Abstract
Coronary artery disease (CAD) and aortic stenosis (AS) share pathophysiological mechanisms and risk factors. Moreover, the prevalence of CAD increases among elderly patients with severe AS since disease progression is strongly associated with age for both CAD and AS. These factors contribute to the frequent coexistence of CAD and AS. Patients with concomitant AS and CAD are characterised by higher baseline risk profiles with a larger number of comorbidities as compared to patients with isolated AS. Therefore, adequate therapeutic strategies are crucial for the treatment of these patients. The number of patients undergoing concomitant coronary artery bypass grafting (CABG) and surgical aortic valve replacement (SAVR) doubled during the last decade. Moreover, the development and rapid integration of transcatheter aortic valve implantation (TAVI) into clinical practice in western European countries has further extended invasive treatment of AS to elderly high-risk patients not considered suitable candidates for SAVR, frequently presenting with CAD. The aim of this review article is to provide an overview on CAD prevalence, impact on clinical outcomes, and treatment strategies in patients with severe AS requiring SAVR or TAVI.
Introduction
Coronary artery disease (CAD) and aortic valve stenosis (AS) share several pathophysiological pathways and risk factors, which contribute to the frequent coexistence of these two pathologic conditions in the same individual1,2. Beyond degenerative changes related to age, both CAD and AS are characterised by similar initiating factors3-5. Subendothelial accumulation of oxidised low-density lipoproteins together with recruitment of lymphocytes and macrophages result in local inflammatory reactions followed by extracellular matrix formation, calcification, fibrosis, and endothelial dysfunction with subsequent disease progression3-5. Mechanical stress accentuates these processes in both CAD and AS. However, AS differs from CAD in terms of the larger accumulation of extracellular calcification and the absence of smooth muscle cell proliferation4,6. Moreover, CAD and AS have common risk factors including age, male gender, diabetes mellitus, hypercholesterolaemia, arterial hypertension, and chronic kidney disease2. In relation to clinical manifestation of the disease, AS is characterised by gradual but continuous progression with clinical events caused by reduced leaflet mobility and subsequent left ventricular outflow obstruction1. Conversely, CAD, rather than following a linear fashion, is characterised by periods of relative quiescence alternating with episodes of clinically apparent or silent plaque rupture7. Clinical events in patients with CAD are a consequence of myocardial ischaemia, typically related to instability of atherosclerotic plaques7.
The prevalence of concomitant CAD increases among elderly patients with severe AS, since disease progression is strongly associated with age for both CAD and AS (Figure 1)2,8,9. Moreover, patients with concomitant AS and CAD are characterised by higher baseline risk profiles with a larger number of comorbid conditions as compared to patients with isolated AS10. Therefore, the management of these patients is complex and individual therapeutic strategies are crucial. The aim of this review article is to provide an overview on CAD prevalence, impact on clinical outcomes, and treatment strategies in patients with severe AS requiring surgical aortic valve replacement (SAVR) or transcatheter aortic valve implantation (TAVI).
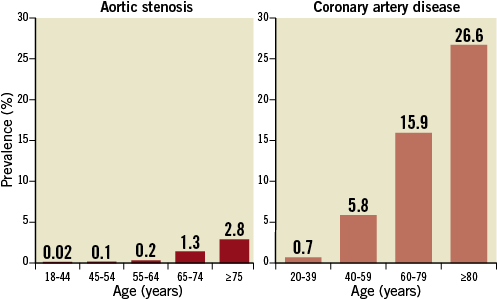
Figure 1. Prevalence of severe aortic stenosis (left) and coronary artery disease (right) according to age. Data are based on available literature.
Coronary artery disease and surgical aortic valve replacement
PREVALENCE
Patients with severe AS undergoing SAVR are a heterogeneous population, ranging from younger patients with isolated bicuspid aortic valve to elderly patients with degenerative AS and several comorbid conditions. CAD is the most common comorbidity and has a prevalence increasing with age, as depicted in Figure 2. According to a large-scale Swedish registry <10% of patients aged <50 years, 30% of patients aged 51-60 years, 41% of patients aged 61-70 years, and 51% of patients aged ≥71 years underwent concomitant CABG at the time of SAVR11. In addition, a 60% prevalence of CAD has been reported among octogenarians undergoing SAVR in two contemporary American registries12,13.
