Abstract
Significant coronary artery disease (CAD) is a frequent finding in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation (TAVI), and the management of these two conditions becomes of particular importance with the extension of the procedure to younger and lower-risk patients. Yet, the preprocedural diagnostic evaluation and the indications for treatment of significant CAD in TAVI candidates remain a matter of debate. In this clinical consensus statement, a group of experts from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) in collaboration with the European Society of Cardiology (ESC) Working Group on Cardiovascular Surgery aims to review the available evidence on the topic and proposes a rationale for the diagnostic evaluation and indications for percutaneous revascularisation of CAD in patients with severe aortic stenosis undergoing transcatheter treatment. Moreover, it also focuses on commissural alignment of transcatheter heart valves and coronary re-access after TAVI and redo-TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) is an established therapy for elderly patients with severe symptomatic aortic valve stenosis (AS), irrespective of surgical risk123. The burden of coronary artery disease (CAD) is significant in patients with severe AS and may impact both the procedural risk as well as the post-procedural prognosis of the patient. Randomised clinical trials and large registries have reported that around half of subjects undergoing TAVI have CAD, although the prevalence decreases in line with the reduction in age and surgical risk profiles4567891011121314151617 (Figure 1). Yet, it should be noted that patients with severe CAD (i.e., SYNTAX score >22, left main CAD) and those with a recent percutaneous coronary intervention (PCI) were excluded from intermediate- and low-risk TAVI trials. Despite TAVI having reached its maturity as the preferred treatment in most patients with tricuspid AS and who are >75 years of age, the preprocedural diagnostic evaluation and indications for treatment of significant CAD in TAVI candidates remain a matter of debate.
Regarding diagnosis, both anatomical and haemodynamic assessments are the mainstay of CAD evaluation. Coronary angiography remains the standard anatomical assessment of CAD in TAVI candidates. Meanwhile, computed tomography angiography, already used routinely for preprocedural planning, has emerged as a reasonable alternative to screen for CAD before TAVI. Few data exist on the invasive haemodynamic assessment (fractional flow reserve [FFR] or instantaneous wave-free ratio [iFR]) of CAD in TAVI patients. Patients with severe AS have systolic left ventricular (LV) outflow obstruction, elevated LV end-diastolic pressures, and significant LV hypertrophy – all of which will preferentially reduce systolic over diastolic coronary blood flow18 (Figure 2) and, hence, impact the invasive physiological measurement.
With respect to CAD treatment, while coronary artery bypass grafting (CABG) at the time of surgical aortic valve replacement (SAVR) is considered the gold standard in surgical candidates with concomitant significant CAD, treatment algorithms for significant asymptomatic CAD in TAVI candidates vary considerably across different institutions, as no consensus exists on the workup nor the management of CAD in patients undergoing TAVI. The guidelines give some recommendations, but the level of evidence is low and based on non-randomised data1. Thus, whether performing PCI before TAVI offers clinical benefit in patients with significant but asymptomatic CAD remains unclear.
Another important point is represented by the unplanned need for coronary intervention after TAVI. This concern increases as TAVI expands to younger patients with longer life expectancy. In these patients, the possible progression of CAD as well as the occurrence of an acute coronary syndrome (ACS) or delayed coronary occlusion makes the issue of coronary re-access after TAVI clinically relevant. Preservation of coronary access during the index TAVI procedure as well as the essentials of obtaining coronary access in the presence of a transcatheter heart valve (THV) are paramount for future invasive coronary diagnostics and interventions.
