Abstract
Aims: We compared the measurement of aortic leaflet calcification on contrast and non-contrast MSCT and investigated predictors of the need for balloon post-dilatation after TAVI.
Methods and results: In 110 patients, who had TAVI with a Medtronic CoreValve prosthesis (MCS) for symptomatic aortic stenosis, calcification of the aortic root was measured on non-contrast MSCT (conventionally) and on contrast MSCT (signal attenuation >450 Houndsfield units). Calcium volume was underestimated on contrast- when compared to non-contrast MSCT: median (IQ-range)=759 (466 to 1295) vs. 2016 (1376 to 3262) and the difference between the two methods increased with higher calcium volumes (correlation coefficient r=0.90). Calcium mass was only slightly underestimated on contrast vs. non-contrast MSCT: median (IQ-range)=441 (268 to 809) vs. 555 (341 to 950) and there was no association between the differences and increasing calcium mass (r=0.17). Balloon post-dilatation was performed for significant aortic regurgitation after TAVI in 11 of 110 patients. When compared to controls, the patients who required balloon post-dilatation had higher aortic leaflet calcium on contrast CT (p<0.01), higher aortic annulus diameters (p<0.01) and higher annulus to prosthesis area ratio (p=0.01). ROC curves demonstrated that aortic root or aortic leaflet calcium measured on either contrast- or non-contrast MSCT showed excellent discrimination for the requirement of balloon post-dilatation (area under ROC >0.80 for all), whereas the discriminatory value of aortic annulus dimensions was moderate (area under ROC=0.69) and that of prosthesis to annulus ratio was poor (area under ROC=0.36).
Conclusions: Dense aortic leaflet calcification measured on contrast MSCT discerned well the need for balloon post-dilatation after TAVI with an MCS for significant PAR. Non-contrast MSCT may no longer be needed to quantify aortic root calcium before TAVI.
Introduction
Transcutaneous aortic valve implantation (TAVI) improves prognosis of patients with severe aortic stenosis who are deemed at too high a risk for surgical valve replacement (SAVR)1. Heavy calcification of the aortic root is an almost universal finding in patients with severe symptomatic aortic stenosis2. The gold standard for the measurement of calcification is standardised non-contrast MSCT, yet the precise anatomical distribution of calcification can be better appreciated on contrast MSCT, which usually shows dense calcification of the aortic leaflets. Balloon valvuloplasty is essential to crack open the calcified valve before it can be crossed with the TAVI prosthesis3,4. The calcified aortic leaflets are then pushed aside and trapped by the prosthesis during implantation. Apposition of a sealing skirt to the disrupted aortic leaflets and or to the surrounding tissues within a few millimetres below them is necessary to prevent paravalvular aortic regurgitation (PAR) for both types of commercially available TAVI prostheses. It is likely that the calcified leaflets also play an important role in preventing device embolisation after deployment. Yet, dense calcification may contribute to deformation of the deployed frame of the prosthesis, which may result in malapposition and paraprosthetic aortic valve regurgitation.
Another factor which may cause PAR after TAVI is undersizing, where the prosthesis is small relative the native annulus5. Prosthesis size selection is based on the measurement of the aortic annulus6,7. Yet, during SAVR the aortic annulus is partially decalcified before sizing proceeds under direct vision, whereas during TAVI sizing relies on non-invasive imaging without prior decalcification.
When significant PAR is encountered after deployment of the prosthesis balloon post-dilatation of the prosthesis (PDP) may reduce the amount of regurgitation, but carries the risks of potential damage to the new valve leaflets as well as of vascular sequelae that may result from the additional instrumentation, for example potentially aortic root rupture and cerebrovascular events8-11. Yet if it were possible to identify a priori which patients would be at risk of requiring PDP, it may be possible to modify the procedure so as to obviate the need for it, for example by selecting a larger pre-dilatation balloon or larger size prosthesis. This study investigates first the measurement of aortic valve calcification on contrast vs. non-contrast MSCT and second, the clinical and anatomical determinants of PDP after TAVI.
