
Abstract
Aims: Limited information exists describing the results of transcatheter aortic valve replacement (TAVR) in patients with symptomatic severe non-calcific aortic stenosis (AS). We aimed to compare procedural, echocardiographic, and clinical outcomes among patients with non-calcific AS with those of senile calcific AS undergoing TAVR.
Methods and results: We retrospectively identified patients with non-calcific AS who received TAVR with self-expanding transcatheter heart valves in our centre. Clinical and echocardiographic outcomes, and post-procedural multi-detector computed tomography (MDCT) measures were compared to those in patients undergoing TAVR for calcific AS. Among 136 patients, 21 patients (15.4%) with native leaflet thickening and minimal calcification were identified (non-calcific group). The patients were significantly younger in the non-calcific group (70.0 [64.0-75.5] vs. 75.0 [69.0-78.0] years) with comparable STS-PROM scores (6.7 [4.8-8.9] vs. 8.2 [4.8-10.9] %). Predilation was performed less frequently (42.9% vs. 93.9%) and post-dilation more often (71.4% vs. 42.6%) in the non-calcific group. Both 30-day and one-year mortality were similar between groups (0% vs. 7.8% and 0% vs. 17.6%). Rates of post-implantation paravalvular leak ≥mild at six months (17.6% vs. 25.7%) were comparable despite lower implantation depth among non-calcific AS patients (10.9±5.7 vs. 7.2±4.3 mm) on post-implantation MDCT.
Conclusions: TAVR with self-expanding transcatheter heart valves appears to be safe and effective in patients with non-calcific AS.
Abbreviations
AS: aortic stenosis
EI: eccentricity index
MDCT: multi-detector computed tomography
PVL: paravalvular leak
RHD: rheumatic heart disease
TAVR: transcatheter aortic valve replacement
TEE: transoesophageal echocardiography
THV: transcatheter heart valve
TTE: transthoracic echocardiography
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve replacement (TAVR) has become an established treatment for patients with symptomatic severe calcific aortic stenosis (AS) deemed inoperable or at intermediate to high surgical risk. In Europe and North America, senile calcific degeneration of the tricuspid aortic valve is the most common cause of AS among the elderly1, a process characterised by progressive fibro-calcific remodelling and thickening of the leaflets2. Progressive aortic valve calcification and leaflet thickening usually occur in tandem, though in most patients leaflet calcification is the predominant pathophysiological process in the development of senile severe AS. There is, however, a proportion of patients that manifests severe aortic leaflet thickening in isolation as a mechanism for AS, without calcium deposition. Several disease processes, such as rheumatic heart disease, systemic lupus erythematosus, valvulopathic drugs (such as benfluorex and fenfluramine) and radiation exposure have been implicated in the development of AS with thickened leaflets but minimal calcification. Non-calcified AS is considered to be a relative contraindication for TAVR, as anchoring and sealing of the transcatheter heart valve could be impaired3. Such cases have been excluded from randomised TAVR trials, and the outcomes of patients with non-calcific AS undergoing TAVR remain unknown.
We sought to address this knowledge gap by comparing procedural, echocardiographic, and clinical outcomes among patients with non-calcific AS with those of senile calcific AS undergoing TAVR.
Methods
PATIENT SELECTION
We retrospectively evaluated the preprocedural multi-detector computed tomography (MDCT) of all patients who received TAVR for evidence of leaflet-thickening dominant presentation with minimal calcification (referred to as non-calcific AS) in a single centre, West China Hospital, between April 2012 and April 2016 (N=136). The indication for TAVR was discussed in all cases by our multidisciplinary Heart Team. Baseline clinical, echocardiographic, and angiographic characteristics, procedural and post-procedural details were collected in a dedicated prospective TAVR database.
ENDPOINTS
TAVR-specific endpoints were defined according to the VARC-2 criteria, including post-implantation paravalvular leak (PVL), which was graded as none, trace, mild, moderate or severe4. Of particular interest were the composite endpoints of valve safety, procedural success and clinical efficacy. A combined endpoint of >mild PVL, post-dilation, or second valve implantation (the latter two being common ways to eliminate an unacceptable amount of PVL) was used to assess the transcatheter heart valve (THV) sizing strategy and its association with suboptimal results.
