Abstract
Aims: We sought to evaluate the effects of significant coronary artery disease (CAD) upon outcome after transcatheter aortic valve implantation (TAVI).
Methods and results: We performed a retrospective study of 271 consecutive patients undergoing TAVI using either the Edwards SAPIEN or Edwards SAPIEN XT valve. Pre-procedural coronary angiograms were analysed by quantitative coronary angiography (defining significant CAD as a stenosis of ≥70% or ≥50% if in the left main stem or a vein graft). Ninety-three out of 271 patients had significant CAD. There was no difference in mortality at 30 days or 12 months between the two groups (6.7% vs. 7.5% and 21.5% vs. 23.7%; log-rank p=0.805). A secondary analysis using the SYNTAX algorithm of coronary anatomy complexity was performed on 189 patients. Those in the high SYNTAX score (>33) group had higher mortality at 30 days and 12 months (14.3% and 57.1%) than the low (5.2% and 23.3%) and intermediate-risk groups (11.1% and 22.2%; log-rank p=0.007). ROC analysis identified a SYNTAX score of >9 at the time of TAVI as the optimal cut-off, with an independent association with mortality (HR 1.95 [95% CI: 1.21-3.13]; p=0.006). Patients with a SYNTAX score >9 had greater 30-day, 12-month and overall mortalities than those with a SYNTAX score <9 (3.7% vs. 11.3% and 20.7% vs. 34.3%; log-rank p=0.005).
Conclusions: Significant CAD, as defined using “real-world” QCA margins, did not have a significant effect upon mortality after TAVI for severe aortic stenosis. However, higher-risk SYNTAX groups, including those with a score >9, had increased mortality.
Introduction
Transcatheter aortic valve implantation (TAVI) has now entered the mainstream as the treatment of choice for patients with severe aortic stenosis (AS) who are unable to undergo surgical aortic valve replacement (sAVR) due to high operative risk or technical feasibility. The early mortality rates have gradually declined as the procedure and devices have developed and the mortality benefits have been successfully demonstrated. The key to success in these patients is careful selection: the current standard is determination of suitability by a multidisciplinary team with expertise in aortic valve disease using a combination of clinical judgement and risk scoring systems1.
One possible risk factor which may contribute towards an adverse patient outcome is the presence of significant coronary artery disease (CAD). In patients undergoing coronary artery bypass grafting (CABG) the presence of severe AS is a class I indication for combined CABG plus sAVR1, though these patients have a higher mortality compared to those undergoing sAVR alone2. Coronary artery disease which is left without bypass grafting has been shown to be associated with increased early and late mortality in small registries, and so complete revascularisation of any vessel diseased by ≥50% is undertaken routinely. Indeed, the current European Society of Cardiology (ESC) guidelines suggest that, in patients requiring surgery for aortic valve disease, revascularisation by CABG for lesions of 50-70% is a class IIa indication (level of evidence C) and for lesions ≥70% it is defined as a class I indication (again only level of evidence C)3.
However, the treatment of CAD prior to TAVI is more heterogenous and no consensus exists. CAD has a high prevalence in these patients4,5 with similar causative risk factors6. Previous coronary revascularisation in the form of CABG or percutaneous intervention (PCI) as a surrogate for CAD has been suggested to increase the risk of death within 30 days in patients receiving TAVI by more than tenfold7; yet the amount of myocardium at risk prior to TAVI was shown not to do so8.
To aid decision making as to whether PCI should be undertaken prior to TAVI we undertook this study to identify whether significant CAD, identified during pre-TAVI coronary angiography, had any effect upon the outcome from TAVI using the Edwards bioprosthesis (Edwards Lifesciences, Irvine, CA, USA), as well as the effects of any PCI performed prior to TAVI.
Methods
We performed a retrospective analysis of 288 consecutive, successful implantations of the Edwards bioprosthesis at St Thomas’ Hospital, London, from 13th March 2008 to 20th June 2012. Each patient had undergone a pre-TAVI assessment incorporating echocardiography (transthoracic [TTE] and/or transoesophageal [TOE]), computed tomography (CT) of the aorta, pulmonary function tests, carotid Dopplers and coronary angiography. Severe aortic stenosis was defined as: peak transvalvular gradient of ≥40 mmHg on either TTE, or TOE or dobutamine stress echocardiography (DSE) and an aortic valve area ≤1.0 cm2. Each case was considered at a multidisciplinary team (MDT) meeting including at least one interventional cardiologist and at least one cardiothoracic surgeon. The patient was accepted for TAVI if deemed unable to undergo sAVR due to excessive risk (EuroSCORE ≥20) or technical considerations precluding surgery. The procedure was performed via either the transfemoral, the transapical or the transaortic approach. Of the 288 TAVI procedures, 275 were performed for severe aortic stenosis and of these 271 patients had pre-TAVI coronary angiograms available for review (Figure 1).