Methods
Population
The study population consisted of 110 consecutive patients who received TAVI with a Medtronic CoreValve prosthesis (MCS) between June 2007 and August 2010 and who had a MSCT for pre-procedure planning at our centre. MSCT was performed in all patients before TAVI, except on patients with an estimated glomerular filtration rate below 20ml/min, or where the MSCT could not be arranged for logistical reasons. No patients were specifically excluded.
MSCT without contrast enhancement
A scan without contrast enhancement was available in 98 of 110patients because it was initially not performed in patients with previous CABG or coronary stents. The non-contrast MSCT acquisition was performed in a prospectively ECG-triggered, sequential (step-and-shoot) mode with a reference tube current of 80 mAs, a tube voltage of 120kV and slice thickness of 3mm in the early or mid diastolic heart phase depending on the heart rate. For analysis on a dedicated cardiovascular CT workstation (MMWP, Siemens AG, Forchheim, Germany) the aortic root was defined as the stretching from the caudal aspect of the aortic annulus to the origin of the left main stem as seen on axial images12. The threshold for the detection of calcium was set at 130HU. Agatston score, calcium volume and mass were measured13-17. In cases where aortic root calcification was confluent with calcium in adjacent structures (mitral annulus, ascending aorta, coronary arteries) only the stack of images that contained the aortic root were selected.
Contrast enhanced MSCT
The MSCT acquisition method using 64-slice dual-source CT has been described before18. In brief, the acquisition was performed using the spiral scan mode, a variable table speed (depending on the heart rate), no ECG-triggered tube output modulation and an ECG-gated image reconstruction. Further acquisition parameters: detector collimation 2×32×0.6mm with rapid alternation of focal spot position in the Z-axis (Z-sharp®), roentgen tube rotation time 330ms, tube voltage 120kV. The scan ranged from the top of the aortic arch to the diaphragm. The volume of iodinated contrast material was adapted to the expected scan time. A 50-60ml bolus of iodixanol (Visipaque® 320mg l/ml; GE Health Care, Eindhoven, The Netherlands) was injected in an antecubital vein at a flow rate of 4.5ml/s followed by a second contrast bolus of 30-40 at 3.0ml/s. Bolus tracking was used to trigger the start of the scan with the arrival of contrast in aortic root. Reconstructions were made in end-systole using a single-segmental reconstruction algorithm with slice thickness 1.5mm; increment 0.4mm; medium-to-smooth convolution kernel (B26f) resulting in a spatial resolution of 0.6-0.7mm in-plane and 0.4-0.5mm through-plane and a temporal resolution of 83ms. The radiation doses ranged from 8 to 20mSv depending on body habitus and table speed.
The aortic annulus was defined as a virtual ring with three anchor points at the bases of the three aortic leaflets19. This definition was visually reproduced by setting up a viewing plane axial to the aortic root as described before20. The minimum and maximum diameters and area of the annulus were measured. Propriety software was developed to allow measurement of aortic leaflet calcification on a contrast MSCT (3mensio Medical Imaging, Bilthoven, the Netherlands). On images axial to the aortic annulus, a region of interest was defined from the aortic annulus to the most cranial aspect of the aortic leaflets. The threshold for detecting calcification in the region of interest was set at 450 Houndsfield units (HU), which was approximately 150HU above contrast density of the blood pool when sampled just above the aortic leaflets in the majority of patients. This was found to be the lowest level at which contrast was not detected as calcium. Non-detection of contrast was visually verified. If necessary, the region of interest was then edited to ensure that calcification detection were limited to the three aortic leaflets (Figure 1). Calcium volume and mass was measured. The density of calcium can be determined from the amount of signal attenuation16,17. Due to the high signal attenuation threshold selected for the detection of calcium it was anticipated that only dense calcium would be measured on contrast MSCT.
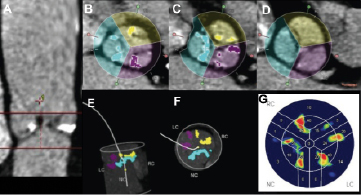
Figure 1. Calcium quantification on aortic leaflets on contrast MSCT. On two longitudinal views the region of interest was defined from the base of the three aortic leaflets to just above the aortic leaflets (Panel A). The threshold for detecting calcification was set at 450 Houndsfield units (HU), which was approximately 150 HU above contrast density of the blood pool when sampled just above the aortic leaflets in the majority of patients. This was found to be the lowest level at which contrast was not detected as calcium. Non-detection of contrast was visually verified at different levels and, if necessary, the region of interest was then edited (Panels B-D) to ensure that calcification detection was limited to and measured individually for the three aortic leaflets. The correct segmented calcium was also verified on 3D views (Panels E, F). Panel G shows a collapsed (2D) calcium density plot after segmentation.
