Abstract
Computed tomography (CT) provides high, isotropic spatial resolution and has become firmly established in pre-procedural imaging for structural heart disease interventions. It allows determination of the exact dimensions of the target structure, provides information regarding the access route and permits identification of fluoroscopic projection angles to provide optimal visualisation for device placement. Several software solutions are available and have been systematically evaluated in the context of transcatheter aortic valve implantation (TAVI). The use of software products to perform automated measurements can be useful, especially when the experience and expertise regarding evaluation of CT in the context of structural heart disease are limited. In scientific studies, software has been demonstrated to provide accurate support for annulus sizing and prosthesis selection, to aid in reliably identifying patients in whom a transfemoral access may be problematic, and to suggest suitable angulations for fluoroscopic imaging to achieve an orthogonal view onto the aortic valve during implantation.
Introduction
Computed tomography (CT) imaging is particularly well suited for morphologic imaging of the heart1. Modern CT scanners, in combination with ECG synchronisation of data acquisition or image reconstruction, provide sufficient temporal resolution to avoid motion artefacts. Spatial resolution is high and, because of very thin slice thickness, virtually isotropic. This means that through-plane spatial resolution is as high as in-plane resolution. Reconstructions in orientations other than the originally acquired axial slices therefore have the same or nearly the same visual appearance and spatial resolution as the axial images (Figure 1). CT data sets hence permit the reconstruction of images in any desired plane without losing the ability to perform exact measurements.
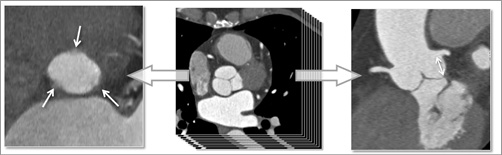
Figure 1. CT imaging of the aortic root. From the originally acquired slices in axial orientation, images in any desired plane can be reconstructed, while retaining high spatial resolution. Here, a plane that is exactly aligned with the aortic annulus is shown on the left (arrows point at the lowest points of the three aortic valve cusps), and a plane that permits measurement of the distance of the coronary ostia from the aortic annulus (arrow) is shown on the right.
Coronary CT angiography is by far the most frequent application of cardiac CT imaging. It is used to detect and rule out coronary artery stenoses and has been incorporated into numerous guidelines2-4. Furthermore, CT can assist in pre-procedural planning of coronary interventions, in particular for the revascularisation of chronic total occlusions5,6.
CT imaging has also gained increasing importance for pre-procedural planning of structural heart disease interventions, again due to its isotropic spatial resolution, which allows analysis of cardiac structures in arbitrary planes1. In this context, the use of CT imaging in the work-up of candidates for transcatheter aortic valve implantation (TAVI) is the most frequent and most prominent application, but there are numerous other interventions which potentially benefit from pre-procedural CT imaging: these, for example, include transcatheter mitral valve implantation, left atrial appendage occlusion, paravalvular leak occlusion or implantation of the Parachute® device (CardioKinetix, Inc., Menlo Park, CA, USA) for left ventricular partitioning in ischaemic heart failure7-10.
Information that CT can provide for structural heart disease interventions
In general, there are three major types of information that CT imaging can provide in the context of planning for structural heart disease interventions: precise information regarding the dimensions of the target structure, information on the access route, and information on optimal fluoroscopic projections to provide visualisation of the target structure without foreshortening (Figure 2). Obviously, not all interventions require CT for planning, not all patients can undergo CT imaging (due to, for example, the need for contrast injection), and not all investigators have equal expertise regarding cardiac CT. At some sites, alternative imaging modalities may be available with higher levels of expertise than CT, such as magnetic resonance tomography or three-dimensional echocardiography. CT can therefore be considered as one of many options for interventional imaging, albeit a robust one that can provide a large amount of information.