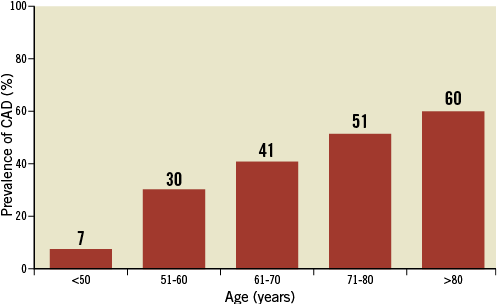
Figure 2. Prevalence of coronary artery disease according to age among patients undergoing surgical aortic valve replacement. Data are based on available literature.
IMPACT ON CLINICAL OUTCOMES
The presence of CAD has been consistently associated with impaired short and long-term outcomes among patients with AS undergoing SAVR10,13,14. Early small-scale studies have reported a negative impact of untreated CAD on survival after SAVR15. Contemporary evidence is mostly based on comparisons between simultaneous CABG and SAVR as compared with isolated SAVR. Indeed, patients undergoing concomitant CABG and SAVR have been shown to be at higher risk of periprocedural events such as stroke, bleeding, and postoperative atrial fibrillation, as compared to patients undergoing isolated SAVR13. In-hospital mortality and periprocedural event rates increase steeply among elderly patients above 80 years of age undergoing concomitant CABG and SAVR16. Moreover, concomitant CABG was identified as an independent predictor of short and long-term mortality among patients undergoing SAVR10,11. It is noteworthy that the association of CAD with age and other major comorbidities may represent an important bias when comparing patients undergoing concomitant CABG and SAVR with isolated SAVR. Recently, a propensity-score based analysis showed a similar long-term survival of 1,082 matched pairs of patients undergoing concomitant CABG and SAVR or isolated SAVR, suggesting that CABG at the time of SAVR neutralises the adverse effects of CAD among patients with otherwise similar non-CAD-related comorbidities (Figure 3)10.
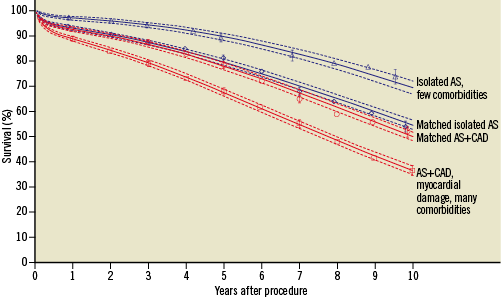
Figure 3. Survival of propensity score matched and unmatched patients with or without coronary artery disease (CAD) undergoing surgical aortic valve replacement due to aortic stenosis (AS). Adapted from reference 10.
RECOMMENDATIONS FOR REVASCULARISATION IN PATIENTS UNDERGOING SAVR
In line with the available evidence, according to current guidelines of the European Society of Cardiology (ESC)17,18 and of the American College of Cardiology/American Heart Association (ACC/AHA)19, CABG is recommended (class I, level of evidence C) in patients with a primary indication for aortic valve surgery with a coronary stenosis ≥70% (≥50% in case of left main stenosis). In addition, CABG should be considered for coronary stenosis ≥50-70% (class IIa, level of evidence C). ESC guidelines also mention the potential use of percutaneous coronary interventions (PCI) in place of CABG in patients with AS. However, the guidelines state that available data are not sufficient to recommend this approach, apart from selected high-risk patients with acute coronary syndromes or in patients with non-severe AS18.
Coronary artery disease and transcatheter aortic valve implantation
PREVALENCE
Patients with severe AS undergoing TAVI are usually not suitable candidates for SAVR due to the high baseline risk profile20-23. Therefore, patients treated with TAVI are frequently elderly with relevant comorbid conditions beyond CAD, such as diabetes, pulmonary hypertension, chronic kidney disease, impaired left ventricular ejection fraction, and previous cardiac surgery. Not unexpectedly, the prevalence of CAD is extremely high in this patient population, ranging from 44% to 75% in major TAVI studies as summarised in Figure 4.
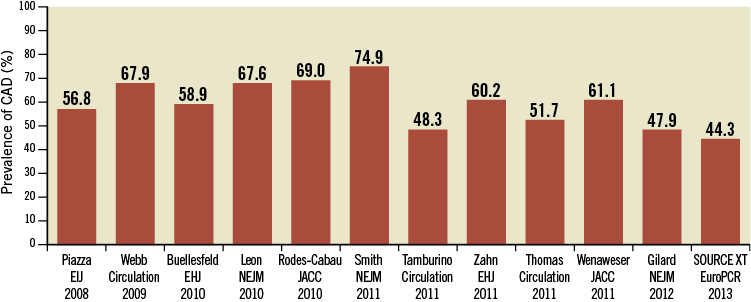
Figure 4. Prevalence of coronary artery disease in major TAVI studies.