Relevant aspects of the TAVI procedure
The patient selection and TAVI implantation procedure has been described before21. Balloon valvuloplasty of the native valve was performed in all cases. As is the case in the majority of TAVI procedures performed worldwide, a cine-aortogram was performed after device deployment to assess device position and to detect and grade aortic regurgitation11. A 6FR pigtail catheter was placed in the outflow part of the frame just above the commissures of the prosthesis leaflets. A radiographic contrast of 20 ml was injected with a power injector at a rate of 20ml/s. Aortic regurgitation (AR) was graded by the operator during the implantation procedure according to the method of Sellers: grade 0=no AR; grade 1=trace; grade 2=mild; grade 3=moderate; grade 4=severe22. In the event of aortic regurgitation grade 3 or 4, one of two approaches was taken: PDP was performed if the operators judged that the AR may be reduced by improved apposition of the sealing skirt to the tissues surrounding the inflow of the prosthesis. Alternatively, if a too deep position of the prosthesis inflow below the annulus was judged to be the cause of AR, then a second valve was implanted (valve-in-valve)23. In practice this meant that if the prosthesis was clearly positioned too deep (inflow >12mm below the annulus given that the sealing skirt stretches for 12mm above the inflow of the frame) a second prosthesis was implanted, whereas in other cases post-dilatation was performed. Snare manoeuvres were not performed in any patients to correct AR.
Comparison of calcium measurement on contrast and non-contrast MSCT
We anticipated that the calcium score would be underestimated on the contrast MSCT relative to the conventional non-contrast method given the much higher threshold for the detection of calcification on a contrast CT (on average 450HU vs. 130HU). In order to compare the measurement of aortic leaflet calcification by the two methods (contrast compared to non-contrast MSCT), 47 patients were selected with calcification largely limited to the aortic leaflets and with none, or limited, calcification in the adjacent structures so as to ensure that the regions of interest corresponded as closely as possible. This was necessary because: 1)aortic leaflet calcification may have an effect on the need for PDP, whereas additional aortic root calcification in the wall of the aorta, mitral valve or coronary ostia would not; 2)the region of interest on the non-contrast images is defined on images axial to the patient and fine anatomical detail cannot be readily appreciated, whereas on the contrast MSCT the region of interest is defined on images axial to the aortic annulus and calcification of individual leaflets can be measured.
Statistical analysis
The MSCT interpreter was blinded to procedural outcome and TAVI operators blinded to calcification data. Prosthesis inflow to annulus area was calculated by dividing the nominal area of the prosthesis inflow (size 26 MCS: 5.32cm2 and size 29 MCS: 6.62cm2) by the cross-sectional area of the aortic annulus. Correlation coefficients and Bland-Altman plots were used for the comparison of measurement of aortic leaflet calcification on contrast and non-contrast MSCT. Calcification score data were not normally distributed, and are given as median (interquartile range). Normally distributed data are given as mean (SD). For comparison between groups Student’s t-test or the Mann-Whitney U-test were used. The odds ratio and 95% confidence interval was used to investigate the association between the exposure variables (calcium volume, mass) and outcome (PDP). To determine which variable best discriminated the need for PDP receiver operator characteristic (ROC) curves were plotted (sensitivity vs. 1-specificity) and the areas under the curve were calculated. Statistical significance was defined as p<0.05. SPSS 15.0 was used.
Results
Demographics
Clinical and anatomical details of the study population are given in Table1. The calcium burden of the aortic root and leaflets was high in all patients, Table1. The degree of calcification was highest for the non-coronary leaflet (calcium mass median [IQR]=187 [121-360]), followed by the right (calcium mass=138 [67-249]) and left coronary leaflets (calcium mass=129 [62-217).