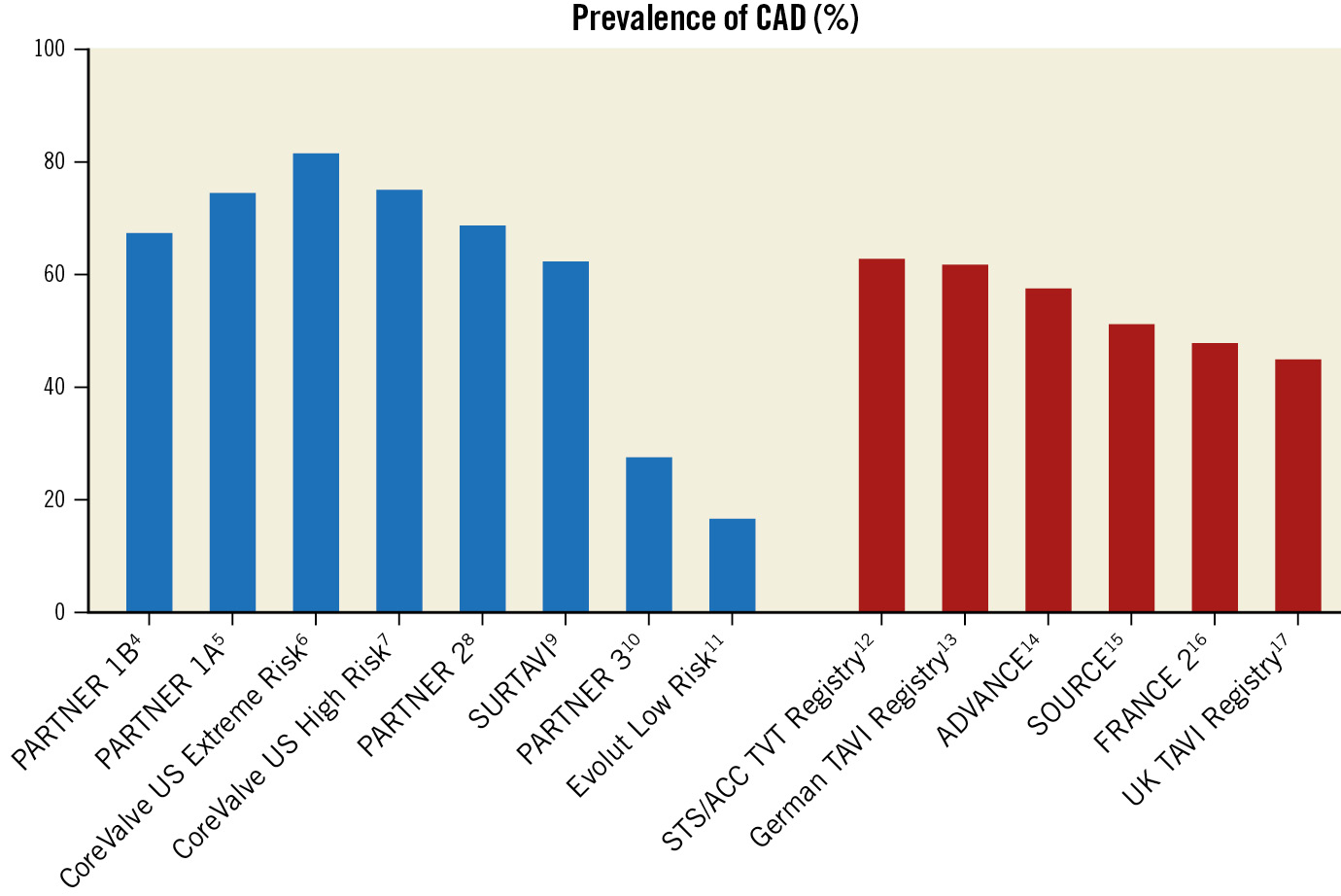
Figure 1. Prevalence of coronary artery disease (CAD) in patients treated with transcatheter aortic valve implantation as reported in randomised clinical trials (blue columns) and real-world registries (red columns). STS/ACC TVT: Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy
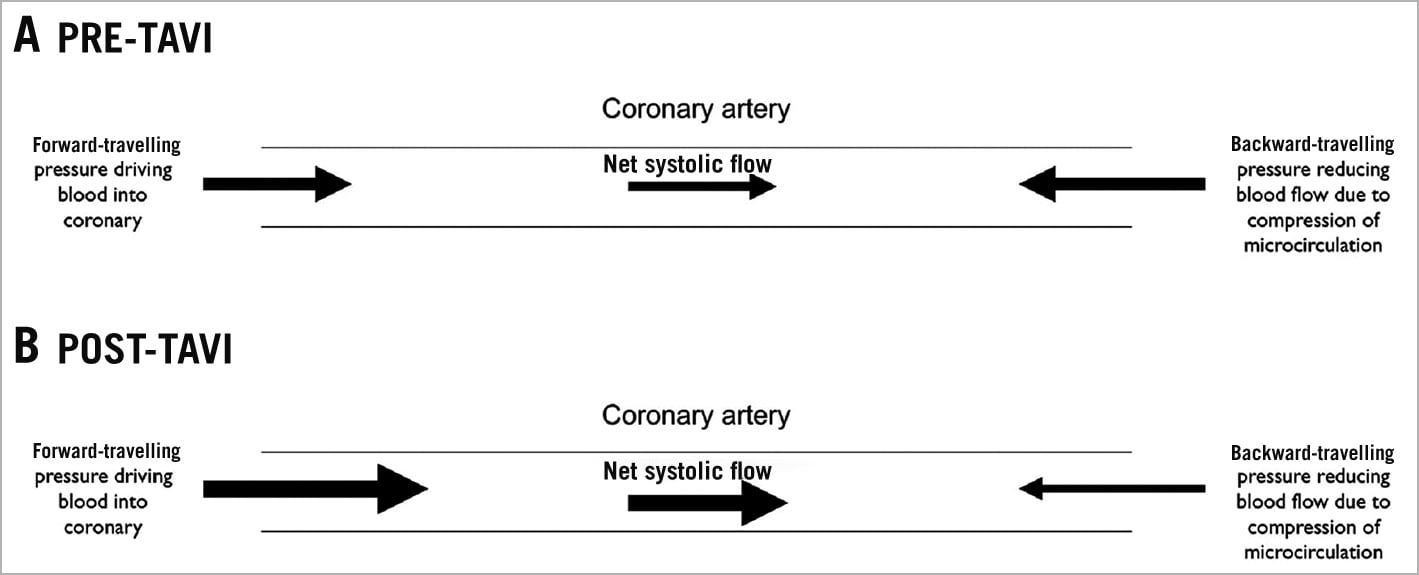
Figure 2. Systolic coronary flow before and after TAVI. TAVI: transcatheter aortic valve implantation. Reproduced with permission18.