MDCT PROTOCOL AND AORTIC ROOT MDCT ANALYSIS
All MDCT scans were performed with a second-generation dual-source CT system (SOMATOM Definition Flash; Siemens Healthineers, Erlangen, Germany) prior to and following TAVR5.
Two independent physicians (T.Y. Xiong and Y.B. Liao) assessed evidence of non-calcific AS. Initially, we performed a semi-quantitative assessment for the presence and severity (grade 1 to 4) of aortic valve calcification as previously described6. Only in patients with grade 1 or 2 calcification were the native leaflets then assessed for substantial thickening, defined as 1) continuous hypoattenuation on all leaflets on axial reconstruction, and 2) on ≥50% of the leaflet height (annulus to the tip) on coronal or sagittal oblique views (Figure 1). Those with focal leaflet thickening also served as controls. The maximal and minimal width of each thickened cusp on axial view was measured. To appreciate further the differences in leaflet thickness between the non-calcific AS and controls, 3D volume-rendering reconstruction using the Hounsfield range of low-density area on MDCT was performed (Figure 2) using OsiriX DICOM Viewer software (OsiriX Foundation, Geneva, Switzerland).

Figure 1. Representative images during patient identification. Three groups of patients. A) Differences in degrees of calcification. A1) A patient with grade 2 calcification. A2) A patient with grade 4 calcification. B) Diffuse (B1) and non-diffuse (B2) leaflet thickening highlighted within red lines. C) Relative height of hypoattenuated opacities on coronal or sagittal oblique views (orange line) in comparison with the distance (green line) from the annulus (yellow line) to the tip of the leaflets (blue line). C1) A patient who met the criteria of non-calcific AS. C2) A patient who did not meet the criteria of non-calcific AS.
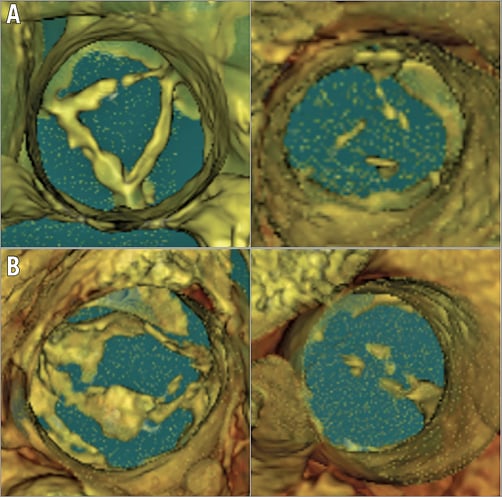
Figure 2. 3D volume-rendering view of thickened leaflets in tricuspid and bicuspid aortic valve with normal controls. A) Non-calcific aortic leaflet thickening in a tricuspid patient (on the left) with a normal control (on the right). B) Non-calcific aortic leaflet thickening in a bicuspid patient (on the left) with a normal control (on the right).
Additional preprocedural and post-procedural measurements of the annulus and stent frame geometry were performed using FluoroCT 3.0 (Circle Cardiovascular Imaging Inc., Calgary, Canada)7. Briefly, we measured the major and minor orthogonal diameters (Dmax and Dmin), perimeter and area of the annulus and stent frame (at levels of inflow, nadir, and coaptation)8. The percentage perimeter oversizing in relation to the annulus, eccentricity index (EI, [1-Dmin/Dmax]×100) and percentage of stent-frame expansion area were calculated7,9. Depth of implantation was measured as the distance between the non-coronary cusp and the lower edge of the stent frame. The volume of aortic root calcification was calculated using FluoroCT.
PROCEDURES
Procedural details have been reported previously10. Balloon predilation and post-dilation were performed according to operator discretion. All cases were performed with self-expanding transcatheter valves (CoreValve® [Medtronic, Minneapolis, MN, USA] or Venus A-Valve® [Venus Medtech Inc., Hangzhou, China])11 through an 18 Fr delivery system (Figure 3). Transthoracic echocardiography (TTE) was performed at 1, 3 and 6 months after TAVR and post-procedural MDCT was arranged before discharge in the absence of contraindications.