IMPACT ON CLINICAL OUTCOMES
In view of the high baseline risk profile of patients undergoing TAVI, it is debated whether the presence of CAD has an impact on clinical outcomes or whether the severe valvular heart disease and the extent of comorbid conditions make any prognostic impact of myocardial ischaemia neglectable. At this point in time available evidence is limited to observational data. In a single-centre study of 136 patients undergoing TAVI, Masson et al reported no impact of CAD on mortality at one-year follow-up. However, mortality rates were higher in patients with CAD as compared to those without CAD (27.7% vs. 18.8%, p=0.63)24. Similar findings were observed by Moat et al in the United Kingdom TAVI national registry including 870 patients, in which CAD was not an independent predictor of mortality at one year (HR 1.23, 95% CI: 0.88-1.73)25. Moreover, D’Ascenzo et al performed a meta-analysis of seven observational studies25-28 including 2,472 patients suggesting no impact of CAD on mortality in patients undergoing TAVI (OR 1.0, 95% CI: 0.67-1.50)29. Conversely, in a retrospective pooled analysis of 201 patients from two small-scale multicentre studies, Dewey et al observed significantly higher rates of death in patients with CAD as compared to patients without CAD undergoing TAVI at 30 days (13.1% vs. 1.2%, p=0.002) and at one-year follow-up (35.7% vs. 18.4%, p=0.01)26. In addition, the presence of CAD has been identified as an independent predictor of death (HR 1.38, 95% CI: 1.07-1.76) in the recently presented large-scale multicentre post-approval SOURCE XT TAVI registry30. Noteworthy, the extent of CAD among patients with AS undergoing TAVI is heterogeneous, ranging from simple single-vessel CAD to complex three-vessel CAD27,31,32. Furthermore, completeness of coronary revascularisation in TAVI patients with CAD is variable24,27,31-33. These two aspects may explain, at least in part, the discordant findings on the impact of CAD on outcomes after TAVI. Moreover, the interpretation of available evidence is limited by the heterogeneous definitions of CAD used in different studies. Last but not least, the limited follow-up duration in most of the available studies prevents any definitive conclusion on whether CAD carries a substantial risk of mortality after successful TAVI.
REVASCULARISATION IN TAVI PATIENTS
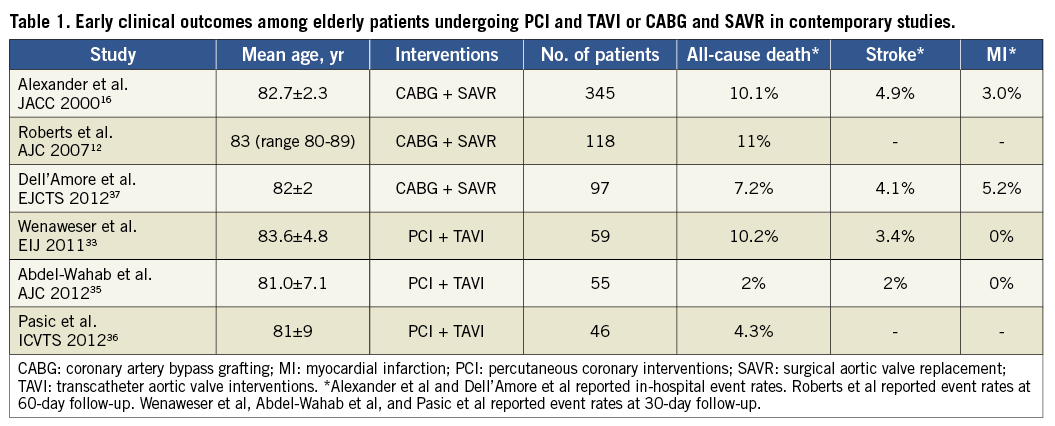
Despite the fact that available randomised trials (i.e., PARTNER A and B) considered CAD requiring revascularisation to be an exclusion criterion21,22, percutaneous coronary interventions (PCI) are frequently performed in patients undergoing TAVI. Available evidence indicates that PCI can be safely performed in patients with severe AS, and a few observational studies have reported on the safety and efficacy of PCI in TAVI patients33-35. In the prospective Bern TAVI registry, 167 out of 257 patients (64.9%) undergoing TAVI had CAD at baseline, and 59 of these (35.3%) underwent concomitant PCI and TAVI or staged PCI followed by TAVI33. Patients undergoing PCI (concomitant or staged) had similar outcomes to patients undergoing isolated TAVI in terms of mortality (10.2% vs. 5.6%, p=0.24) as well as myocardial infarction (0.5% vs. 0%, p=1.00) and major stroke (4.1% vs. 3.4%, p=1.00), defined according to the Valve Academic Research Consortium (VARC) criteria at 30-day follow-up. These findings support the feasibility and safety of PCI in TAVI patients33. Similarly, Abdel-Wahab et al