Objectives
This position paper, developed by a group of international experts invited by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Scientific Documents and Initiatives Committee, aims to provide a rational and practical approach to the management of CAD in patients with symptomatic severe AS undergoing TAVI. Specifically, it is focused on the diagnosis and treatment of significant CAD in TAVI candidates, as well as on THV commissural alignment and coronary re-access after TAVI and redo-TAVI, with each considered topic based on literature review .
Diagnostic evaluation of CAD in patients undergoing TAVI
INVASIVE CORONARY ANGIOGRAPHY
Invasive coronary angiography (ICA) remains the current standard of care in the assessment of CAD among patients undergoing TAVI and has several benefits. Firstly, elderly patients scheduled for TAVI often have coronary arteries with a significant burden of calcium – limiting the diagnostic performance of coronary CT angiography19. Secondly, clinical practice guidelines on valvular heart disease recommend that decisions on coronary revascularisation be based primarily on the angiographic degree of disease severity120. Thirdly, in cases of intermediate CAD severity, ICA may allow immediate invasive physiological and imaging assessment of CAD, as well as the potential to proceed directly to PCI in the same sitting, where appropriate.
Current clinical practice guidelines do not address the timing of ICA in patients undergoing TAVI20. Most operators perform ICA in advance of the TAVI procedure to minimise procedure time and contrast volume, as well as to inform the Heart Team on the need for revascularisation, which could influence the choice between SAVR+CABG versus TAVI+PCI. However, concomitant ICA at the time of TAVI may be considered in patients who are not surgical candidates and/or with a low probability of significant CAD.
However, ICA does have disadvantages, including an increased risk of vascular complications, contrast nephropathy, additional burden on the healthcare system and a potential to delay the treatment of AS. Current clinical practice guidelines recommend ICA for the assessment of CAD prior to consideration of either SAVR or TAVI – particularly in patients with a history of cardiovascular disease, with at least one cardiovascular risk factor, suspected myocardial ischaemia, LV systolic dysfunction, or men >40 years of age and postmenopausal women (Class I, Level of Evidence [LoE] C)1.
INVASIVE CORONARY PHYSIOLOGY ASSESSMENT
Invasive physiological assessment of coronary lesions using FFR or iFR is an established and well-validated approach to guide PCI and has a Class 1, LoE A recommendation in international guidelines. However, there are no randomised trial data and only limited observational studies addressing invasive physiological assessment in patients with severe AS.
Aortic stenosis generates LV pressure overload, leading to ventricular remodelling and hypertrophy. The consequent increase in LV mass and intracavity pressure induces changes in coronary anatomy and physiology which potentially impact the assessment of ischaemia using both FFR and iFR (Table 1)2122.
Furthermore, while the safety of both intracoronary and intravenous adenosine has been demonstrated in numerous studies18232425, some data suggest that its effect may be blunted in patients with AS, leading to a potential underestimation of the severity of coronary artery stenosis with FFR18. Moreover, AS acts as an effective tandem lesion downstream of an epicardial coronary stenosis, and this might represent another pitfall of FFR in this setting.
Studies that evaluated the impact of severe AS on FFR, by performing pressure wire assessment both before and after TAVI, have presented conflicting findings. While some investigations have shown a reduction in FFR immediately after TAVI, with a further decline in the long term attributable to increased systolic hyperaemic flow2627, an increase in FFR immediately after TAVI has also been described28. The largest available study to date found no significant overall change in FFR values after TAVI, but while lesions with a positive baseline FFR decreased after TAVI, negative values increased. However, the changes in FFR were small and only affected the indication for revascularisation in a minority of lesions24.
iFR may represent a better option in patients with AS since it does not require adenosine and is independent of systolic flow. However, observational data are also inconsistent. While several studies found no significant changes in iFR immediately before and after TAVI1827, the same authors reported significant differences, leading to a change in the indication for intervention in more than a quarter of patients29. Limited data on the correlation of invasive coronary physiology assessment with clinical outcomes in AS patients are promising, but long-term data are scarce30. Some studies have suggested that the cut-off values for physiological assessment of coronary lesions which correlate with clinical outcomes differ in patients with AS25. However, current evidence is inconclusive, hence use of the conventional thresholds of FFR ≤0.80 and iFR ≤0.89 is advisable.