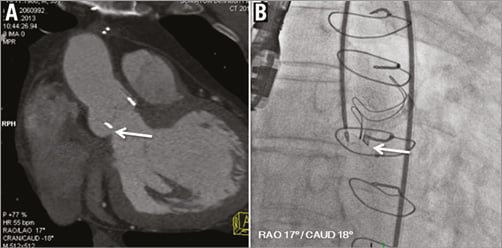
Figure 2. CT-assisted planning of structural heart interventions. Visualisation of a paravalvular leak next to an aortic valve bioprosthesis (A, arrow). The optimal angulation for visualisation of the leak (here: RAO 17°/caudal 18°) can be obtained from CT and used as C-arm angulation during the interventional placement of an occlusion device (B, arrow).
CT data acquisition
For CT image acquisition, certain recommendations can be made in order to optimise image quality in the context of structural heart disease interventions. In order to achieve high and isotropic spatial resolution, it is desirable to choose a protocol that provides a reconstructed slice width of 0.6 to 0.75 mm, resulting in approximately 300 slices for covering the entire heart. Additional acquisitions or reconstructions may be necessary to visualise the access route, for example the iliac arteries. While the peripheral vessels and aorta can be visualised without ECG synchronisation, ECG-synchronised imaging, either through retrospective gating or prospective triggering, and suspended respiration during acquisition are indispensable in order to minimise motion artefacts for the heart itself. If desired, the entire cardiac cycle can be covered to provide systolic and diastolic information, albeit at the cost of increased radiation exposure. In high heart rates, motion artefact may be present, so that short-acting beta-blockers are often used to optimise imaging conditions (and are recommended for heart rates >60-65 beats/min, if clinically possible). Typically, diastolic imaging is used for cardiac CT (triggered at approximately 70% of the cardiac cycle). However, in high heart rates and in irregular rhythms, systolic imaging may be better (e.g., 250-350 ms after the R-peak). It has further been suggested that imaging for TAVI evaluation should be performed in systole11. The intravenous application of iodinated contrast (50-100 ml) is mandatory. For pre-procedural TAVI imaging, protocols that require substantially lower amounts of contrast agent have been published, given the high proportion of elderly patients with renal failure in that patient cohort12,13. Radiation exposure strongly depends on the image acquisition protocol, but typically ranges from 1 to 10 mSv14.
CT data evaluation
When analysing the acquired CT data sets, one particularly important step is to define and create an imaging plane that is exactly aligned with the target structure. This is a prerequisite for the exact measurement of dimensions and for the determination of optimal viewing angles. For example, in case of pre-procedural TAVI imaging, an image plane must be created that is exactly aligned with the aortic annulus (Figure 3)15. Multiplanar reconstruction with careful interactive manipulation of imaging planes is frequently used for this purpose. While this is easily possible with sufficient experience16, specific semi-automated or fully automated software products are available to aid with the required image processing17,18. They have been evaluated in a number of clinical trials, the results of which will be outlined below. Most of the available software products have been developed specifically for TAVI, since this is by far the most frequent structural heart disease intervention for which CT imaging is used as a reference. Hence, in the subsequent text, the evaluation of software products specifically in the context of TAVI planning will be outlined.
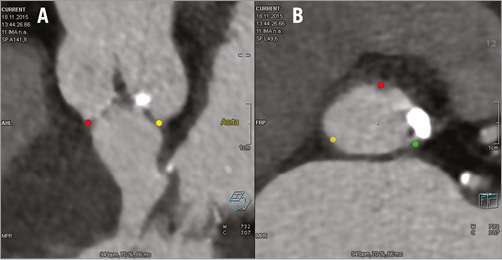
Figure 3. Pre-procedural semi-automated assessment for TAVI procedure using Siemens Valve Pilot software. The three lowest points of the aortic valve cusps (“nadirs”) are automatically identified (A) and a plane is created that contains these three points and hence corresponds to the aortic annulus plane (B).