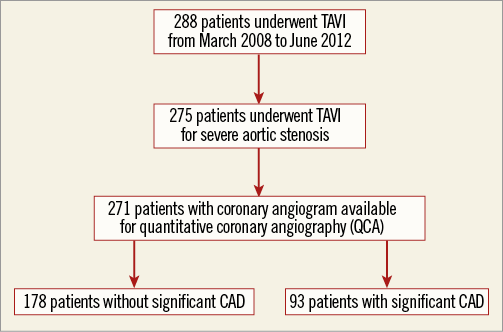
Figure 1. Study design.
Data were interrogated from the database of patients, including patient demographics and baseline characteristics, procedural results and long-term outcome. The coronary angiograms were assessed for epicardial coronary artery lesions of ≥70% severity to denote significant coronary artery disease using quantitative coronary angiography (QCA) (or ≥50% for left main stem [LMS] or saphenous vein graft lesions [SVG]). This was performed independently by two trained operators blinded to the results and outcomes. Patients were then divided into two groups: those without significant CAD (group 1) and those with (group 2). Vessels which had been revascularised by CABG were deemed “not significant”. Outcomes were defined as in-patient mortality, death within 30 days, death within 12 months, overall death, acute kidney injury (according to the updated Valve Academic Research Consortium [VARC] Acute Kidney Injury Network [AKIN] criteria9). PCI was performed as per standard practice with all patients receiving bare metal stents and dual antiplatelet therapy for 30 days afterwards, continuing on a single antiplatelet thereafter. One procedure was performed as a hybrid immediately prior to TAVI. A secondary analysis was also performed using classification of patients who had not undergone CABG on the basis of the anatomical complexity of coronary disease using the SYNTAX scoring algorithm10. Three independent cardiologists analysed the available angiograms and inter-rater agreement was assessed by Fleiss’ kappa statistic11.
Data analysis was performed using SPSS 21.0 (IBM Corp., Armonk, NY, USA) and PRISM 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) with t-tests used for comparison of continuous variables and χ2 tests for categorical variables. Non-normally distributed data were tested with appropriate non-parametric statistical tests. Survival statistics and curves were created using the Kaplan-Meier method. Univariate Cox regression was performed using an unadjusted model for each covariate and a multivariate model was constructed using a forward elimination method and an entry criterion of 0.05.
Results
BASELINE DEMOGRAPHICS
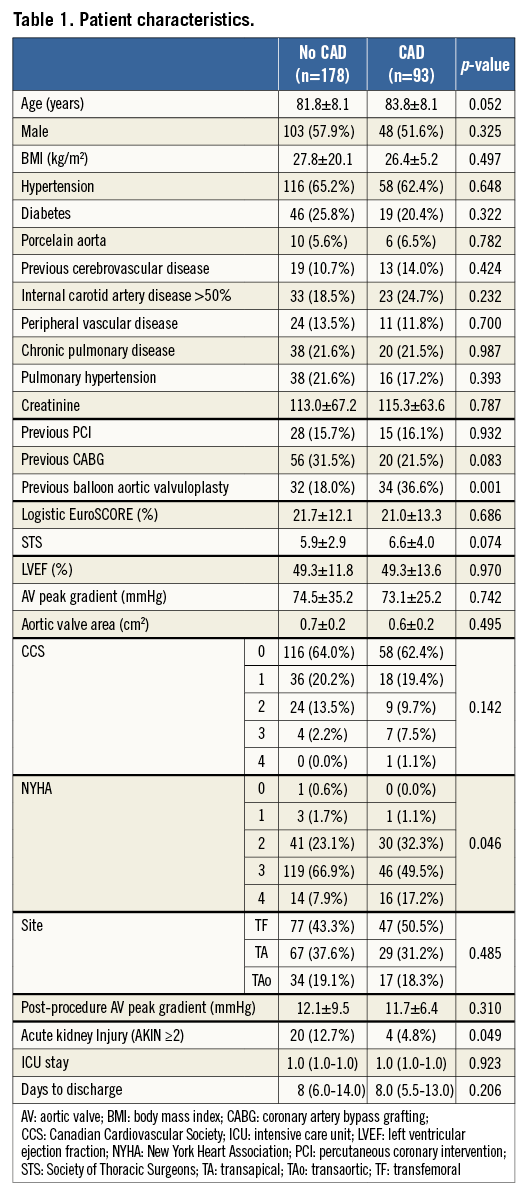
The final study population consisted of 271 patients (Figure 1) who underwent TAVI using the Edwards bioprosthesis for severe aortic stenosis and for whom pre-TAVI coronary angiography was available for QCA. Group 1 comprised 178 patients free of significant CAD (65.7%), and group 2 of 93 patients with significant coronary disease (34.3%). The demographics are summarised in Table 1 and show that the groups were well matched with similar perioperative risk scores (p=0.686 for LES), barring a higher rate of previous balloon aortic valvuloplasty (BAV) in the CAD group (36.6% vs. 18.0%; p=0.001).