Overall, further research on the clinical value of invasive coronary physiology in the context of severe AS is needed, particularly for validation of (non-) hyperaemic cut-offs to guide revascularisation.
Table 1. Effects of aortic stenosis on coronary anatomy and physiology.
| Effects of AS on coronary artery anatomy and physiology |
|---|
| Reduction in stroke volume, systolic and mean arterial pressure, which may cause reduced coronary perfusion pressure |
| Decreased density of coronary microvasculature |
| Attenuated and delayed systolic forward compression wave of coronary blood flow |
| Increased resting diastolic backward expansion wave |
| Reduction in resting microvascular resistance, with inability to reduce further in response to hyperaemia |
| Upregulation of vasoactive factors, leading to increased resting blood flow |
| Microvascular dysfunction impairing hyperaemic response |
| Reversal of normal endocardial-epicardial blood flow ratio at rest |
| Reduced diastolic coronary perfusion phase |
| Attenuated coronary flow reserve |
NON-INVASIVE CAD ASSESSMENT
Avoiding ICA by a non-invasive assessment of CAD in TAVI patients is appealing. Multislice computed tomography (MSCT) is routine prior to TAVI for procedural planning. The MSCT may be used for concomitant CT coronary angiography (CTCA), allowing for an evaluation of CAD without a significant additional burden to the patient or healthcare system, and may potentially expedite the treatment of AS.
CTCA might be considered in patients with a low pretest probability of CAD and in whom reasonable image quality is expected. Given that the prevalence of significant CAD among elderly TAVI recipients approaches 50%31, including prior PCI in 20%32, and that significant coronary calcification is frequent, CTCA is unlikely to be of adequate diagnostic quality for the majority of pre-TAVI CAD assessments33.
Another study found it was possible to use the pre-TAVI MSCT to evaluate the presence of significant CAD in 76.3% of their TAVI cohort, while 21.7% required ICA due to suboptimal CT imaging or clinically significant disease on MSCT34. There was no significant difference in 30-day or 1-year major adverse cardiac events between patients who required ICA and those who did not. A contemporary meta-analysis including 7 studies and 1,275 TAVI patients compared the diagnostic accuracy of the pre-TAVI MSCT to ICA35. The pooled sensitivity, specificity, and positive (PPV) and negative predictive values (NPV) of the non-invasive approach were 95%, 65%, 71% and 94%, respectively.
Fractional flow reserve-computed tomography (FFR-CT) provides both anatomical and functional assessments of coronary arteries and substantially improved diagnostic accuracy in a recent prospective comparison with invasive FFR in 42 patients (68 vessels) with severe AS36. The CT data were interpretable in 92.3% of patients; sensitivity, specificity, PPV and NPV were 73.9%, 78.4%, 68.0%, and 82.9%, respectively.
These data suggest that pre-TAVI CTCA, potentially in combination with FFR-CT, may offer potential advantages in time, cost, and risk and may become an alternative first-line screening strategy in selected subsets of AS patients.
Cardiac magnetic resonance (CMR) imaging can be used to evaluate myocardial perfusion, myocardial viability, and proximal coronary anatomy and can provide important prognostic information such as the extent of myocardial fibrosis and characterisation of LV remodelling37. A single, small study showed a high sensitivity and specificity for stress-perfusion CMR when compared to ICA in 23 patients with severe AS38. Notably, adenosine stress-perfusion was performed without complications despite the presence of severe AS. When compared to MSCT, however, the CMR scan is technically more challenging and represents an additional step in the pre-TAVI pathway; hence, it should be reserved for specific indications, rather than as part of a routine workup39.
Consensus points:
1) Invasive coronary angiography should remain the mainstay of CAD assessment in the majority of TAVI candidates.
2) Assessment of coronary artery disease by means of pre-TAVI CTCA may be considered in younger patients with a low cardiovascular risk profile.
3) More data are needed to assess the clinical value of invasive coronary physiology assessment in patients with severe aortic stenosis.
Management of CAD in patients undergoing TAVI
While it is clear that AS patients presenting with an ACS should undergo revascularisation of the culprit vessel, many questions still linger about the management of significant stable CAD in subjects undergoing TAVI. Should PCI be pursued in every patient with severe stable CAD? Should it be performed before, during or after TAVI? What is the role of physiology-guided PCI in the setting of AS? Should we always aim for complete revascularisation?