reported a consecutive series of 125 patients undergoing TAVI at a single centre, in which a strategy of pre-procedural PCI of all coronary stenoses >50% was applied throughout the study period35. Patients with CAD and AS undergoing concomitant PCI and TAVI had a similar risk of death (2% vs. 6%, p=0.27), cardiac death (2% vs. 4%, p=0.44), myocardial infarction (0% vs. 0%), and stroke (4% vs. 5%, p=0.27) according to VARC criteria as compared to patients with isolated TAVI at 30 days35. Moreover, all-cause death occurred with similar rates after concomitant PCI and TAVI compared with isolated TAVI during six-month follow-up (9% vs. 14%, p=0.42). In order to put available data in perspective, early clinical outcomes among elderly patients undergoing PCI and TAVI or octogenarians undergoing CABG and SAVR in contemporary clinical studies are summarised in Table 112,16,33,35-37.
TAVI and PCI: open issues
The clinical management of patients with CAD and AS undergoing TAVI is complex and requires a global evaluation of individual patient risk profiles (Figure 5). With respect to treatment of CAD in TAVI patients, a few considerations are needed beyond the actual safety and feasibility of PCI in this clinical scenario.
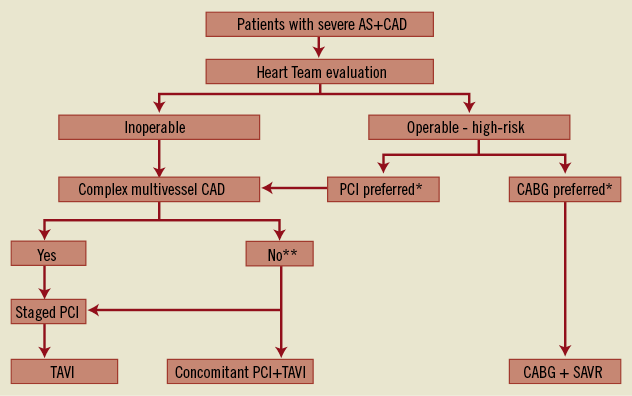
Figure 5. Treatment options for inoperable and high-risk patients with severe aortic stenosis (AS) and coronary artery disease (CAD). CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; SAVR: surgical aortic valve replacement. *Decision according to Heart Team. ** In patients without complex CAD, the decision to perform staged PCI or concomitant PCI and TAVI should be based on individual clinical evaluation.
ASSESSMENT OF CAD AND COMPLETENESS OF REVASCULARISATION
Evaluation of induced myocardial ischaemia is laborious and eventually impossible in patients with AS, and the performance of any stress test is contraindicated in patients with symptomatic severe AS. Therefore, assessment of CAD is mostly limited to angiography. However, the ischaemic relevance of flow-limiting stenoses defined by angiography is debated, and PCI should only be performed in patients in case of significant coronary lesions with evidence of ischaemia17,38. Patients with AS presenting with acute coronary syndromes have a clear indication to undergo revascularisation. Moreover, the presence of angina is generally considered as an incentive to perform PCI in TAVI patients with stable CAD. However, at this point in time, it is unclear whether complete revascularisation of all angiographically significant coronary stenoses should be performed in patients undergoing TAVI. Indeed, a more selective strategy with PCI limited to significant stenoses of proximal segments of major epicardial vessels may be a valid alternative, allowing procedure time to be shortened, the use of contrast media to be optimised, and the risk of procedural complications to be reduced. Nevertheless, it needs to be underscored that cannulation of coronary ostia may be difficult after implantation of an aortic valve prosthesis in case of subsequent need of PCI due to a lesion initially left untreated. Overall, the advantages and disadvantages of revascularisation should be evaluated on an individual basis, taking into consideration comorbidities, severity of CAD, extent of myocardium at risk, and anticipated complexity of PCI. Of note, in patients with severe AS and diffused CAD not amenable to revascularisation, TAVI should still be considered.