CAD VERSUS NO CAD
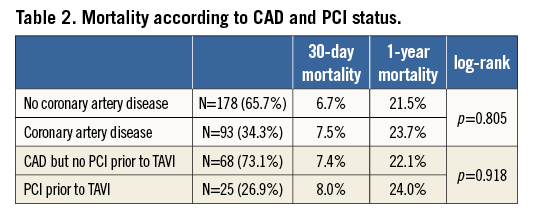
No difference was seen in either pre-TAVI or post-TAVI peak AV gradient between the CAD and no CAD groups (Figure 2). At a median follow-up of 683 days (interquartile range [IQR] 319.0-1,126.3) there was no difference in overall mortality (log-rank p=0.805; Figure 3A). Table 2 describes the mortality rates for the two groups and shows no statistically significant difference at either 30 days or 12 months. Rates of stroke were similar between the CAD and no CAD groups at 3.9% and 4.3%, respectively (p=0.884), and rates of major vascular complications were also not significantly different (4.5% vs. 2.2%, p=0.331).
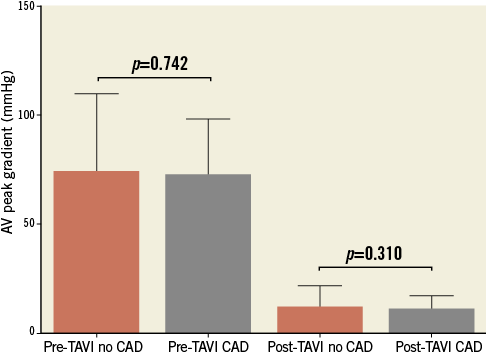
Figure 2. Pre-TAVI and post-TAVI peak aortic valve (AV) gradients.
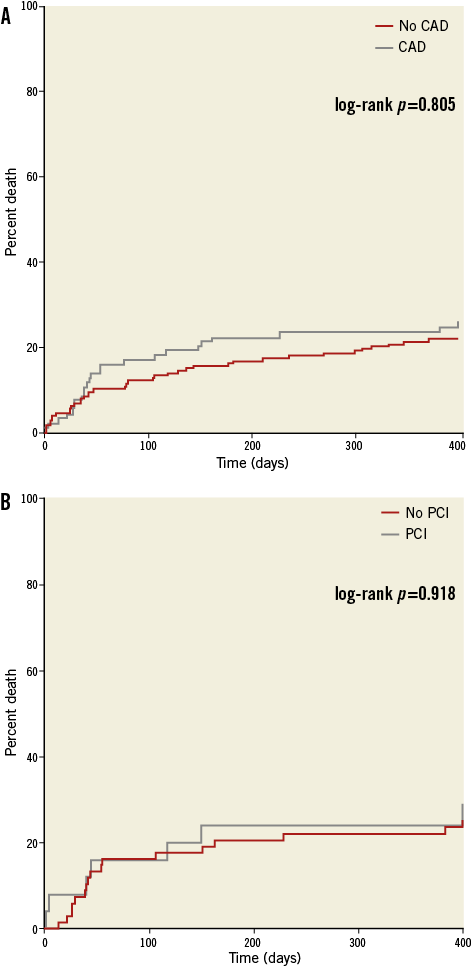
Figure 3. Kaplan-Meier curves showing the effects of significant coronary artery disease (CAD) upon survival (A), and the effects of percutaneous coronary intervention (PCI) prior to TAVI upon survival (B).