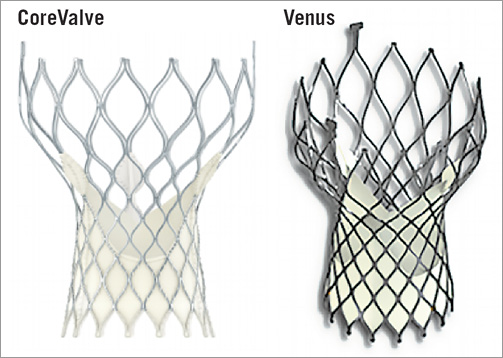
Figure 3. The two self-expanding devices used in this cohort.
STATISTICAL ANALYSIS
Due to the small sample size and the concern of a type II error, we restricted our analysis to the descriptive level. Continuous variables are presented as mean±standard deviation or medians (25th-75th quartile). Categorical variables are presented as frequencies and percentages. All computations relied on commercially available software, SPSS, Version 21 (IBM Corp., Armonk, NY, USA).
Results
NON-CALCIFIC AS IDENTIFICATION
Among 136 patients, 31 (22.8%) were identified with minimal (grade 1 or 2) calcification on pre-TAVR MDCT analysis. The mean calcium volume of these 31 cases was 87.9 (47.8-150.8)mm3 compared to 596.8 (394.4-876.1) mm3 in the remaining 105 patients. Among those with minimal calcification, we identified 21 cases meeting the predefined criteria for diffuse leaflet thickening who were included in the non-calcific AS group. In these patients, the maximal and minimal leaflet dimensions were 4.4 (3.8-4.9) mm and 1.44±0.5 mm, respectively. The remaining 115 (84.6%) patients comprised the control group.
PATIENT CHARACTERISTICS
The average age of the non-calcific AS group was lower than that of the control group (70.0 [64.0-75.5] vs. 75.0 [69.0-78.0] years) (Table 1). The mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was numerically lower (6.7 [4.8-8.9] vs. 8.2 [4.8-10.9] %) and bicuspid aortic valve morphology was less common (28.6% vs. 63.5%) in the non-calcific AS group.
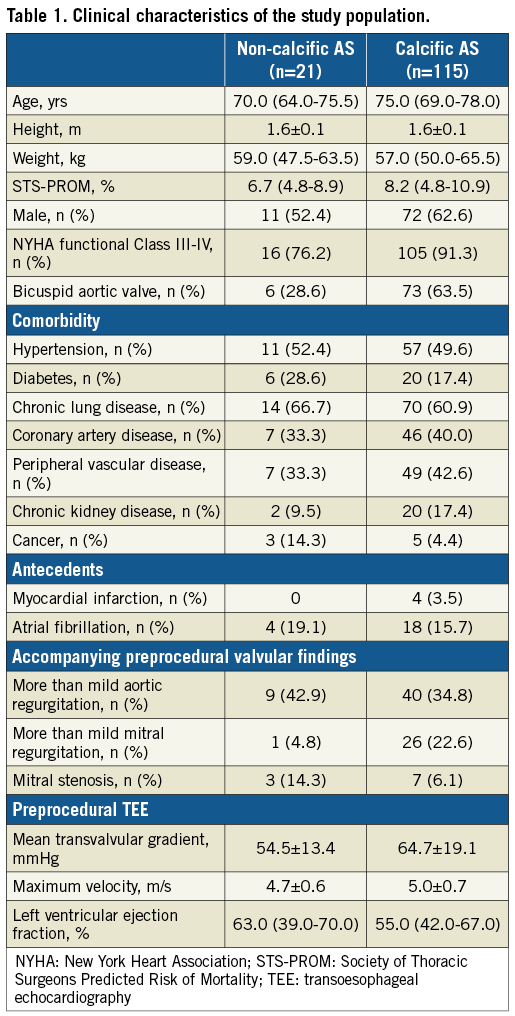
Thickened leaflets were reported on preprocedural TTE imaging in all patients in the non-calcific AS group. The maximum velocity (4.7±0.6 vs. 5.0±0.7 m/s) and mean transvalvular gradient (54.5±13.4 vs. 64.7±19.1 mmHg) were higher in the control group.