AR grade 3-4 was seen immediately after device implantation in 15 of 110 patients. In four of these 15 patients a second MCS was immediately implanted (valve-in-valve), which resulted in AR grade 1-2 in all four patients. In 11 of these 15 patients PDP was performed, which resulted in AR grade ≤2 in eight of 11 patients. One further patient received a second prosthesis (valve-in-valve) that improved the AR grading to trivial. The grade of AR spontaneously reduced to grade 2 in the first week after TAVI in another patient. One other patient with persistent AR grade 3 despite PDP died 12 days after the procedure. With the exception of this patient, there were no patients with PAR grade >2 on echocardiography one week after TAVI. PDP was associated with the development of aVSD in one patient who had an unusually long ventricular membranous septum24.
Comparison of calcium volume and mass on contrast and non-contrast MSCT
In the 47 patients with calcification limited to aortic leaflets the measurements of both calcium volume and mass were lower on contrast when compared to non-contrast MSCT: calcium volume median (IQ-range)=759 (466 to 1295) vs. 2016 (1376 to 3262); calcium mass=441 (268 to 809) vs. 555 (341 to 950). There was a high degree of correlation between the contrast and non-contrast MSCT measurement of both calcium volume and mass (respectively r=0.93 and r=0.95), see Figure2. The Bland-Altman plot showed that there was a negative bias for the measurement of calcium volume on contrast scans and the difference between the two methods increased as calcium volume increased (correlation coefficient r=0.90), Figure2. On the other hand, the calcium mass was also underestimated on contrast MSCT, but there was no association between the difference between the two methods and increasing calcium values (correlation coefficient r=0.17), Figure3.
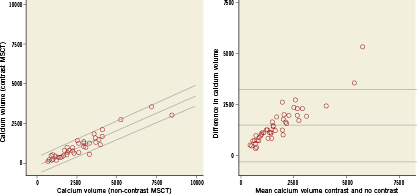
Figure 2. Scatter plot (left panel) and Bland-Altman plot (right panel) of calcium volume measured on contrast and non-contrast MSCT. The linear regression lines with 95% confidence intervals are shown for scatter-plot (left panel: intercept -99, slope 0.45, R-squared =0.86). Reference lines represent the mean difference±2SD for the Bland-Altman plot (right panel).
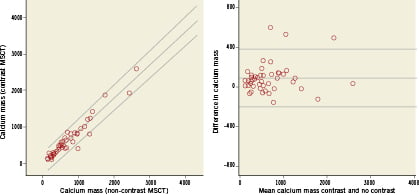
Figure 3. Scatterplot (left panel) and Bland-Altman plot (right panel) of calcium mass measured on contrast and non-contrast MSCT. The linear regression lines with 95% confidence intervals are shown for the scatterplot (left panel: intercept -26, slope 0.91, R-squared =0.93). Reference lines represent the mean difference±2SD for the Bland-Altman plot (right panel).
Characteristics of patients who underwent PDP
There were no differences in the clinical characteristics of patients who did and did not have PDP (Table1). On preprocedural MSCT the patients who required PDP had larger annulus dimensions (minimum, maximum, mean diameters), higher degrees of aortic root calcification on non-contrast MSCT and higher levels of aortic leaflet calcification on contrast MSCT, Table1. The ratio of nominal prosthesis inflow to annulus dimensions was lower in patients who received PDP when compared to those who did not, Table1. There was no difference depth of implantation between the two groups.