Systematic evaluation of software to support TAVI planning
Various commercially available software products provide automated or semi-automated measurements of parameters that are useful for TAVI planning. Among the most common, 3mensio Structural Heart (Pie Medical Imaging BV, Maastricht, The Netherlands) is a standalone software for, amongst other applications, semi-automated aortic root measurements that runs on personal computers17,19-21. This software automatically segments the ascending aorta and stretches the vessel. The user then manually adjusts the lumen centreline and defines the annulus plane by identifying the three nadirs of the leaflets. Subsequently, aortic root measurements, including diameters of the aortic annulus, sinotubular junction, left ventricular outflow tract and the distance to the coronary ostia, angulation of the aortic arch and calcium scoring of the valve are manually performed and the angulation of the aortic valve plane can be determined (Figure 4). Other vendors provide similar or more automated solutions, sometimes integrated into image processing workstations and not available as separate products, such as syngo.via CT Cardiac Function – Valve Pilot (Siemens Healthcare, Forchheim, Germany) (Figure 3, Figure 5) and HeartNavigator (Philips Healthcare, Best, The Netherlands)18,22.
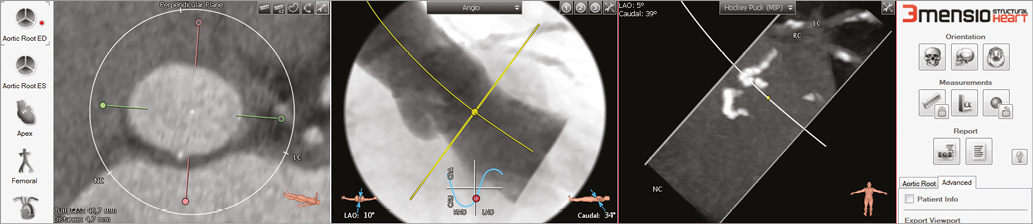
Figure 4. Pre-procedural assessment for TAVI procedure using 3mensio Structural Heart software. Shown here is the determination of angulations that correspond to an orthogonal view onto the aortic valve plane.
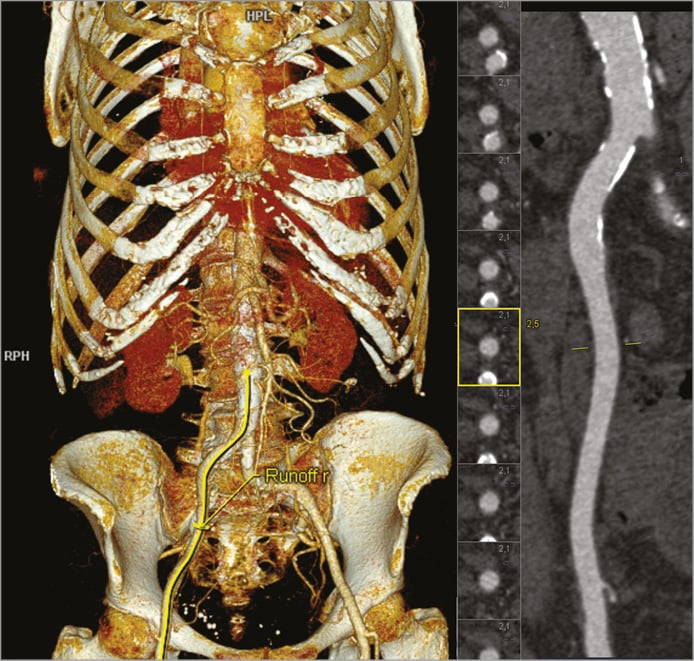
Figure 5. Assessment of the peripheral vessels using syngo.via CT Vascular Analysis. A three-dimensional model of the aorta and the peripheral vessels (left), and a stretched view of the right iliac artery plus serial cross-sectional views of the vessel (right) are shown.
