Abstract
The treatment of both aortic and now mitral valvular disease has been transformed through transcatheter valvular interventions. TAVR has rapidly become the treatment of choice for symptomatic severe aortic stenosis in both high, and now intermediate-risk patients. Building upon this success the last two years have seen the clinical introduction of transcatheter mitral valve replacement (TMVR), with a number of devices being utilised in first-in-man and feasibility studies. These experiences have helped determine the anatomical requirements and specifications that enable successful device deployment and avoidance of complications. A unique strength of cardiac CT is the ability to identify and characterise calcification of the aortic and mitral apparatus, enabling a deeper understanding of the relationship of calcification and procedural complications.
Introduction
Although some of the data obtained from computed tomography (CT) in pre-procedural planning can be obtained from echocardiography, there are a few elements that are in fact uniquely provided by CT. While calcification has been proven to be somewhat of an Achilles heel for coronary CT angiography owing to the preferential display of calcification and the obscuration of the coronary lumen on CT, this robust identification and characterisation has proven helpful for transcatheter interventions. The confident localisation, quantification, and characterisation of calcification has proven extremely helpful not only in procedural planning but also in helping to understand potential mechanisms of complications related to transcatheter aortic and mitral interventions.
Calcification of the aortic valve
– Diagnosis of aortic stenosis (AS)
– Anatomical localisation (leaflets, annular, subannular, left ventricular outflow tract [LVOT]), calcified raphe
– Quantification methods
– Paravalvular aortic regurgitation (PAR)
– Rupture
Calcification of the aortic valvular apparatus is both common and at times complicated in the setting of severe symptomatic AS. The pathophysiology and distribution of aortic valvular calcification in severe tricuspid senile AS has been extensively studied and discussed. While important to help confirm the diagnosis of AS, particularly in the setting of low gradient and poor contractile reserve, the extent and distribution of cusp calcification has not been shown, consistently, to be as important for sizing nor to be a driver of procedural complications. Annular and subannular calcification, on the other hand, have both been shown to play an important role in individualising device selection and sizing and to be drivers of procedural complications1,2. While data have been consistent in highlighting the importance of aortic root and LVOT calcium as a predictor of procedural outcomes, the assessment on contrast-enhanced pre-TAVR multi-detector computed tomography (MDCT) scans is challenging. To begin with, the nature of the anatomy of the aortic root makes it challenging to perform three-dimensional (3D) regional calcium quantification accurately and in a consistent fashion. In addition, contrast enhancement is essential in order to obtain detailed anatomical information regarding the aortic root and annulus. In addition, typically, when quantifying calcium on post-contrast CT examinations, threshold-based calcium quantification is used as a fixed calcium detection level and does not take into account variability in contrast attenuation due to acquisition- and patient-related factors. To optimise calcium quantification methodology across different scanners/acquisition protocols, a patient-specific calcium detection threshold, adjusting for variation in contrast attenuation, should be considered. Unfortunately, for various practical reasons and owing to the complexity of establishing a patient-specific threshold, most previous studies have applied various empirical fixed calcium-detection thresholds ranging from 500 to 850 Hounsfield units (HU).
Aortic valve calcium quantification for the diagnosis of aortic stenosis
It is now well established that, while the diagnosis of AS according to the guidelines is largely dependent on haemodynamic measures of mean gradient and calculated aortic valve area from echocardiography, there is commonly discordance amongst Doppler criteria. Importantly, while flow and aortic valve area play a large role in the determination of mean gradient, there are other factors, in particular vascular compliance and the severity of valvular calcification, both of which can reduce the mean gradient. In the setting of echocardiographic haemodynamic discordance, particularly in the absence of contractile reserve, the quantification of aortic valvular calcification from non-contrast CT has been shown to be highly discriminatory of severe AS. Clavel et al have provided thresholds that have been validated as being both helpful diagnostically and prognostically informative, specifically with thresholds of aortic valve calcium (AVC) ≥1,274 arbitrary units (AU) in women and 2,065 AU in men or with AVC density (indexed to annulus cross-sectional area) ≥292 AU/cm2 in women and 476 AU/cm2 in men3. Beyond the diagnostic value of severe AVC load, it has also been linked independently and incrementally to lower survival, which has been shown to be improved through aortic valve intervention4. Following adjustment for typical predictors of risk, severe absolute aortic valve calcification, as measured from non-contrast CT, conferred an increased hazard for mortality under medical treatment (adjusted hazard ratio [HR] 1.75, 95% confidence interval [CI]: 1.04-2.92; p=0.03).