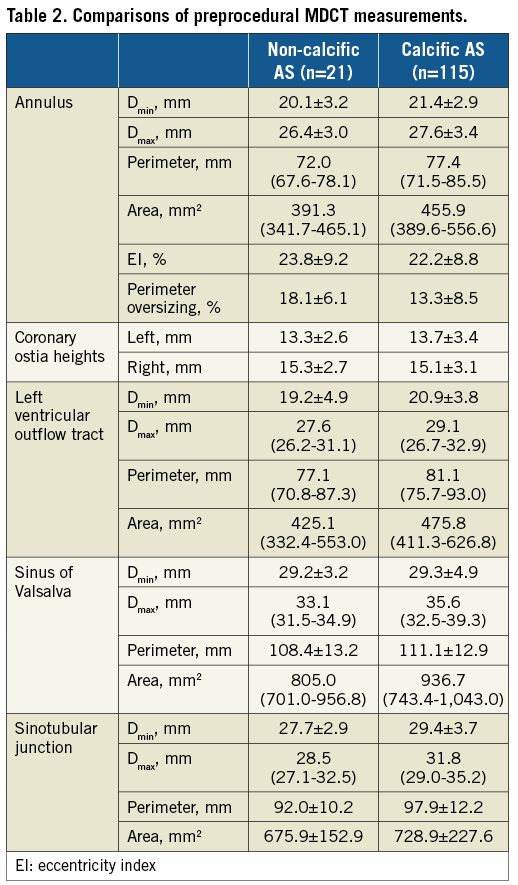
Annular and aortic root dimensions were smaller among those with non-calcific AS compared to controls (Table 2).
PROCEDURAL DATA
A transfemoral approach was feasible in all but one patient (Table 3). The median valve size selected was 26 mm in the non-calcific AS group and 29 mm among controls. Given the aforementioned smaller annular dimensions in the non-calcific AS cohort, there was greater perimeter oversizing among these patients (18.1±6.1 vs. 13.3±8.5%). Predilation was performed less frequently in patients with non-calcific AS than in controls (42.9% vs. 93.9%). In contrast, post-dilation was more commonly required in the non-calcific AS group (71.4% vs. 42.6%). The need for a second valve implantation was similar (14.3% vs. 18.3%). Two cases (1.7%) of prosthesis embolisation occurred in the control group.
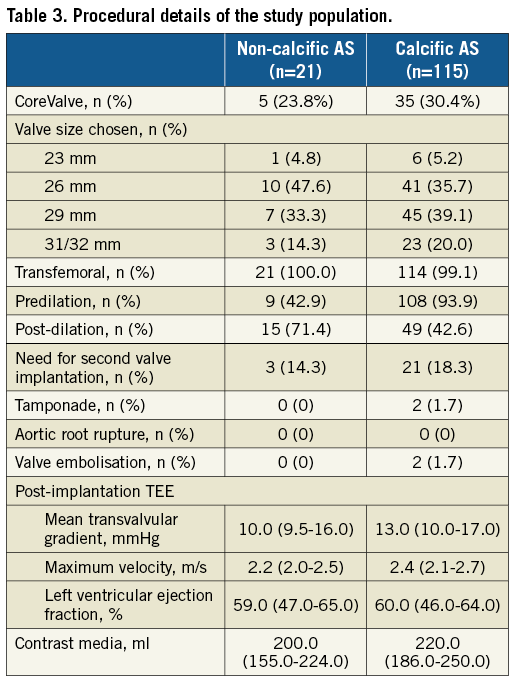
CLINICAL AND HAEMODYNAMIC OUTCOMES
Thirty-day (0% vs. 7.8%) and one-year (0% vs. 17.6%) mortality was similar between the non-calcific AS and control groups, respectively (Table 4). Similar rates of stroke (4.8% vs. 2.6%), vascular complications (19.0% vs. 11.3%), and new permanent pacemaker implantation (28.6% vs. 23.5%) were observed.