In the following, we address in detail published data regarding the evaluation of annulus and aortic root dimensions, peripheral access vessels, and optimal fluoroscopic angulations for the TAVI procedure.
Annulus size and aortic root dimensions
Numerous studies have compared automated software tools to manual measurements by expert users regarding size of the aortic annulus as well as aortic root dimensions17,20,23,24. In a cohort of 105 patients, Watanabe et al used 3mensio software and showed a good correlation and concordance for aortic annulus and root dimensions between semi-automated software and fully manual assessment20. The average bias of annulus dimensions between automated and manual assessment was 0.27 mm (95% confidence interval [CI]: –2.25 to 2.80 mm) for the short diameter, 1.30 mm (95% CI: –1.20 to 3.80 mm) for the long diameter, 1.15 mm (95% CI: -0.97 to 3.27 mm) for the mean diameter, and, regarding distance to the aortic annulus, 1.61 mm (95% CI: –3.40 to 6.62 mm) for the left coronary artery ostium and 3.45 mm (95% CI: –0.58 to 7.47 mm) for the right coronary artery ostium. Delgado et al reported an excellent agreement for aortic annulus and root dimensions with intraclass correlation coefficients ranging from 0.97 to 0.99 (p<0.001) between semi-automated software and manual assessment using the same 3mensio software in a cohort of ninety patients17. Van Linden et al demonstrated close correlation (correlation coefficient 0.995, p<0.001) for measurements of the aortic annulus diameter between the fully automated syngo.CT software (Siemens Healthcare) and the semi-automated 3mensio software with manual annular plane adjustment in seventy-three image data sets18. Lou et al compared fully automated and semi-automated software algorithms using syngo.CT software to manual annulus measurements and reported a significantly smaller mean annulus area if assessed manually (p<0.001). Similar values for mean annulus area were described for fully automated and semi-automated measurements. Furthermore, they reported that the frequency of concordant recommendations for valve size increased if manual analysis was replaced with the semi-automated method (60% agreement was improved to 82.4%; 95% CI: 69.1-83.4%)23. In particular, fully automatic annulus plane detection using syngo.CT software provided robust and reliable results and, according to the authors, minor manual adjustments of the detected plane were necessary in only 4% of patients18. A high intra- and inter-observer reproducibility of automated software for measurement of aortic annulus dimensions has been demonstrated in several studies17,20,21,24. Typically, automated software provided better inter-observer reproducibility than manual measurements, and image processing time was significantly reduced (3±1 vs. 7±1 minutes for the experienced observer and 5±1 vs. 10±1 minutes for the less experienced observer)17,24. Similarly, Blanke et al assessed a semi-automated prototype analysis software (Heart Valve Analysis Protocol; Siemens Healthcare Sector, Forchheim, Germany) and reported a significantly lower mean analysis time for the model-based measurement both for experienced (26±8 vs. 98±12 seconds, p<0.001) and inexperienced (34±11 vs. 123±18 seconds, p<0.001) observers25.
Peripheral access vessels
Assessment of peripheral access vessels for transfemoral TAVI mainly requires very thorough measurement of iliac and femoral artery diameters for reliable determination of the minimal lumen size. CT is a better predictor of complications than angiography since it allows accurate measurement of luminal dimensions26. The lumen-to-sheath ratio has been identified as a major predictor of vascular complications, with more recent data suggesting that the minimum vessel area may be more relevant than the minimum vessel diameter27,28. Determination of the minimal lumen, in turn, necessitates creating cross-sections that are exactly orthogonal to the vessel along its entire course and especially where measurements are made. This can be time-consuming and prone to error when done manually. The major advantage of automated software programmes is the fact that they can generate vessel centrelines without or with minimal user input and, based on the centreline, automatically create orthogonal cross-sections at every location of the arteries (Figure 5). Vessel contours can then be automatically detected. Automated measurement of vessel cross-sections along the entire path of the artery permits the generation of a lumen profile and alerts the reader to areas of small luminal dimensions that may pose an obstacle to advancing the prosthesis.