PCI IN PATIENTS WITH STABLE CAD UNDERGOING TAVI
A meta-analysis of observational studies including over 5,000 patients comparing the outcomes of patients with CAD undergoing TAVI with versus without prior PCI found no benefit from routine PCI before TAVI for either 30-day major adverse cardiovascular events (MACE; death, myocardial infarction, stroke, acute kidney injury) or one-year mortality40. The ACTIVATION (PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION) non-inferiority trial is the only randomised study comparing a PCI versus no-PCI strategy in patients with severe symptomatic AS and significant CAD41. In this study, the primary composite endpoint of all-cause death and rehospitalisation occurred in 41.5% of the PCI arm and 44.0% of the no-PCI arm (the requirement for non-inferiority was not met), with a higher incidence of bleeding in the PCI arm41. Nevertheless, the results of the ACTIVATION trial should be viewed in the light of its many limitations (e.g., the study was underpowered for the primary endpoint, it included only subjects with stable CAD – of whom 69% were completely asymptomatic – and more than two thirds of patients had single-vessel CAD).
In the Evolut Low Risk trial, fewer patients with CAD received PCI with TAVI than SAVR with CABG, with similar mortality, disabling stroke, or myocardial infarction at 2 years (Kini A, et al. Two-year outcomes following TAVI or SAVR in patients stratified by need for revascularisation. PCR London Valves 2021. https://eposter.europa-organisation.com/2021/pcrvalves/index/slide/abstract/44. Last accessed: 6 February 2023).
Management may be based on the clinical presentation, with PCI performed in patients with angina. However, this approach can be challenging given the overlap between AS- and CAD-related symptoms. The location of the coronary stenosis may also be important in selecting which patients should undergo PCI. In the French national registry of TAVI (FRANCE 2), only significant lesions of the left anterior descending artery (LAD) were associated with increased 3-year mortality (hazard ratio [HR]: 1.42, 95% confidence interval [CI]: 1.10-1.87)42. However, all these approaches deserve validation in future large-scale randomised trials, and several studies are ongoing, including COMPLETE TAVR (ClinicalTrials.gov: NCT04634240). As of today, according to the ESC/European Association for Cardio-Thoracic Surgery (EACTS) Clinical Practice Guidelines on Myocardial Revascularization, PCI should be considered in patients undergoing TAVI with a coronary artery diameter stenosis >70% in proximal segments (Class IIa, LoE C)20.
The approach to percutaneous coronary revascularisation in patients undergoing TAVI suggested by this consensus was developed based on the available literature, which largely refers to patients older than 75 years of age. Yet, guideline recommendations regarding coronary revascularisation in TAVI patients do not differ in subjects <75 versus >75 years of age. The presence of CAD, together with the life expectancy of the patient undergoing TAVI, should be weighted by the Heart Team with regard to the choice of transcatheter heart valve (THV), aiming at preserving future coronary access and the possibility of repeat TAVI.
Notably, guidelines and surgical consensus state that in patients undergoing SAVR, revascularisation of proximal significant CAD should be pursued (LoE C). A more extensive revascularisation is usually performed with the surgical approach in order to avoid a repeat sternotomy. Yet, the mortality risk of SAVR+CABG is higher than that of SAVR alone. In this regard, it should be acknowledged that robust evidence for a definitive recommendation about indication and completeness of revascularisation is lacking also for the surgical approach.
TIMING OF PCI
The optimal timing of PCI (before vs concomitant vs after TAVI) in patients undergoing TAVI remains uncertain, and the available evidence is based on retrospective registry data only. Each strategy has its pros and cons (Table 2).
The potential challenges in reaccessing the coronary arteries after TAVI have been suggested as a reason to advise systematic PCI before TAVI. This strategy is supported by a study where failure to access the coronary arteries was reported in 17.9% of patients treated with a certain THV platform43. Concordantly, PCI is mostly performed before THV implantation – either as a staged or concomitant procedure44454647. According to an American nationwide sample of 22,344 patients, in-hospital mortality was higher if TAVI and PCI were performed during the same hospitalisation (10.7% vs 4.6%; p<0.001)48. However, the authors could not differentiate if both procedures were staged or concomitant, nor if PCI was planned or performed urgently because of ischaemic complications during or after TAVI. A substudy of the SURTAVI trial showed similar rates of all-cause mortality and stroke but a lower rate of acute kidney injury in patients undergoing staged as compared to concomitant PCI49. A meta-analysis of 5 observational reports, including 1,634 patients, showed that concomitant TAVI and PCI procedures were associated with similar 30-day and 1-year mortality as compared to isolated TAVI44. Recently, Ochiai et al reported a single-centre experience with 2-year follow-up of 258 patients who underwent elective PCI before TAVI (n=143), simultaneously with TAVI (n=77) or post-TAVI (n=38), and found no differences in clinical outcomes50.