Paravalvular regurgitation
Annular and subannular calcification have been consistently shown to confer an increased hazard for PAR. The mechanism is felt to represent non-apposition of the transcatheter heart valve (THV) device driven by landing zone calcification. The pattern of calcification has consistently been shown to play a role. Azzalini et al introduced the TAVR community to the concept of the AVC nodule score (AVCNS)5. This score was defined as AVC mass×mass of the largest calcium nodule. Those patients with a higher score exhibited a greater likelihood of greater than mild PAR (odds ratio [OR] 2.269, 95% CI: 1.433-3.593; p<0.001). Khalique et al also looked at this question, and showed that upper LVOT calcium, but not overall LVOT calcium, predicts post-dilation (PD)/PAR independently of THV sizing. Interestingly, as reported in this and the other studies referenced, aortic valve calcification itself does not drive significant PAR to the same extent as LVOT calcification6. It would seem that the leaflets are almost certainly well removed for the landing zone and displaced to the sinus of Valsalva. Recently, Jilaihawi et al published data which emphasised the importance of aortic root calcium to predict PAR but also highlighted the importance of looking carefully at the imaging variables and attenuation values in identifying patients at risk7. For non-contrast studies, a threshold of 450 HU was most discriminatory with a volume of ≥235 mm3 and a Hounsfield value of 850 on post-contrast exams with a calcium volume of ≥626 mm3. Interestingly, they found that LVOT calcification predicts PAR but only as a binary variable without any incremental benefit derived from calcium quantification.
Annular rupture
Annular rupture is one of the most feared immediate complications of TAVR, with a reported mortality associated with free rupture of 75%2. While this complication was historically poorly understood, over time and through the integration of CT into procedural planning, signals began to emerge. In 2013, Barbanti et al established a multicentre registry of 27 patients who experienced annular rupture and had undergone CT prior to the procedure. Through a caliper matching process with a larger historical cohort of patients who did not experience rupture, it became clear that moderate to severe subannular calcification was a strong predictor of annular rupture, particularly when combined with annular oversizing in excess of 20% by area. The primary analysis in this study was qualitative and semi-quantitative in nature2. The LVOT was analysed for the presence, amount, and location of calcification. Patients with aortic root rupture had a higher burden of LVOT/subannular calcification (calcium score, 181.2±211.0 vs. 22.5±37.6, p<0.001) (Figure 1), whereas no difference in the rate of moderate/severe aortic cusp calcification was noted (83.9% vs. 87.1%, p=0.892). Building upon these results, Hansson et al extended this analysis by performing a more thorough quantitative analysis on these CT data sets8. By using a calcium segmentation (3mensio Structural Heart; 3mensio Medical Imaging BV, Bilthoven, The Netherlands) algorithm, the authors were able to discriminate further the patterns and distribution of calcium associated with annular injury. In this study, a patient-specific calcium detection threshold was used. There is increasing awareness of the importance of using varied thresholds for calcium assessment, as chest wall thickness, tube potential, cardiac output, and the iodine concentration of the contrast medium used can have a significant impact on the attenuation of the blood pool and ability to segment calcification. Beyond quantification, the calcium distribution was evaluated, further segmenting the LVOT into the overall LVOT and upper and lower LVOT. Patients with aortic root injury had significantly more overall LVOT calcium: median volumes were 74 [5-326] mm3 vs. 4 [0-63] mm3 (p=0.0001). Median high LVOT/subannular calcium volume was higher in the aortic root injury group as compared to the control group, i.e., annular rupture: 28.7 [3.1-65.9] mm3 vs. control: 0 [0-8.5] mm3 (p<0.0001). What was of particular interest was that calcification below the non-coronary cusp was significantly more predictive of rupture compared to calcium below either the right or left cusp (NC-AUC: 0.81, 95% CI: 0.72-0.90; RC-AUC: 0.67, 95% CI: 0.58-0.77; LC-AUC: 0.65, 95% CI: 0.55-0.75) (p=0.01). The reasons for this finding remain unclear but it has been hypothesised that, given that the most vulnerable region of the aortic root is below the left cusp, calcification opposite the left cusp may result in significant resistance and cause the THV to migrate away from a protruding nodule during balloon inflation. This rapid contralateral movement could potentially be a mechanism for annular rupture.