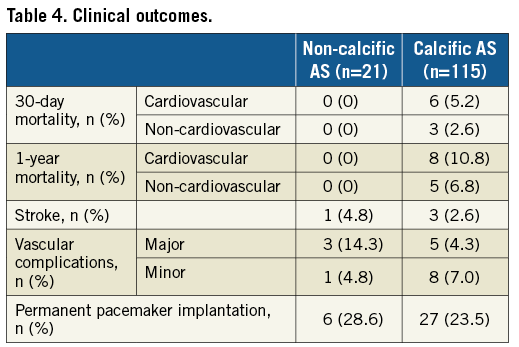
From hospital discharge to six-month follow-up, the mean transvalvular gradient was equivalent between groups (11.0 [9.5-15.0] vs. 12.0 [9.0-16.8] mmHg and 10.0 [8.0-14.5] vs. 11.0 [9.0-16.0] mmHg, respectively) (Figure 4). The proportion of patients with PVL ≥mild was numerically lower in the non-calcific AS group at hospital discharge (23.8% vs. 37.2%) and at six months (17.6% vs. 25.7%).
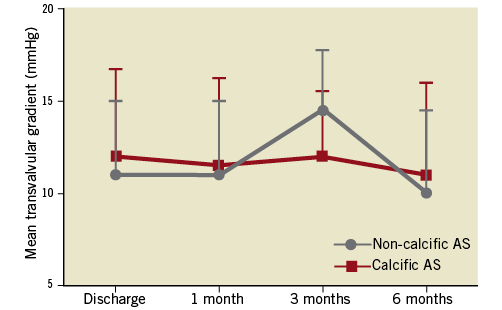
Figure 4. Haemodynamic comparisons between the two groups.
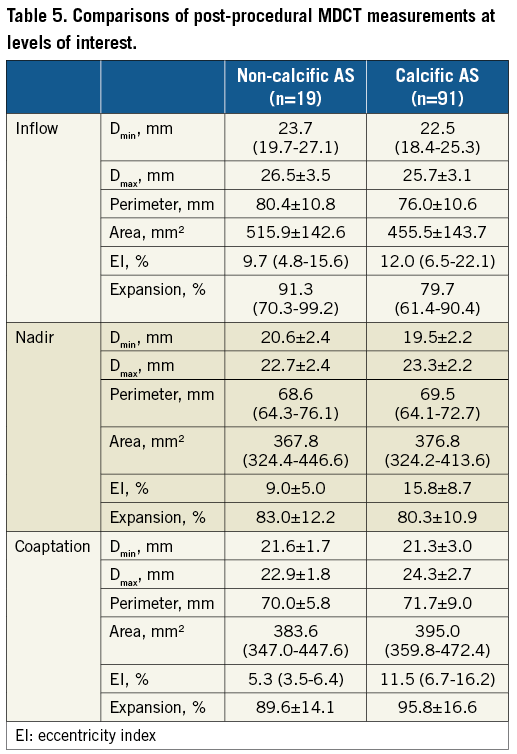
POST-IMPLANT MDCT
The majority of patients in both groups (90.5% vs. 79.1%) underwent post-procedural MDCT at a median of six (five to seven) days. Patients with non-calcific AS had lower mean implantation depth than controls (10.9±5.7 vs. 7.2±4.3 mm). The stent frame EI was lower in the non-calcific AS group at nadir (9.0±5.0 vs. 15.8±8.7%) and leaflet coaptation (5.3 [3.5-6.4] vs. 11.5 [6.7-16.2]%) (Table 5).
THV OVERSIZING
Figure 5 illustrates the rates of the combined endpoint of >mild PVL, post-dilation, or second valve implantation, stratified according to MDCT-based perimeter oversizing. In the non-calcific AS group, patients with an oversizing ≥20% had numerically fewer adverse events than those with an oversizing <20% (62.5% vs. 84.6%). Importantly, the depth of implantation was higher in those with ≥20% oversizing (7.4±3.5 vs. 12.9±5.9 mm).
