Abstract
Aims: To describe the characteristics of coronary artery disease (CAD) in high-risk patients with aortic stenosis (AS), and its impact on indications for transcatheter aortic valve implantation (TAVI), and on outcomes.
Methods and results: Of 240 patients referred for TAVI, 230 had documented CAD status. Mean age was 81.5±8 years. Hundred and forty-four (63%) had CAD. Compared to patients with no CAD, those with CAD had a higher risk of mortality (EuroSCORE: 31±18%, vs. 23±11%, p=0.004). Overall, 145 patients (63%) underwent TAVI, 31 (13%) surgery, and 54 (24%) medical treatment. No patient was denied intervention because of the CAD. CAD led to re-orientateone patient (0.4%) towards surgery. PCI was performed before TAVI in 11 (7%). Survival rates were respectively 90% and 85% in the CAD and non-CAD groups (p=0.37) at 30 days, and 76.4±5.4% and 70.6±6.8% (p=0.28) at 1-year. At follow-up, functional status was similar in both groups and no further revascularisation was needed.
Conclusions: In high-risk patients referred for TAVI, CAD is frequent and associated with worse baseline characteristics. It has a limited impact on indications for TAVI. It seldom requires revascularisation anddoes not preclude satisfactory outcomes afterTAVI.
Introduction
Prevalence of coronary artery disease (CAD) in patients with aortic stenosis (AS) is high, ranging from 30 to 50%.1-5 The presence of CAD has an impact on the indications for surgical aortic valve replacement (AVR), and interventions combining AVR and coronary artery bypass grafting (CABG) are associated with an increase in post-operative mortality, compared to AVR alone.6-8
In the last few years, transcatheter aortic valve implantation (TAVI) has emerged as an attractive therapeutic option in patients with severe symptomatic AS and high risk for, or contraindications to conventional AVR. Although 30,000 patients have received TAVI to date, there is limited data on the clinical impact of CAD in the setting of TAVI.9,10
Thus, we sought to describe 1) the characteristics of CAD in a population of high-risk patients referred for severe AS; 2) CAD impact on AS treatment strategy; 3) the management of CAD in patients treated by TAVI; and 4) its influence on early and mid-term outcomes after TAVI.
Methods
Study population
From October 2006 to October 2009, 240 patients were consecutively referred for evaluation and management of high-risk severe symptomatic AS. Screening included multidisciplinary clinical evaluation, transthoracic and transoesophageal echocardiography (TEE), systematic coronary and femoro-iliac angiography, multidetector computed tomography scanner for coverage of the entire aorta, iliac and common femoral arteries. A heart team evaluated the risk profile of the patients on the basis of these evaluations combined with the use of the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) and the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM). Most of high-risk patients presented with an EuroSCORE >20%, or a STS-PROM >10%, or with a contraindication to surgery (e.g., porcelain aorta, chest radiation…)
The algorithm for management of high-risk patients with severe symptomatic AS has been previously described.11
Of these 240 high-risk patients, 10 were excluded from the study because of uncertain coronary status. Patients were included in the CAD group if they had a history of prior myocardial infarction (MI) or coronary revascularisation (percutaneous intervention [PCI] or coronary artery bypass grafting [CABG]), or significant coronary stenosis on screening coronary angiogram. Significant coronary stenosis was defined by quantitative coronary angiography as ≥70% luminal diameter narrowing of an epicardial artery measured in the “worst view” angiographic projection (≥50% for left main). Decision making for PCI was clinically driven, and restricted to patients presenting angina, and/or threatening ostial or proximal coronary lesions covering a large myocardial area. Study flow chart is shown in Figure1.
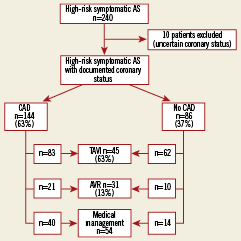
Figure 1. Management of high-risk patients with aortic stenosis according to their coronary status. Algorithm for treatment in 240 high-risk aortic stenosis patients, with and without coronary artery disease, referred for transcatheter aortic valve implantation.
Transcatheter aortic valve implantation
Procedures were performed under general anaesthesia, using either the Edwards SAPIEN transcatheter heart valve, via the transfemoral or the transapical approach, or the Medtronic CoreValve System, via the transfemoral or the subclavian approach. Technical aspects of the procedures have been detailed previously.12-18
Outcomes
Outcomes were described according to the guidelines for reporting mortality and morbidity after cardiac valve interventions. In patients who underwent TAVI, periprocedural MI was defined according to the joint ESC/ACCF/AHA/WHF guidelines as an increase in troponin of more than five times the 99th percentile upper range limit (URL) plus either new pathological Q-waves, documented new coronary artery occlusion, or evidence of new loss of viable myocardium.19 Non-Q MI was defined as an increase in troponin of more than five times the 99th percentile URL associated with transient ST or T-wave changes without new Q-waves. All electrocardiograms were recorded before and after the procedure. All clinical events were prospectively recorded during the hospital stay and the follow-up.