For the work-up of peripheral vascular access assessment in TAVI, Wiegerinck et al compared a CT-based semi-automated segmentation software (3mensio Vascular; Pie Medical Imaging BV) with projection angiography and found that vessel diameters measured semi-automatically on CT were statistically smaller. They reported that 18% of the patients would even have been denied a transfemoral TAVI based on the CT measurements, whereas projection angiography rated the diameters as sufficient for the transfemoral approach29.
Angulation
For most available transcatheter aortic prosthetic valves, an exactly orthogonal fluoroscopic view onto the aortic annulus plane during the implantation procedure is of high importance. Conventionally, repeated aortograms are used to identify suitable angulations. However, information about suitable viewing angles that will provide an exactly orthogonal view onto the aortic valve, preferably with the right coronary cusp in front and the left and non-coronary cusp arranged symmetrically behind it, can easily be obtained from the volumetric CT data set (Figure 4). The slightly different position of the patient between CT acquisition and the TAVI procedure itself poses no noticeable problem in clinical practice. It has been shown that the prediction of suitable fluoroscopic viewing angles based on CT reduces the amount of contrast agent and it has even been suggested that it may improve implantation results19,30,31. Manual extraction of suitable angulations is possible but requires expertise15,16. Hence, the fact that software products can automatically suggest suitable viewing angles is potentially useful. For example, Delgado et al reported an excellent agreement between automated post-processing software and angiography, with an intraclass correlation coefficient of 0.97 for both the left anterior oblique and the craniocaudal projection in a cohort of 90 patients using 3mensio software17. For the use of the semi-automated prototype analysis Siemens software (Heart Valve Analysis Protocol), Blanke et al found no significant difference between manual and model-based assessment of the angulation prediction; however, no validation with angiography was performed32. In a cohort of 71 patients, Samin et al investigated whether automated three-dimensional analysis using 3mensio Valves software could accurately predict the line of perpendicularity of the aortic annulus and the corresponding C-arm angulations required for prosthesis delivery19. For those 36 patients included in the CT cohort, the position of the C-arm was chosen among the proposed positions included in the predicted line of perpendicularity, and the first angiogram was performed only when the prosthesis was already inserted, to validate its height relative to the annulus. There was one patient in whom the alignment of the annulus appeared to be inaccurate and an additional angiogram was performed in order to improve C-arm angulation before proceeding to the delivery. The intra-observer and inter-observer reproducibility of CT analysis for prediction of the line of perpendicularity of the aortic annulus was reported to be excellent (kappa=1 and 0.94, respectively)19.
In summary, software products that can substantially aid in the preparation of patients for TAVI exist by numerous manufacturers and their application has been evaluated relatively thoroughly. According to published results, they provide robust information and can be a useful tool, especially when experience and expertise regarding evaluation of CT in the context of TAVI are limited. Importantly, since many other interventions require similar information, it can be assumed that CT may be equally helpful for the entire spectrum of structural heart disease interventions. For example, for transcatheter mitral valve implantation, Blanke et al were able to show that segmentation of CT images using semi-automated software (3mensio Structural Heart) can help predict optimal fluoroscopic angulations to achieve a coplanar view of the mitral annulus9,32.
Conclusion
CT is an increasingly important imaging tool for coronary and structural heart disease interventions. The most important areas of information concern the access route, measurement of dimensions of the target structure and prediction of an optimal viewing angulation. The use of specific software for interpretation is not a prerequisite, but can be helpful, especially when expertise is limited. Several software programmes are available. Most have been thoroughly evaluated and perform sufficiently well.
Conflict of interest statement
S. Achenbach has received research grants (to the institution) from Siemens Healthcare. M. Marwan has received lecture fees from Siemens Healthcare and Edwards Lifesciences. The other authors have no conflicts of interest to declare.