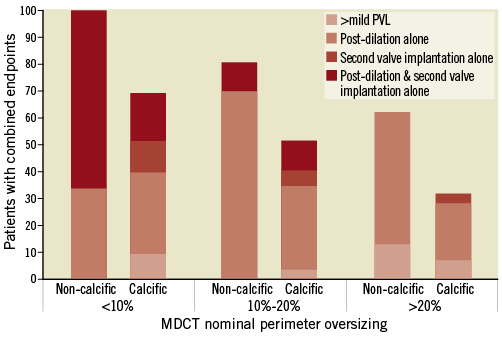
Figure 5. Comparisons of combined endpoints stratified by percentage perimeter oversizing between groups. Combined endpoints were more than mild PVL at discharge, post-dilation or second valve implantation due to PVL.
Discussion
Our study represents the little information currently available regarding the use of THVs in the setting of non-calcific AS. We demonstrated that TAVR in this setting appears safe and efficacious, with similar midterm haemodynamic and clinical outcomes to TAVR for calcific AS. Importantly, patients presenting with non-calcific AS were younger, required THV post-dilation more often, and had a lower depth of implantation than those with calcific AS. When interpreting our results, it should be noted that bicuspid aortic valve morphology was less common in the non-calcific AS group compared to the senile calcific AS group (28.6% vs. 63.5%).
Aortic valve sclerosis, characterised by leaflet calcification and thickening, is common among the elderly12. Leaflet thickening and calcification represent disparate pathophysiological mechanisms of valve obstruction which potentially pose unique challenges for transcatheter therapies where the character of native leaflet tissue is important for valve positioning, anchoring, and sealing. The paucity of information for TAVR in non-calcific AS therefore represents an important data-free zone.
In this study, we identified patients with non-calcific AS using novel MDCT-based criteria. The criteria encompassed semi-quantitative assessment for the burden of calcification and appreciation of hypoattenuated thickening on native leaflets as a three-dimensional structure, which could be practical to guide the identification of such patients. The degree of leaflet thickening observed was dramatic. When considered in parallel with minimal valvular calcification (<20% calcium volume of calcific AS), these patients represent a previously unidentified TAVR population. The underlying pathology was unclear but could be rheumatic heart disease, early stage of calcific AS, or a unique disease process of non-calcific aortic valve degeneration.
In the current study, only first-generation TAVR devices were used. Such devices could be challenging for managing non-calcific AS. Potential procedural problems relating to the thickened and non-calcific leaflets include: ventricular movement of the THV due to non-compliant leaflet tissue; insufficient anchoring due to minimal calcium; challenging positioning in the absence of calcific markers; coronary occlusion by thick and elongated leaflets; high post-procedural gradients caused by frame malexpansion; high pacemaker rates due to the direct transmission of expansion forces against the pacing tissue; and PVL. Not surprisingly, available data on this type of pathology have been limited to sporadic case reports13.
Acute procedural results in our study among non-calcific AS patients were satisfactory, without procedural death, THV embolisation, or coronary occlusion. The requirement for a second prosthesis among non-calcific AS patients was higher than in contemporary series (14.3%) but similar to that of calcific AS in our institution (18.3%). Such high rates probably reflect our learning curve with first-generation devices.
We performed post-implantation imaging in 80.9% of cases, for further understanding of the interactions between the native anatomy and implanted stent frame. Lower depth of implantation among the non-calcific AS cohort was identified. Explanations for this observation may be related to the anatomy: (A) “slipping” due to the absence of calcium to anchor at the intended site; (B) “squeezing” the valve caudally on interaction of the frame with non-compliant fibrotic leaflets; (C) impaired visualisation of the implant plane in the absence of calcium; or (D) excessive oversizing. Early in our experience of non-calcific AS cases, we infrequently performed balloon predilation for fear of further impairing THV anchoring. More recently, we have considered gentle predilation a prerequisite even in the absence of calcium, as this affords an opportunity to assess THV sizing and the anchor plane and, most importantly, to open the commissures of the fibrotic leaflets creating space for THV expansion. We believe that higher THV implantation could be achieved by this manoeuvre while also reducing the necessity to perform post-dilation. On the other hand, lower EI observed at the nadir and coaptation level for the non-calcific AS group might decrease the risk of dynamic leaflet deformation and THV thrombosis, which needs longer surveillance to be confirmed.