Statistical analysis
Continuous variables were expressed as mean ±standard deviation. Categorical data were expressed as percentages. The Student t test was used to compare continuous variables and the 2-tail Fisher’s exact test or chi-square test to compare categorical variables. Ap-value <0.05 was considered to indicate a statistically significant difference. Survival curves were obtained by the Kaplan-Meier method. Statistical analysis was performed using statistical software JMP 7.0.1 ©2007 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
Frequency of CAD was 63%. Patients with CAD were more frequently men, and had more frequent multiple extracardiac comorbities. These baseline characteristics are described in Table1.
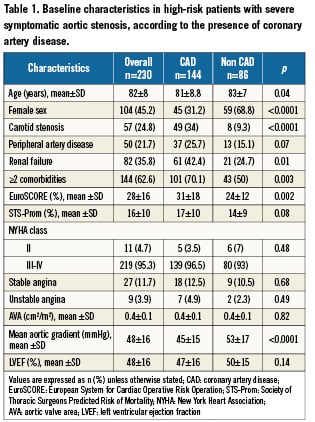
Among the 144 patients with CAD, 101 (70%) had one or several of the following: previous revascularisation in 91 (63%), 39 (43%) underwent CABG only, 28 (31%) PCI only, and 24 (26%) both techniques; a history of MI in 55 (38%). CAD was diagnosed during the screening for TAVI in 43 patients (30%). Overall, 3patients (2%) had a left main lesion, 78 (54%) 3-vessel disease, 23 (16%) 2-vessel disease, and 40 (28%) single vessel disease.
The baseline characteristics of patients who underwent TAVI according the presence or absence of CAD are presented in Table2.
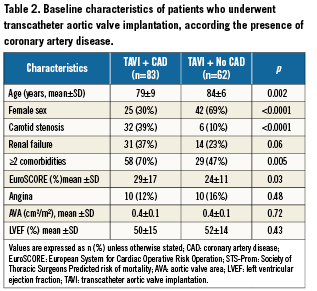
Impact of CAD on AS treatment strategy
No patient was denied any intervention because of CAD. Only onepatient was re-oriented towards combined AVR and CABG because of an unprotected left main stenosis. Four patients died before intervention, because of sudden death (n=3), or refractory cardiogenic shock (n=1). In the 36 patients who underwent medical treatment only, reasons for denying intervention were related to: frailty (n=8), a too large aortic annulus diameter (n=7), malignancy (n=4), cognitive dysfunction (n=4), too small femoral arteries and contraindication to the transapical approach (n=4), patients’ refusal (n=2), left ventricular thrombus (n=2); associated severe obstructive hypertrophic cardiomyopathy (n=2), associated severe organic mitral regurgitation (n=1), previous stroke with disabling sequelae (n=2).
Management of CAD in the setting of TAVI
Out of the 83 CAD patients who underwent TAVI, 16 (19%) were free of any residual significant coronary stenosis. Among the 67 patients with ≥1 coronary stenosis, 56 (83%) did not undergo any revascularisation prior to TAVI; the other 11 patients (17%) underwent PCI prior to TAVI (n=9) or just before TAVI, at the beginning of the procedure (n=2). Their baseline characteristics are summarised in Table3. In 5/11 cases, PCI concerned patients with severe stenoses of the proximal left anterior descending artery.
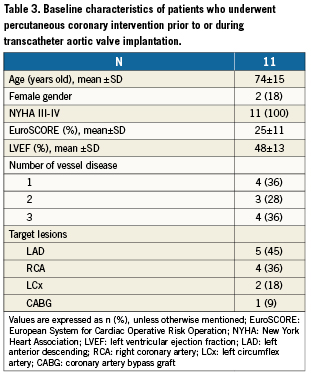
Mean delay between PCI and TAVI was 6±6 weeks. Among the nine patients who had PCI prior to TAVI, one had balloon aortic valvuloplasty during the same procedure. There were no severe adverse events after PCI. No revascularisation was required after TAVI during index hospital stay.