Cannulation of the coronary ostia may be more challenging after TAVI, especially after implantation of self-expanding THVs with a supra-annular leaflet position5152. In a group of 41 patients implanted with balloon-expandable valves who underwent angiography/PCI both before and after TAVI, there were no differences in the rate of successful selective coronary cannulation or any procedural variables (i.e., duration or contrast volume)53. Zivelonghi et al performed successful angiography in 65 out of 66 subjects after TAVI (25 self-expanding THVs with a supra-annular leaflet position and 41 balloon-expandable THVs with an intra-annular leaflet position); only one artery was not accessible because of a high implantation of an Evolut R (Medtronic) THV54. However, Blumenstein et al reported that selective cannulation of the coronary ostium was feasible in all patients who received balloon-expandable valves but only in 3 out of 15 treated with supra-annular self-expanding prostheses55.
Thus, decision-making on PCI in patients scheduled for TAVI should be based on patient characteristics, including the presence or absence of angina, CAD severity, lesion location and complexity, and type of implanted THV. The evidence base is limited but, in general, does not support routine PCI prior to TAVI in asymptomatic lesions and suggests that when PCI is performed, a separate staged procedure prior to TAVI is preferable.
The ongoing 900-patient TAVI PCI Trial (ClinicalTrials.gov: NCT04310046) randomises patients 1:1 to FFR-guided PCI 1-45 days before or after implantation of a SAPIEN 3 (Edwards Lifesciences) THV. This study will also inform current practice on the value of invasive coronary physiological assessment of intermediate coronary lesions in AS patients, as a second iFR measurement in the group undergoing PCI after TAVI is planned. The aforementioned COMPLETE TAVR trial is currently randomising 4,000 TAVI patients with severe CAD (i.e., at least one coronary artery lesion of ≥70% visual angiographic diameter stenosis in a native segment that is at least 2.5 mm in diameter, excluding chronic total occlusions) to staged complete revascularisation after TAVI with a balloon-expandable valve versus medical management alone.
Table 2. Advantages and disadvantages of different PCI timing in patients undergoing TAVI.
| PCI before TAVI | PCI after TAVI | Combined PCI and TAVI | |
|---|---|---|---|
| Advantages | - Easier coronary access (especially for self-expanding THV with a supra-annular leaflet position)- Lower risk of ischaemia-induced haemodynamic instability (i.e., during rapid pacing)- Reduced contrast use compared with concomitant PCI and TAVI | - More reliable FFR/iFR of intermediate lesions- Lower risk of haemodynamic instability during complex PCI (i.e., with rotational atherectomy and impaired LV function)- Reduced contrast use compared with concomitant PCI and TAVI | - Use of the same arterial access- Lower cost |
| Disadvantages | - Less reliable FFR/iFR assessments of borderline lesions- Higher risk of haemodynamic instability due to AS | - More challenging and potentially compromised coronary access- Less stability and support of the coronary guiding catheter- Potential THV dislodgement | - Larger amount of contrast and higher risk of AKI- Prolonged procedure- Need for DAPT at the time of TAVI, hence increased bleeding risk |
| AS: aortic stenosis; AKI: acute kidney injury; DAPT: dual antiplatelet therapy; FFR: fractional flow reserve; iFR: instantaneous wave-free ratio; LV: left ventricular; PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve | |||
ANGIOGRAPHY-GUIDED VERSUS PHYSIOLOGY-GUIDED PCI
Angiography alone versus physiology-guided revascularisation was compared in a propensity score-matched analysis of 318 patients with AS. Following FFR, the number of diseased vessels was downgraded (from 1.85±0.97 to 1.48±1.00; p<0.01) and significantly lower than in the angiography-alone group (1.48±1.00 vs 1.80±0.97; p<0.01) without significant differences in MACE at 5 years56.
The FAITAVI (Functional Assessment In TAVI; ClinicalTrials.gov: NCT03360591) trial is randomising patients with severe AS and CAD to percutaneous myocardial revascularisation dictated according to an angiography-guided versus physiology-guided (i.e., FFR/iFR) strategy and evaluating the incidence of MACE, bleeding and target lesion failure at 24 months. The TCW Trial (TransCatheter Valve and Vessels; ClinicalTrials.gov: NCT03424941) is a prospective, randomised, controlled, open-label, multicentre, international, non-inferiority trial of patients with multivessel disease and AS. Patients will be randomised in a 1:1 fashion to TAVI and FFR-guided PCI (experimental arm) and CABG and SAVR (comparative arm).