Haemodynamic results among non-calcific AS cases were similar to those of calcific AS and were sustained during midterm follow-up. The incidence of ≥mild PVL was 23.8% at hospital discharge and stable at six months. It has been hypothesised that the thickened leaflets could potentially yield lower rates of PVL than calcific disease, as the leaflet tissue may itself act as a sealing skirt. In this context, it is interesting to note that, despite a considerably lower depth of implant, the rate of ≥mild PVL was numerically lower in those with non-calcific AS. A lower depth of implant and lower rate of ≥mild PVL in the non-calcific group could have been impacted by the imbalanced proportion of bicuspid morphology, as the implant technique in bicuspid cases is different: the valve tends to be implanted higher which could also impact on PVL.
Valve sizing is of crucial importance for optimal TAVR outcomes. Oversizing the prosthesis relative to the native aortic root (annulus, supra-annular anchoring zone) is required for anchoring and sealing14. Most recently, an analysis of the US CoreValve Evolut R study revealed higher rates of ≥mild PVL with perimeter oversizing <20%15. Although limited by small sample size, we observed that oversizing ≥20% was associated with numerically lower rates of more than mild PVL, and requirement for post-dilation or second valve implantation. Among patients with pure aortic regurgitation, Roy et al reported rates of ≥mild PVL and requirement for second valve implantation similar to those observed in our non-calcific AS patients16. We cautiously advocate ≥20% perimeter oversizing when performing TAVR with a self-expanding prosthesis for non-calcific AS. Further validation of this sizing strategy is required.
The challenges associated with treating non-calcific AS, according to our experience, might be solved by gentle predilation and ≥20% perimeter oversizing when using self-expanding devices in order to open the fibrotic leaflets fully, creating space for THV expansion. The same holds true for new-generation TAVR devices. The ability to reposition the THV during deployment allows more precise positioning, reducing the frequency of THV embolisation and requirement for a second valve, and lower rates of PVL and permanent pacemaker implantation15,17. Such devices should be strongly considered when treating this non-calcific pathology.
Limitations
Our results have limitations inherent to single-centre retrospective studies, including small sample size, selection and treatment bias, the absence of blinded core laboratory-adjudicated echocardiographic and MDCT data, and self-reporting of clinical outcomes. Only first-generation self-expanding valves were used in this analysis; our results should not be extrapolated to balloon-expandable or mechanically expandable systems, or repositionable devices. Measurement of valve implant depth on post-TAVR MDCT is challenging and, as no standardised method exists, we have chosen the most frequently used technique in this study. The fact that the non-calcific AS group had fewer balloon aortic valvuloplasty (BAV) patients will impact on the interpretation of our results. Patients with non-calcific AS were selected solely on imaging rather than clinical criteria, and the exact underlying pathology behind native leaflet thickening was not explored in our study.
Conclusions
Among patients with non-calcific AS identified using novel MDCT criteria, TAVR with first-generation self-expanding transcatheter heart valves appears to be safe and effective.
| Impact on daily practice AS patients with thickened native leaflets and minimal valvular calcification can be identified using MDCT criteria. TAVR with first-generation self-expanding transcatheter heart valves appears to be safe and effective in non-calcific AS patients. Procedural strategies such as prosthesis oversizing ratio and predilation and post-dilation need to be adjusted according to patient characteristics. |
Guest Editor
This paper was guest edited by Nicolas M. Van Mieghem, MD, PhD, FACC, FESC; Thoraxcenter, Erasmus Medical Center, Rotterdam, the Netherlands.
Funding
This work was supported by the National Natural Science Foundation of China (81370219, Beijing, China); Science and Technology Innovative Research Groups Program of Sichuan province (2017TD0004); “13th Five-Year” National Key Research and Development Program of China (2016YFC1102200); West China Hospital “1·3·5” Discipline of Excellence Project - Percutaneous transcatheter aortic valve implantation.
Conflict of interest statement
M. Chen and Y. Feng are consultants/proctors for Venus Medtech and MicroPort. N. Piazza is a consultant/proctor for Medtronic and MicroPort, and is a co-founder of FluoroCT. D. Mylotte is a consultant/proctor for Medtronic and MicroPort. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

