tract calcium, etc.). If CT simulation predicts that a patient can have two TAVI procedures, but only if the patient receives a particular THV first, then this may inform the choice of initial THV. We recognise, of course, that these decisions are highly individualised, and lifetime management is just one factor that must be weighed by the Heart Team when recommending surgery vs TAVI or BEV vs SEV. In the coming years, additional data regarding THV durability, structural valve deterioration, and THV thrombosis (e.g., from the pivotal low-risk trials) are also likely to impact the decision-making of TAVI vs SAVR (surgical aortic valve replacement) and SEV vs BEV in younger patients.
Study limitations
We recognise that this is a simulation study and, therefore, subject to certain limitations. The VTC and VTA cut-offs used may have been overly conservative. We simulated THV implantation depth per contemporary recommendations, and hence, findings may not be applicable to patients who underwent TAVI in the past with deeper THV deployment. In some patients, CT can exaggerate obstruction risk, due to imaging artefact/blooming from the metallic stent frame. Also in some patients, THV deployment may not be perfectly coaxial, with the aortic root leaning away from (or towards) a particular coronary ostium. Invasive aortography can be a useful adjunctive modality to evaluate risk of coronary obstruction. Unfortunately, fluoroscopic images were not collected for central core lab analysis in the LRT trial and the EPROMPT registry. Additionally, 30-day CT acquisition protocols were not always optimised for the determination of commissural alignment. For obvious reasons, we could not test the accuracy of sinus sequestration risk predictions by performing redo TAVI in every patient. However, our predictions based on the baseline CT scans were very similar to those reported by our group and others using post-TAVI CT scans, suggesting that the CT simulation model is reproducible12345678. Coronary perfusion after redo TAVI is potentially preserved by flow down to one coronary sinus and then around to the other sinuses, but this is difficult, if not impossible, to model using CT. Predictions of the need for leaflet modification are based on our interpretation of the available literature and data. Other strategies to prevent coronary obstruction, such as “snorkel stenting”, were not considered because, in the setting of redo TAVI, the risk of coronary stent compression/crush and stent thrombosis is high, and the protruding stent obstructs coronary access for angiography or percutaneous coronary intervention. Finally, we recognise that our findings cannot necessarily be extrapolated to other THVs (e.g., ACURATE neo2 [Boston Scientific], Portico [Abbott]) which have different stent frame and leaflet geometries.
Conclusions
Using CT simulation, it is possible to predict whether a patient can undergo multiple TAVI procedures in their life time or not. Those who cannot may prefer to undergo surgery first. CT simulation could provide a personalised lifetime management strategy for younger patients with symptomatic severe AS and inform shared decision-making.
Impact on daily practice
The optimal lifetime management of patients with aortic stenosis with bioprosthetic valves (surgical or transcatheter) remains unclear. Serial transcatheter aortic valve implantation (TAVI)-in-TAVI risks coronary obstruction. Using baseline computed tomography scans, it is possible to plan not only the first TAVI but also to predict the feasibility of future re-intervention with redo TAVI should the first valve fail. This could provide a personalised lifetime management strategy for younger patients with symptomatic severe aortic stenosis.
Funding
MedStar Health Research Institute, Washington, D.C., USA and Medtronic, Minneapolis, MN, USA contributed funding to this study.
Conflict of interest statement
R. Torguson reports being a consultant for Pi-Cardia Ltd. J. C. Wang reports being a consultant for Edwards Lifesciences, and Cordis; and has received speaker and consultancy fees from Boston Scientific. P. Parikh has served as a consultant for Medtronic; and has received a research grant from Edwards Lifesciences. F. Asch is Director of the MedStar Health Academic Echocardiography Core Laboratory, which has institutional contracts with Medtronic, Edwards Lifesciences, Abbott, Boston Scientific, Biotronik, and LivaNova, but has no personal disclosures. G. Weissman is the director of an academic cardiac computed tomography core lab with institutional contracts with Ancora Heart and LivaNova, but has no personal disclosures.. H. M. Garcia-Garcia reports receiving institutional grants from Medtronic, Biotronik, Neovasc, Boston Scientific, Abbott, Shockwave, Chiesi, and Philips, but has no personal dsiclosures. R. Waksman has served on an advisory board for Abbott, Boston Scientific, Medtronic, Philips IGT, and Pi-Cardia Ltd.; has been a consultant for Abbott, Biotronik, Boston Scientific, Cordis, Medtronic, Philips IGT, Pi-Cardia Ltd., Swiss Interventional Systems/SIS Medical AG, Transmural Systems Inc., and Venous MedTech; reports grant support from AstraZeneca, Biotronik, Boston Scientific, Chiesi, Medtronic, and Philips IGT; is part of the speakers bureau of AstraZeneca; and is an investor in MedAlliance, and Transmural Systems Inc. T. Rogers is a proctor and consultant for Medtronic, and Edwards Lifesciences; has served on the advisory board for Medtronic; and reports equity interest in Transmural Systems. He is also a co-inventor on patents, assigned to NIH, for transcatheter electrosurgery devices. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