Multivariate Cox proportional hazards modelling identified a porcelain aorta (hazard ratio [HR] 3.36 [95% confidence intervals [CI] 1.72-6.56]; p<0.001), chronic pulmonary disease (HR 2.01 [95% CI: 1.29-3.13]; p=0.002), significant MR (HR 1.92 [95% CI: 1.20-3.07]; p=0.007) and glomerular filtration rate (HR 0.99 [95% CI: 0.98-1.00]; p=0.047) as independently associated with death after TAVI. Neither CAD status, nor the use of pre-TAVI PCI nor previous revascularisation by either PCI or CABG demonstrated either a significant or a “promising” association with death in the initial univariate analysis.
PCI PRIOR TO TAVI
Twenty-five patients underwent PCI prior to TAVI (6.3%) and their mortality rates are detailed in Table 2. The median time between PCI and TAVI was 49.5 days (interquartile range 25-127 days). There was no significant difference in 30-day or 12-month mortality in those patients who received PCI and those with CAD who did not. The Kaplan-Meier survival curve is presented in Figure 3B and shows no statistically significant difference between the two groups. No patients underwent post-TAVI PCI during the perioperative period or index admission.
SYNTAX SCORE
One hundred and eighty-nine patients without CABG had coronary angiograms available for calculation of SYNTAX scores at the time of TAVI. The three independent raters had a Fleiss’ kappa statistic of 0.736 (p<0.001), suggesting a very good agreement. The median score was 0 (the interquartile range was 0-12 with a maximum SYNTAX score of 52). Ninety-one point five percent (n=173) of patients had a SYNTAX score of 0-22; 4.8% (n=9) had a SYNTAX score of 23-32 and 3.7% of patients had a score of ≥33. The Kaplan-Meier survival analysis revealed that this highest tertile of SYNTAX score had significantly higher mortality at 30 days (14.3%) and one year (57.1%) when compared to the others (log-rank p=0.007; Figure 4A and Table 3). Receiver operating characteristic curves were fitted identifying a SYNTAX score of 9 as the optimum cut-off point for predicting mortality. When used to separate patients into two groups, it was a good discriminator of risk with significantly divergent Kaplan-Meier curves: those with a SYNTAX score >9 had higher 30-day and one-year mortalities than those with a SYNTAX score ≤9 (11.3% vs. 3.7% and 34.3% vs. 20.7%, respectively [log-rank p=0.005]; Figure 4B and Table 3). Twenty-eight percent of patients had a SYNTAX score of >9 (Figure 5 for spread).
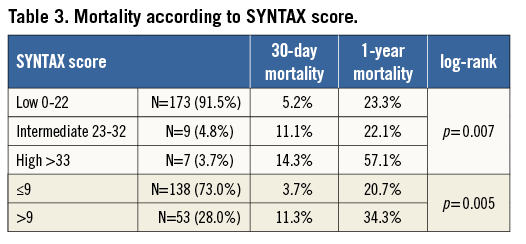
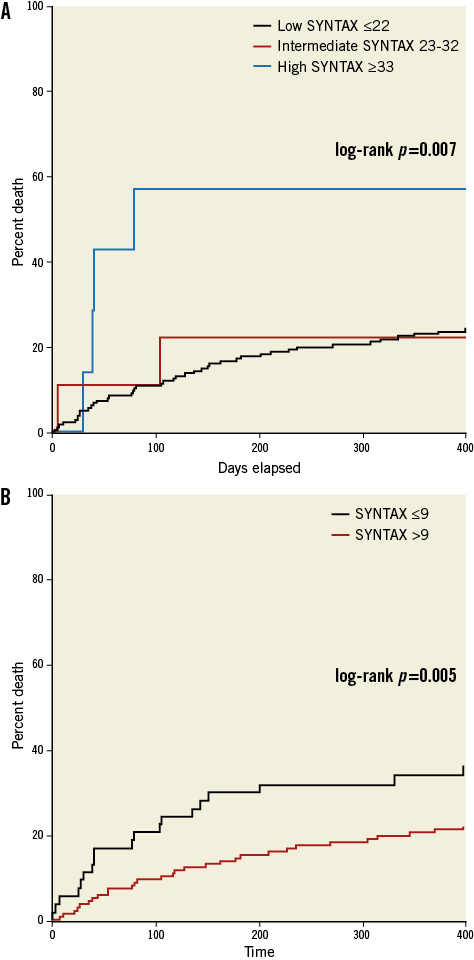
Figure 4. Kaplan-Meier curves showing the effects of complexity of coronary artery disease anatomy according to SYNTAX risk group (A), and the effects of a SYNTAX score >9 upon cumulative mortality (B).