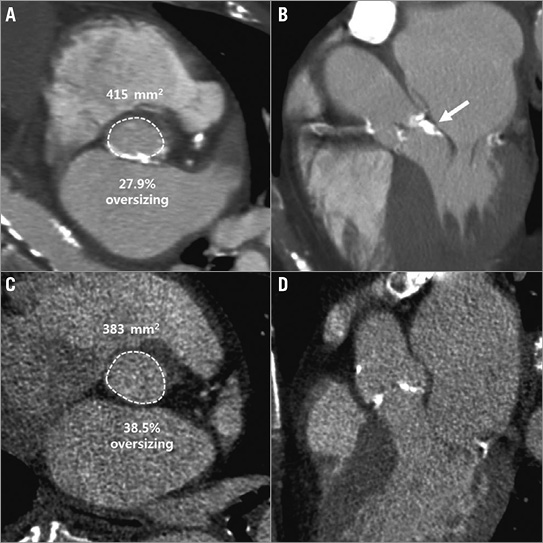
Figure 1. Two examples of annular calcification greater than 20% with different clinical outcomes. A) & B) This patient underwent a 26 mm SAPIEN XT deployment with 27.9% oversizing but in the setting of severe LVOT calcification and experienced annular rupture. C) & D) This patient underwent TAVR with a 26 mm SAPIEN XT which, based on native annular geometry, represented even greater oversizing (38.5% oversizing), but did so in the absence of LVOT calcification and experienced an uneventful TAVR. Copyright © American Heart Association, Inc. All rights reserved.
Calcification of the mitral apparatus
Calcification of the mitral apparatus can occur as mitral annular calcification (MAC), mitral leaflet calcification and as calcifications of the subvalvular apparatus, i.e., chordae and papillary muscles.
While calcification can be identified on echocardiography due to its echo-dense appearance and acoustic shadowing, echo suffers from limited tissue characterisation and discriminatory power against dense collagen. In contrast, high spatial resolution, and the inherently different CT values for calcified versus non-calcified tissue favour CT for the detection, quantification, and characterisation of calcifications of the mitral apparatus, as well as its truly 3D depiction.
Among calcification of the mitral apparatus, MAC is by far the most common, and is estimated to have a prevalence of approximately 6% in an unselected, general population9. MAC affects more commonly the posterior portion of the annulus as opposed to the anterior portion. Along the posterior portion, MAC involves the fibrous base of the mitral annulus and is thus located along the atrioventricular junction, more specifically on the ventricular side of the posterior mitral valve leaflet (PML) (Figure 2). Similar to AVC, MAC can be spotty or confluent, and can be characterised as protruding or non-protruding. If confluent, limited to the posterior annulus, the calcification takes the form of a U. If the anterior annulus is also involved, the calcification is circular.
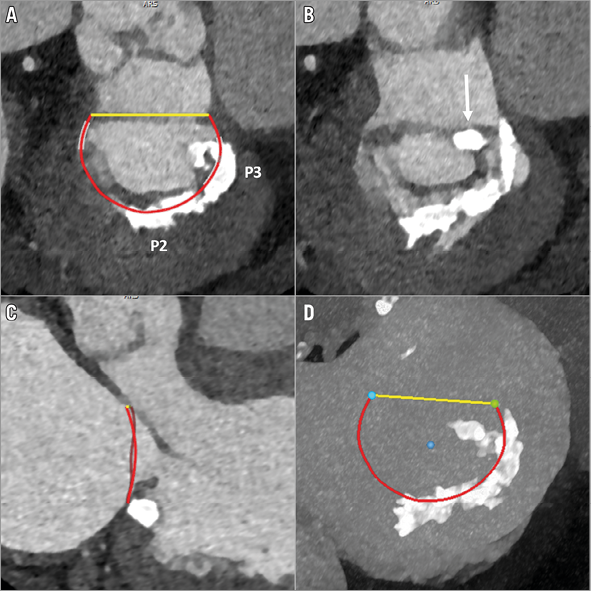
Figure 2. Patient with mitral regurgitation and MAC. A) & B) Short-axis images at the annular and subannular level, demonstrating the non-circular but confluent, space-occupying calcifications of P2 and P3 as well as a bulky calcification at the posteromedial commissure (arrow). C) Long-axis view, illustrating the bulky nature of MAC. D) En face maximum intensity projection, demonstrating the extent of the leaflet calcification.