At present, there are no randomised studies comparing complete versus incomplete revascularisation in patients with stable CAD undergoing TAVI. Thus, no recommendations can be made. A Heart Team-based decision of the most appropriate revascularisation strategy for TAVI patients should be aimed for in all cases.
ANTITHROMBOTIC THERAPY POST-TAVI AND PCI
Antithrombotic therapy after TAVI remains a field of rapidly evolving evidence. Recent data suggest that monotherapy (either single antiplatelet therapy [SAPT] or oral anticoagulant therapy [OAC]) is associated with better clinical outcomes than dual therapy (dual antiplatelet therapy [DAPT] or SAPT+OAC)5758. Consequently, the standard treatment after TAVI is aspirin, while patients with an indication for OAC should receive this as monotherapy. However, treatment should also be adjusted to patient-specific factors such as CAD, and in particular recent PCI, with DAPT prescribed according to existing clinical practice guidelines for patients with recent acute coronary syndromes and/or PCI. The balance between the benefit of reduction of ischaemic events and the risk of bleeding remains the major determinant in decision-making, as TAVI patients often have concomitant high bleeding risk factors including age >75 years59. According to those guidelines, in patients with high bleeding risk and no indication for OAC, DAPT for 3 months should be followed by SAPT. When long-term OAC is indicated, triple therapy should be stopped after 1 week and followed by OAC+SAPT for 6 months. In cases of high or very high bleeding risk, triple therapy should be avoided, and SAPT+OAC can be withheld after 1-3 months and followed by OAC alone60.
Consensus points:
1) PCI before TAVI should be performed in patients with severe CAD (i.e., coronary artery diameter stenosis >70%, >50% for the left main) only in proximal segments, particularly if presenting with an acute coronary syndrome, symptoms of angina pectoris or subocclusive lesions (i.e., >90% diameter stenosis).
2) The timing of PCI with respect to the TAVI procedure should be based on clinical presentation, the patient’s anatomical characteristics and coronary lesion complexity.
3) If PCI is planned after TAVI, the THV choice (i.e., low-frame versus high-frame) and implantation technique (i.e., commissural alignment) should be aimed at preserving easy coronary access.
Coronary access and TAVI
NEED FOR CORONARY ACCESS AFTER TAVI AND CLINICAL INDICATIONS
Data from large-scale TAVI registries show that ICA and/or PCI may be required in approximately 2% of TAVI patients within the first year after TAVI526162. ICA is performed in up to 16% of TAVI patients within 5 years of follow-up, and approximately 5% of these patients undergo PCI of one or more coronary lesions63. Around two-thirds of post-TAVI ICA/PCI procedures are performed in the setting of ACS, mainly due to unstable angina or non-ST-segment elevation myocardial infarction, whereas silent ischaemia, stable angina or workup for heart failure serves as an indication for the remaining 1/3 of cases526163. However, even though historically the reported frequency of ICA/PCI procedures after TAVI is low, this may change, due not only to the recently introduced more conservative strategy for asymptomatic lesions but also to the treatment being used more frequently in younger patients with longer life expectancies who may eventually develop symptomatic CAD.
TRANSCATHETER HEART VALVE SELECTION AND IMPACT ON FUTURE CORONARY ACCESS
The choice of THV is subject to multiple factors including the patient's individual anatomical suitability, availability, and operator experience64. The overarching purpose of an optimal THV selection must be to achieve the best possible immediate outcome with the least likelihood of major complications. However, in view of improved procedural results in recent years, long-term considerations, including THV durability, feasibility of redo-TAVI, conduction abnormalities, and the possibility for future coronary access, also become important.
THV design, in particular the stent frame height and leaflet position, can affect future coronary access. Thus, familiarity with available THV devices is essential, including for interventional cardiologists not performing TAVI (Figure 3, Figure 4). This is particularly important, since in SAVR, native leaflets are resected and surgeons routinely align the surgical valve commissural posts to native commissures to make coronary access straightforward65. However, commissural alignment has not been uniformly performed in TAVI, and certain THV designs may make coronary access more challenging.
The balloon-expandable SAPIEN 3 and SAPIEN 3 Ultra (Edwards Lifesciences) THVs have a low stent-frame height (15.5-22.5 mm, according to valve size) with an upper row of open cells as well as an intra-annular leaflet position. Accordingly, coronary cannulation is often possible either above the outflow of the THV or through the large cells in the upper row of the stent, depending on the THV implant depth in relation to the patient’s anatomy.
The stent frame of the self-expanding Evolut R, Pro and Pro+ (Medtronic) THVs extends beyond the coronary ostia. The leaflets are supra-annular with a 26 mm commissural post height from the THV inflow. Therefore, coronary access is achieved in most cases through the diamond-shaped prosthesis frame cells.