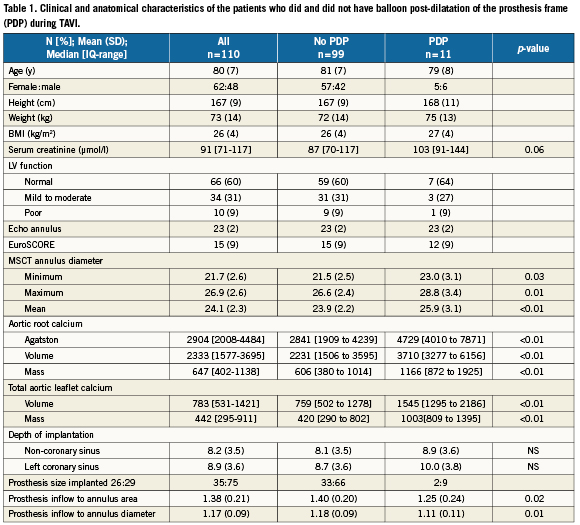
The implanted prosthesis was correctly sized or over-sized based on the sagittal annulus measurement from echocardiography in all patients who subsequently required PDP. Yet if sizing was based on the maximum annulus diameter obtained from MSCT, which is not accurately measured on 2D echocardiography, the selected prosthesis would have been undersized in eight out of 11 patients who required PDP. If the sizing was based on the mean annulus diameter obtained from MSCT, the selected prosthesis would have been undersized in five out of 11 patients who required PDP and oversized in one patient. Undersizing, defined as a mean annulus diameter ≥23.5 for a size 26 and ≥27.5 for a size 29 MCS implant, was associated with PDP: odds ratio (95% CI)=11.0 (2.7-45).
An aortic leaflet dense calcium mass ≥800 (contrast MSCT) was more frequent in cases of patients who had PDP than those who did not (91% vs. 25%), and was significantly associated with PDP: odds ratio (95% CI) = 29.6 (3.6 to 242.9). Of patients with an aortic leaflet dense calcium mass ≥800, 29% required PDP compared to 1% of those with a dense calcium mass <800. Similar data were seen for an aortic leaflet dense calcium volume ≥1200 (contrast MSCT), which was seen respectively in 91% and 26% of patients who did and did not have PDP. On non-contrast MSCT an Agaston score ≥3000 was seen in all patients who had PDP and in 43% of those who did not.
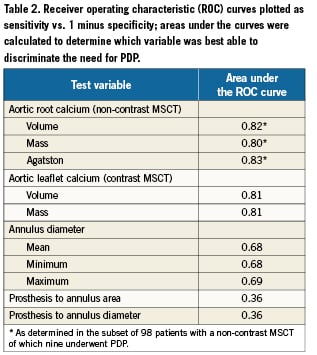
When evaluated by ROC curve, all measures of aortic root or aortic leaflet calcium showed excellent discrimination for the requirement of PDP (all >0.80, Table2), whereas the discriminatory value of aortic annulus dimensions was moderate (0.68) and that of prosthesis to annulus ratio was poor (0.39), Table2.
Discussion
This study shows firstly that quantification of mass and volume of dense calcium of the aortic leaflets on contrast MSCT is feasible and, secondly, that it allows the detection of dense calcifications that increase the risk of needing post-dilatation after TAVI. Dense calcium was a predictor of PDP in the present study and others25. Less dense calcification did not affect this procedural outcome. This concept is supported by the observation in the present study that calcifications measured on both contrast and non-contrast MSCT discriminated the need for PDP equally, despite the fact that spots of calcium with mild to moderate density (<450HU) are not detectable on contrast MSCT.
Although semi-quantitative estimation of calcification burden on the aortic leaflets on echocardiography has been described2, the measurement of calcium on non-contrast MSCT (Agatston score, volume, mass) has to be considered the gold standard26,27. We report that calcium volume is underestimated on contrast when compared to non-contrast MSCT, and the degree of underestimation increases with increasing levels of root Ca volume. In comparison, the mass measurement did not show a similar increasing bias. This may be explained by the fact that less dense calcium that will not be detected on contrast scans (<450HU) may have a substantial volume but relatively little mass. These data indicate measurement of calcium on contrast MSCT may lead to significant misclassification when small amounts of calcification have prognostic implications for example in the coronary arteries13. Yet only dense aortic leaflet calcification (Agatston score >3000 on non-contrast MSCT, mass ≥800, volume ≥1200 on contrast MSCT) was a risk marker for PDP during TAVI in this and another study25. The ability to easily measure dense aortic root calcification contrast MSCT may eliminate the need for an additional non-contrast MSCT before TAVI. This would reduce the radiation dose of a pre-TAVI MSCT scan by approximately 1-2mSv on average, depending on the scanning protocol used.