Besides merely describing the location of MAC, its severity can be graded, using a semi-quantitative, subjective grading scale - none, mild, moderate and severe.
Caseous calcification of the mitral annulus is a rare variant of MAC with central liquefaction necrosis, which forms as bulky, often rounded, space-occupying lesion predominantly along the posterior annulus. Compared to typical MAC, caseous annular calcifications are less echo-dense and may present echolucent zones with less acoustic shadowing. On contrast-enhanced CT, caseous calcifications can exhibit homogenous areas of attenuation similar to the contrast-enhanced blood, but can ultimately be well distinguished on non-contrast-enhanced image data.
MAC and TMVI
Early results with transcatheter mitral valve implantation (TMVI) have shown real promise in selected patient populations10. To date, little is known about the relevance of MAC for devices currently under investigation. While early feasibility trials tend to exclude severe annular calcification, it is conceivable that severe and bulky MAC will interfere with the apposition of the self-expanding TMVI systems, potentially creating a substrate for paravalvular leak (PVL), in particular if the extent of calcium is non-symmetric and bulky. This being said, besides the focal extent of MAC, its overall distribution (homogeneous vs. heterogeneous) will probably be important.
THV for calcific mitral disease
In contrast, treatment of calcific mitral valve disease with transapical, transseptal or direct implantation of aortic THVs into the mitral position actually requires severe MAC for device anchoring, ideally in a homogeneous, circumferential pattern. In this scenario, MAC provides the mechanical counter-bearing for implantation of both self-expanding and balloon-expandable devices. CT has been shown to be helpful with sizing for these procedures, although precisely how to segment the mitral orifice in the setting of exuberant calcification given the inevitable blooming of calcification remains uncertain. CT has also been shown to be helpful in predicting the risk of neo-LVOT obstruction. Importantly, for determining procedural feasibility, it is currently not the exact quantification of the MAC, but rather the quantification of the landing zone itself, which is necessary to achieve appropriate device sizing in order to prevent PVL or, even worse, device migration. However, moving forward in the optimisation of transcatheter treatment of calcific mitral disease, further quantitative measures such as calcium density may be defined. Besides MAC, the presence of leaflet calcification also has to be considered for this intervention, as bulky calcifications along the anterior mitral valve leaflet, in particular along the A2 segment, may be displaced into the LVOT, ultimately resulting in LVOT obstruction.
Current evaluation of MAC with CT has been limited to visual inspection but, as we have learnt in the coronary space, density and other quantitative measures may be important to understand further which patients may benefit from which therapies. In addition, given the rapid engineering developments in this field, there will almost certainly be new devices that can adapt to more exuberant and complex patterns of MAC.
Conclusion
CT has rapidly become an essential tool for the procedural planning and guidance of transcatheter valvular interventions and has offered an understanding of many of the important anatomical drivers of varied clinical outcomes. We have, however, only begun to scratch the surface in our understanding of how to evaluate and measure and predict the clinical impact of calcification in the heart. In the future, advance analyses with 3D and eventually biological tissue printing in combination with computational fluid dynamics may help significantly to advance our understanding of the impact of calcification on transcatheter heart valve expansion, tissue friability, mechanical forces and, ultimately, procedural outcomes.
Conflict of interest statement
J. Leipsic and P. Blanke are consultants for Edwards Lifesciences and provide core lab services through UBC to Edwards Lifesciences, Medtronic, Neovasc, and Tendyne Holdings. P. Blanke also serves as an imaging consultant for Neovasc and Tendyne.