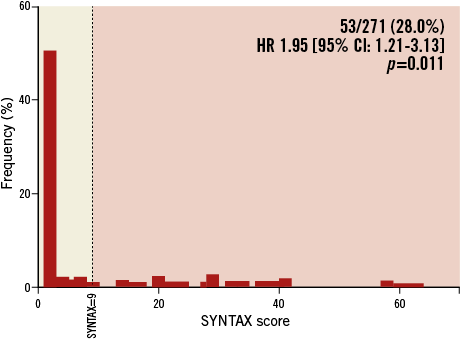
Figure 5. Frequency of SYNTAX scores with the optimal cut-off of a score of 9.
In those patients without CABG eligible for SYNTAX scoring, multivariate Cox proportional hazards modelling revealed that two factors independently associated with mortality were a SYNTAX score >9 (HR 1.86 [95% CI: 1.15-3.02]; p=0.011) and chronic pulmonary disease (HR 1.84 [95% CI: 1.12-3.04]; p=0.016). When SYNTAX >9 is replaced by SYNTAX >33 (the highest tertile) in the model it also demonstrates an independent, strong association with mortality (HR 4.26 [95% CI: 1.68-10.8]; p=0.002).
Discussion
Since the introduction of the two commercially available TAVI systems, the CoreValve Revalving® system (Medtronic CoreValve, Luxembourg) and the Edwards SAPIEN® valve (Edwards Lifesciences), improvements have been made in deliverability and access technologies. The mortality benefits of TAVI in this higher-risk cohort have now been successfully demonstrated12,13. Beyond this, we should now consider the next stage in the development of the technique, aiming to improve patient safety, benefits and outcomes. The literature surrounding sAVR has successfully identified a number of risk factors associated with adverse outcomes in those undergoing the procedure: recent myocardial infarction, increasing age14-16, diabetes17, poor left ventricular function14-16,18, female sex15,16 and impaired renal function15. Concomitant revascularisation with CABG increases the procedural and cross-clamp time for sAVR19. When compared to patients receiving isolated sAVR alone, who did not have significant coronary artery disease, patients undergoing a combined sAVR and CABG have been shown to suffer increased rates of periprocedural myocardial infarction and early postoperative mortality20-23. Other series have refuted this and demonstrated no adverse outcome upon mortality in this period24-26. However, a more pertinent comparison is between those patients with significant CAD undergoing sAVR with and without CABG. Indeed, the evidence here suggests that there is a protective effect against periprocedural MI and mortality and late measures of mortality and morbidity24,26,27. Patients who do not receive “full” revascularisation are also affected by more LV systolic dysfunction postoperatively28. This breadth of evidence has led to concomitant CABG surgery becoming the “standard of care” in patients receiving sAVR with CAD.
Reflecting the example of our cardiac surgical colleagues in improving patient safety and outcome, the purpose of this study was to identify the effects of significant CAD upon survival in all patients receiving an Edwards SAPIEN or SAPIEN XT TAVI at our centre. This was defined as stenoses of ≥70% in major epicardial coronary arteries identified during pre-TAVI coronary angiography (or ≥50% in SVG or LMS lesions). The baseline demographics of the two groups (CAD versus no CAD) were broadly similar. The overall rate of significant CAD in this cohort was 34.3% (93/271) – in keeping with the other studies on similar groups4,7,8. This is a significant proportion of patients undergoing TAVI.
However, the CAD status was not found significantly to affect the 30-day or 12-month mortalities or the survival as per the Kaplan-Meier analysis. Neither previous PCI nor previous CABG, nor a combined “previous revascularisation” variable, was associated with greater mortality, as suggested by previous studies using these as a surrogate for CAD7,29. However, a raised SYNTAX score was found to be independently associated with mortality after TAVI – whether in the very high risk group with a score of >33 or a more useful cut-off of 9.