PAR is common post-TAVI, but is mostly mild whereas haemodynamically significant AR (grade 3 to 4) is reported in approximately 1 to 5% of patients8,25. This is in part because haemodynamically significant PAR is not well tolerated in elderly patients with stiff ventricles. Consequently it is almost always ameliorated by additional measures during the implantation procedure, even when it does not lead to acute haemodynamic compromise. Treatment options include PDP in order to improve apposition of the sealing skirt at the inflow of the frame to surrounding tissues, or, if a too deep implantation is the principle cause, a second prosthesis may be deployed (valve-in-valve). We observed haemodynamically significant AR (grade ≥3) in 15 patients (14%) after TAVI, of which 10% was first treated with PDP and 4% were treated with implantation of a second valve. The reported incidence of PDP after implantation of a MCS ranges between 9 and 34%8,25. Another study of similar size reported PAR grade ≥3 in 9% of patients of which 3% were treated with implantation of a second valve after PDP failed to improve the AR25. In that study, PDP was performed in a larger proportion, i.e., 34% of patients, because the threshold was lower, i.e., patients with PAR grade 2 also underwent PDP. In our study, PAR grade ≥3 was seen in only one patient on follow up echocardiography one week after TAVI. It is thought that the nitinol frame of the MCS continues to expand up until 48hours after device release. Consequently PAR may be expected to reduce further within this period without PDP. The additional instrumentation during PDP may carry an incremental risk of complications such as aortic root rupture, although the risk may be small and substantially lower than for predilatation of the native valve (PABV) that is not followed immediately by TAVI25,28. A case report suggested that caution may be warranted when post-dilatation is performed in patients with a ventricular membranous septum that extends a relatively long way below the aortic annulus24. However, as there is currently no technique to eliminate the calcium during TAVI, and the current frame technology does not fully accommodate the surrounding anatomy, additional PDP is integral part of TAVI, fortunately a low risk. It is conceivable that more aggressive predilatation of the native valve in patients with very dense aortic leaflet calcification may reduce PAR and the need for post-dilatation, yet this may come at a greater risk of root rupture and needs further study. In patients who receive a surgical aortic valve prosthesis, aortic calcification is a marker of adverse procedural outcome. There is currently no evidence that this calcification predicts an adverse outcome after TAVI, although the case series have been relatively small.
We report that aortic annulus dimensions and the ratio of nominal prosthesis to annulus size were higher in patients who required PDP when compared to those who did not. Two studies of respectively 74 and 53 patients reported similar findings based on 2D echocardiographic measurements5,29. In the present study there was substantial overlap between the two groups. However, annulus dimensions discriminated less well the need for PDP than did quantification of calcifications. These data suggest that relative undersizing, if not too extreme, may exacerbate the effects of dense calcification rather than being the primary cause of haemodynamically significant AR requiring PDP. Furthermore, the availability of larger prosthesis sizes in combination with sizing based on mean annulus dimensions from a 3D imaging modality such as MSCT, 3DTEE or CMRI may reduce the need for PDP, although this would require further study.
Limitations
This is a relatively small study and should be viewed as hypothesis generating. Since the analysis method for the quantification of calcium on the contrast and non-contrast scans are different and non-contrast scans do not allow appreciation of fine anatomical detail, it is not possible to exactly match the regions of interest on the two types of scan. Other factors that may affect the detection of calcium between contrast and non-contrast scans include differences in scanner type, body habitus, the radiation dose, slice thickness, reconstruction increment in addition to the presence or absence of contrast17,30-32. Despite the fact that these differences can not be adjusted, the ability to discriminate the need for PDP was similar for calcium measured on both contrast and non-contrast MSCT. This study included only patients who received an MCS, and we cannot comment on the implications for the Edwards system.
Conclusions
Dense aortic leaflet calcification can be quantified sufficiently on contrast MSCT to allow prediction of haemodynamically significant PAR during TAVI requiring PDP release of the MCS. On contrast MSCT, an aortic leaflet calcium mass >800, a calcium volume >1200, larger annulus dimensions and nominal prosthesis inflow to annulus ratio were associated with PDP during TAVI. Aortic root or aortic leaflet calcium on respectively non-contrast and contrast MSCT showed excellent discrimination for the requirement of PDP, whereas the discriminatory value of aortic annulus dimensions was moderate.
Conflict of interest statement
The authors have no conflicts of interest to declare in relation to this paper.