Impact of CAD on 30-day outcomes after TAVI
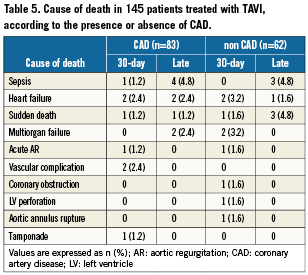
Results are displayed in Tables4 and 5. There were no periprocedural Q-wave MIs. The rate of periprocedural non-Q-wave MI was similar in the two groups. None of these MIs led to any clinically relevant events. Overall, mean troponin level (mcg/L) after procedure was 4.5±5.5. It was not significantly lower in the non-CAD group (3.5±5.4 versus 5.2±5.5, p=0.09) Thirty-day mortality was similar in the two groups. There were eight deaths in the CAD group: tamponade (n=1), cardiogenic shock (n=1), massive aortic regurgitation (n=1), low cardiac output syndrome (n=1), vascular complication (n=2), sudden unexplained death at day 1 (n=1) and septic shock (n=1). There were nine deaths in the non-CAD group: post-procedural ventricular fibrillation (n=1), coronary obstruction by the native leaflets (n=1), massive aortic regurgitation (n=1), left ventricular perforation (n=1), aortic annulus rupture (n=1), heart failure (n=2), and multiorgan failure (n=2). Non-fatal complication rates were similar in the two groups.
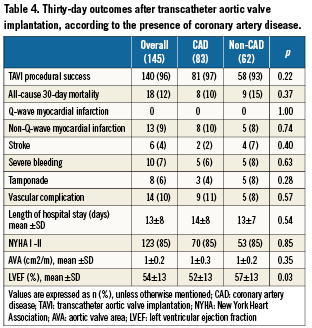
The rate of NYHA class I or II patients 30 days after TAVI was high (85%) and similar in both the CAD and non-CAD groups. Echocardiographic findings at 30 days were also similar in both groups.
Impact of CAD on 1-year outcomes after TAVI
Follow-up was obtained in 100% of the patients. Mean follow-up was 248±239 days. Overall, 1-year survival rate was 74%±4.2: 70.6%±6.8 in the non-CAD group and 76.4%±5.4 in the CAD group (p=0.28). Kaplan-Meier survival curves are displayed in Figure2. The causes of deaths are detailed in Table5.
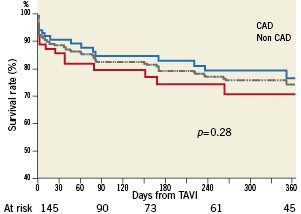
Figure 2. One-year survival after transcatheter aortic valve implantation, according to the presence or absence of coronary artery disease. Kaplan-Meier survival curves after TAVI in overall (dotted gray line), CAD (blue full line) and non-CAD (red full line) subgroups.
Among hospital survivors, NYHA I-II rates at last follow-up were high (78%), and not significantly different between the CAD and non-CAD groups (72% versus 87%, p=0.09). The number of hospital readmissions for cardiac causes at last follow-up was low and similar in both groups (respectively 14% and 9%, p=0.65). No patient had recurrent angina or underwent coronary artery revascularisation during follow-up.
Discussion
Our study confirmed that CAD is frequent among high-risk AS patients. Baseline characteristics of patients with CAD revealed higher risk profiles than in those without CAD, due to comorbidities. To our knowledge, this study is the first one to suggest that, despite this higher baseline risk, CAD has a limited impact on the decision to perform TAVI. Finally, CAD does not influence the short- and mid-term prognosis after TAVI.
CAD characteristics in high-risk symptomatic AS patients
Definition of CAD status was based on prior coronary revascularisation, documented MI, or coronary angiogram showing significant artery stenosis. The few publications on this topic used various definitions of CAD, which could account, in part, for the differences in results and findings.5,9,10
In accordance with previous studies, frequency of CAD was high in the overall study population (63%), and not significantly different in patients who underwent TAVI (57%).4,5 This is consistent with previous observations, and tends to be higher than AVR series of AS patients, where frequency of CAD ranges from 25% to 50%.20-26 This can be explained in part by the fact that high-risk patients with prior CABG tend to undergo TAVI rather than have redo surgery for AVR.
Impact of CAD on treatment strategy in high-risk AS patients
To our knowledge, this is the first report of the impact of CAD on treatment strategy in a high risk AS population. Published studies on CAD in the setting of TAVI are scarce, and tend to focus on patients who actually underwent TAVI, but not on the entire screened population.9,10
In the present study, access to any intervention was never denied on the sole basis of the patient’s CAD status. Although AVR was primarily considered in patients with multivessel CAD, this option was rarely chosen in the present population: only one patient was redirected to surgery because of an unprotected left main lesion.