Twenty-five patients underwent PCI in preparation for their TAVI. Survival analysis by the Kaplan-Meier method demonstrated no significant mortality benefit for PCI in the CAD cohort. These patients were identified to be at high risk due to either the prognostic implications of their lesions, anatomy more amenable to PCI or presentation with more severe angina, and so there may be a selection bias. Cox proportional hazards modelling did not reveal PCI to be an independent risk factor for death, in accordance with the Bern experience30, although both studies may have been underpowered to detect this.
Retrospective analyses of the effects of CAD upon outcome after TAVI have not provided a definitive answer to the question of the significance of CAD in TAVI patients7,8,29,31. The German TAVI registry suggested greater mortality in the presence of CAD, defined as previous revascularisation or a stenosis of ≥50%, but the two cohorts were significantly different, perhaps accounting for this finding29. A recent meta-analysis also demonstrated no effect of CAD upon outcome in the midterm, but again the studies included had variable definitions of CAD, including previous revascularisation and lesions of 50% and above32. Goel et al have recently shown that PCI in patients with significant CAD is feasible across a variety of presentations including acute coronary syndromes, but not in the context of preparing for valvular intervention33. Van Mieghem and colleagues demonstrated no significant difference between complete and incomplete revascularisation, nor in patients with SYNTAX scores greater than or less than 834. Defining which lesions merit intervention is also problematic: physiology-guided PCI using fractional flow reserve is difficult, given that it is not ratified in the severe AS cohort, in whom there is impairment of the normal coronary flow regulation35, and there is a paucity of data on microvascular resistance and other determinants of coronary haemodynamics.
Our study analysed the effects of actual stenoses of ≥70% in the major epicardial vessels. This would seem to be the most germane cut-off, representing the usual threshold for a flow-limiting lesion. However, we could identify no statistically significant relationship between CAD and death at 30 days, 12 months or death overall. Whilst the existence of previous revascularisation in the form of previous PCI or CABG was shown in another study to be associated with 30-day and overall mortality, in improving the TAVI technique this is not a modifiable risk factor. We have also demonstrated that while patients with high SYNTAX scores (>33) may represent a very high-risk cohort for TAVI, a SYNTAX score of greater than 9 has a significant independent association with mortality after TAVI and may be a target for revascularisation to reduce risk. The next phase of TAVI development requires data from randomised controlled trials of the effects of pre-TAVI PCI upon outcome, such as the percutAneous Coronary intervention prIor to transcatheter aortic Valve implantation (ACTIVATION) trial now enrolling, which hypothesises that non-revascularisation of lesions of ≥70% severity prior to TAVI is non-inferior to revascularisation.
As with any such study there are limits to observational data, including the selection bias in the PCI cohort. Whilst every effort was made to maintain a complete database, we may not have been informed of subsequent events in patients from outside our direct region, beyond our usual post-TAVI follow-up period.
In conclusion, CAD, classified using “real-world” cut-offs of 70% for major epicardial stenoses and 50% for LMS and SVG lesions, does not have a significant effect upon mortality after TAVI. However, a SYNTAX score greater than 9 was independently associated with increased mortality. Definitive guidance as to the optimal treatment strategy requires randomised data.
| Impact on daily practice Our data suggest that the angiographic significance of coronary artery disease using a “real-world” threshold of 70% does not affect mortality in patients undergoing TAVI. However, more complex coronary artery disease, as described by SYNTAX score, increases the risk of death. This should perhaps be taken into account in the “Heart Team” decision-making process. Whether patients should undergo percutaneous intervention prior to TAVI will be addressed in the ongoing ACTIVATION trial. |
Funding
There was no funding for this specific study. M.Z. Khawaja (FS/12/15/29380) and K.N. Asrress (FS/11/43/28760) are in receipt of British Heart Foundation Clinical Research Training Fellowship funding.
Conflict of interest statement
M.Z. Khawaja, M. Thomas and S. Redwood have received research funding from Boston Scientific and Edwards Lifesciences and C.P. Young, V. Bapat, M. Thomas and S. Redwood are proctors for Edwards Lifesciences. The other authors have no conflicts of interest to declare.