TIMING OF PCI
If the selected treatment strategy includes coronary revascularisation and TAVI, timing of PCI may represent a critical decision. It is noteworthy that patients screened for TAVI usually undergo coronary angiography prior to the Heart Team discussion. Therefore, ad hoc PCI at the time of coronary angiography may be an option only for patients known to be inoperable due to coexisting comorbid conditions. Performing PCI and TAVI during the same session may be a practical solution and does not expose patients to risks associated with an additional invasive procedure. On the other hand, a staged strategy - with PCI and TAVI performed in separate sessions - allows reduction of the procedure duration as well as contrast media volume used during TAVI. At this point in time, PCI timing strategies are different among centres. Taking into consideration the lack of evidence, it appears feasible to perform concomitant PCI and TAVI in patients with CAD requiring revascularisation if PCI complexity is expected to be low. Conversely, staged interventions may be a valuable option among patients with a greater extent of CAD for which a higher PCI complexity is foreseen.
CORONARY STENT SELECTION
Coronary stent selection may also be critical among TAVI patients requiring PCI, in view of the subsequent need for dual antiplatelet therapy. The use of drug-eluting stents has been shown to be safe in a wide spectrum of clinical conditions and provides a markedly reduced risk of repeat revascularisation compared to the use of bare metal stents39. However, up to 40% of TAVI patients have coexisting atrial fibrillation and may require long-term oral anticoagulation, with a subsequent increased bleeding risk in case of triple therapy with dual antiplatelet therapy in addition to oral anticoagulation40. Of note, the optimal duration of dual antiplatelet therapy after coronary stent implantation is still a matter of debate. According to the current guidelines of the ESC, dual antiplatelet therapy should be continued for at least one month after bare metal stent implantation and at least six months after drug-eluting stent implantation17. However, these recommendations are based on little observational data. Moreover, the use of drug-eluting stents has been shown to be safe among patients requiring long-term oral anticoagulation41. In view of these considerations, drug-eluting stents represent the first choice in TAVI patients undergoing PCI in order to reduce the risk of repeat interventions. In patients with atrial fibrillation and high bleeding risk, additional interventions such as left atrial appendage occlusion or ablation of atrial fibrillation may be considered in order to eliminate the need for oral anticoagulation. Finally, the use of newer anticoagulants such as direct thrombin inhibitors may be considered to reduce the risk of bleeding complications.
Conclusions
CAD is a frequent comorbid condition in patients presenting with severe AS, with a prevalence increasing along with age. Among patients undergoing SAVR, CAD has consistently been shown to have a negative prognostic impact during short and long-term follow-up. In line with available evidence, current guidelines recommend coronary revascularisation of all significant stenoses in patients undergoing SAVR. Conversely, the impact of CAD on clinical outcomes after TAVI is subject to ongoing debate. In the absence of definitive randomised studies, coronary revascularisation by means of PCI appears reasonable in TAVI patients with significant CAD, particularly in case of proximal stenosis of major coronary arteries. However, several issues remain open and will require future investigations, including completeness and timing of revascularisation in TAVI patients with CAD. Moreover, current revascularisation strategies are reasonable for high-risk elderly patients who are candidates for TAVI in contemporary clinical practice, and may be different among intermediate-risk patients considered for TAVI in the future. Ongoing randomised trials such as SURTAVI (ClinicalTrials.gov identifier NCT01586910) will provide further insights into coronary revascularisation among patients undergoing TAVI.
Funding
Supported by an unrestricted research grant from Medtronic to the University of Bern.
Conflict of interest statement
B. Meier has received educational and research support to the institution from Abbott, Cordis, Boston Scientific, and Medtronic. S. Windecker has received honoraria and consultant fees from Edwards Lifesciences and Medtronic. P. Wenaweser is proctor and receives honoraria from Medtronic and Edwards Lifesciences. G. G. Stefanini and S. Stortecky have no conflicts of interest to declare.