CAD management in the setting of TAVI
Several options can be considered, using medical treatment or PCI. We never considered hybrid interventions combining TAVI and CABG. Our most frequent choice was TAVI combined with medical management of CAD. Only a minority of patients underwent PCI combined with TAVI. In most cases, we planned PCI one month prior to TAVI. In two patients, we performed PCI during TAVI, in the context of severe congestive heart failure concomitant with acute coronary syndrome. This option should be restricted to a minority of patients, because of the greater length of the procedure and the increased risk of renal failure. Finally, PCI may be performed after TAVI, but we never considered this option.28
Overall, this strategy reflects a trend towards less systematic coronary revascularisation in the setting of TAVI when compared to AVR. Despite the absence of any evidence based data to support this approach, it seems to be shared by most teams and to be associated with satisfactory outcomes in this patients’ population.5,9,10,20,22
Impact of CAD on outcomes after TAVI
As others, we observed no periprocedural Q-wave MI.5 CAD and non-CAD groups had similar post-procedural non-Q-wave MI rates, with no clinical impact.
Overall, the present 12% TAVI 30-day mortality rate was similar to that published in most other studies and there was no significant difference between CAD and non CAD patients.20-23,29-31 Published studies specifically addressing the issue of the impact of CAD on outcomes after TAVI are rare.9,10 Contrary to our findings, Dewey etal showed a 10 fold higher 30-day mortality rate among CAD compared to non-CAD patients (13.1%, vs. 1.2%, p=0.002).9 However, in this study, CAD was defined only as previous coronary artery revascularisation, which excluded all the patients with CAD treated medically or newly discovered. Our results are closer to those of Masson et al, who observed similar 30-day mortality in patients with, and without CAD (11.5%, vs. 6.3%, p=0.39).10 In The PARTNER US Trial, subgroup analysis did not show any significant interaction between the presence of prior coronary revascularisation and the effect of TAVI treatment on 30-day mortality.5 Consistent with these results, our findings suggest that CAD has alimited impact on the periprocedural risk of TAVI, and that a strategy using restricted coronary revascularisation by PCI does not preclude patient safety.
Overall, the present 1-year survival rate was similar to that observed in recently published studies.5,21-23,30 We observe in this study that CAD patients do not have excess mortality one year after TAVI, compared to non CAD patients. Predictors of cumulative late mortality after TAVI in the Multicentre Canadian Experience were essentially extra-cardiac comorbidities, and not coronary status.22,23 Furthermore, among 130 patients included in the European PARTNER trial, only two late deaths were definitely related to coronary events.30
Finally, the majority of survivors remained in NYHA class I or II, with no difference between the CAD and non-CAD groups. We found no more readmissions for cardiovascular causes in the CAD than in the non-CAD group during follow-up. There was neither recurrence of angina, nor need for later coronary revascularisation during follow-up after TAVI.
Study limitations
Our study included several patient subsets: patients with previously treated CAD, untreated CAD, undergoing recent PCI and hybrid PCI-TAVI. Limitations are also due to its monocentric and observational design. The fact that our cohort was monocentric however gives a clear picture of the impact of a homogeneous treatment strategy. Given the number of statistical tests relative to the sample size, a type 1 or a type 2 error cannot be excluded. The one-year follow-up is too short to determine the long-term impact of CAD in this population. Further randomised studies are needed to evaluate the best treatment strategy for CAD in TAVI patients. To analyse post-procedural MI rates, we used the definition for post-operative MI after cardiac surgery. However, specific definitions for TAVI would help in standardising outcomes and allowing relevant comparisons between various series. The recent availability of the definitions established by the Valvular Academic Research Consortium should play a key role.31 However, as our data collection was prospectively carried out since October 2006, we could not use these definitions.
Conclusion
In high-risk patients with severe symptomatic AS referred for TAVI, CAD is frequent and associated with higher risk features. CAD in isolation did not significantly restrict TAVI indications. Despite limited use of PCI, the presence of CAD had no statistically significant effect upon the early and mid-term outcomes after TAVI.
Conflict of interest statement
Dr. Himbert is a proctor physician for Edwards Lifesciences and Medtronic Inc. Dr. Nataf is a proctor physician for Edwards Lifesciences. Drs Iung received speaker’s fees from Edwards Lifesciences. Dr Vahanian received speaker’s fees from Edwards Lifesciences and is a consultant for Medtronic Inc. The other authors have no conflict of interest to declare.