The self-expanding ACURATE neo and neo2 (Boston Scientific) THVs with a supra-annular leaflet position have a commissural post height of 25-31 mm, according to valve type and size, but have an open-cell architecture in the upper part of the frame that allows easier access to the coronary ostia.
The Portico and Navitor (Abbott) THVs with an intra-annular leaflet position have a tall stent-frame design with a commissural post height of 21-25 mm and an open-cell design.
It should be noted that, more than the commissural post height per se, what influences coronary access after TAVI (and after redo-TAVI) is the THV leaflet height with respect to the recommended annular positioning, which is lowest for the SAPIEN 3/Ultra and highest for the Evolut R/Pro THVs (Figure 3).
Importantly, coronary access after TAVI is also influenced by several critical anatomical factors, such as sinus sizes (particularly in relationship to the THV size), height and width of the sinotubular junction (STJ), and coronary location in terms of the height and relation with native commissures.
Available data on coronary access following TAVI demonstrate an overall high success rate of coronary engagement (Table 3), which is reassuring and suggests that coronary access should not be the decisive factor in the THV selection algorithm616667. Nonetheless, there is increasing evidence in the literature that high stent-frame THVs with a supra-annular leaflet position are more challenging in regard to selective coronary cannulation, especially in the case of severe commissural misalignment68 or a high implant THV position69 – the latter being pursued for most THV types to reduce rates of permanent pacemaker implantation70717273.
Coronary access may be even more challenging in the case of TAVI in surgical bioprostheses (TAV-in-SAV) or TAVI-in-TAVI procedures74. In these specific situations, altered sinus geometry, the neoskirt of the leaflets from the first THV being pushed aside by the second THV, and commissural misalignment are factors that may contribute to compromising the engagement of the coronary arteries in up to half of cases6875. Catheters may also interact with the THV leaflets, which, in the extreme case of leaflet pinning, may lead to acute haemodynamic compromise.
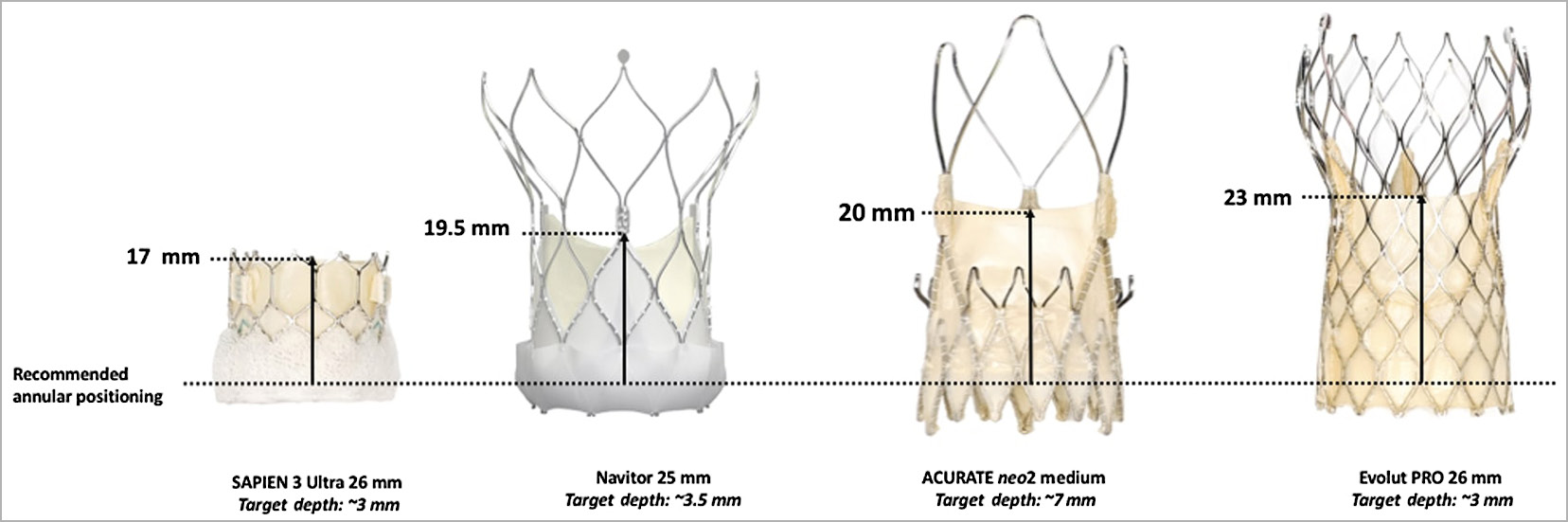
Figure 3. THV leaflet height with respect to recommended annular positioning. THV: transcatheter heart valve
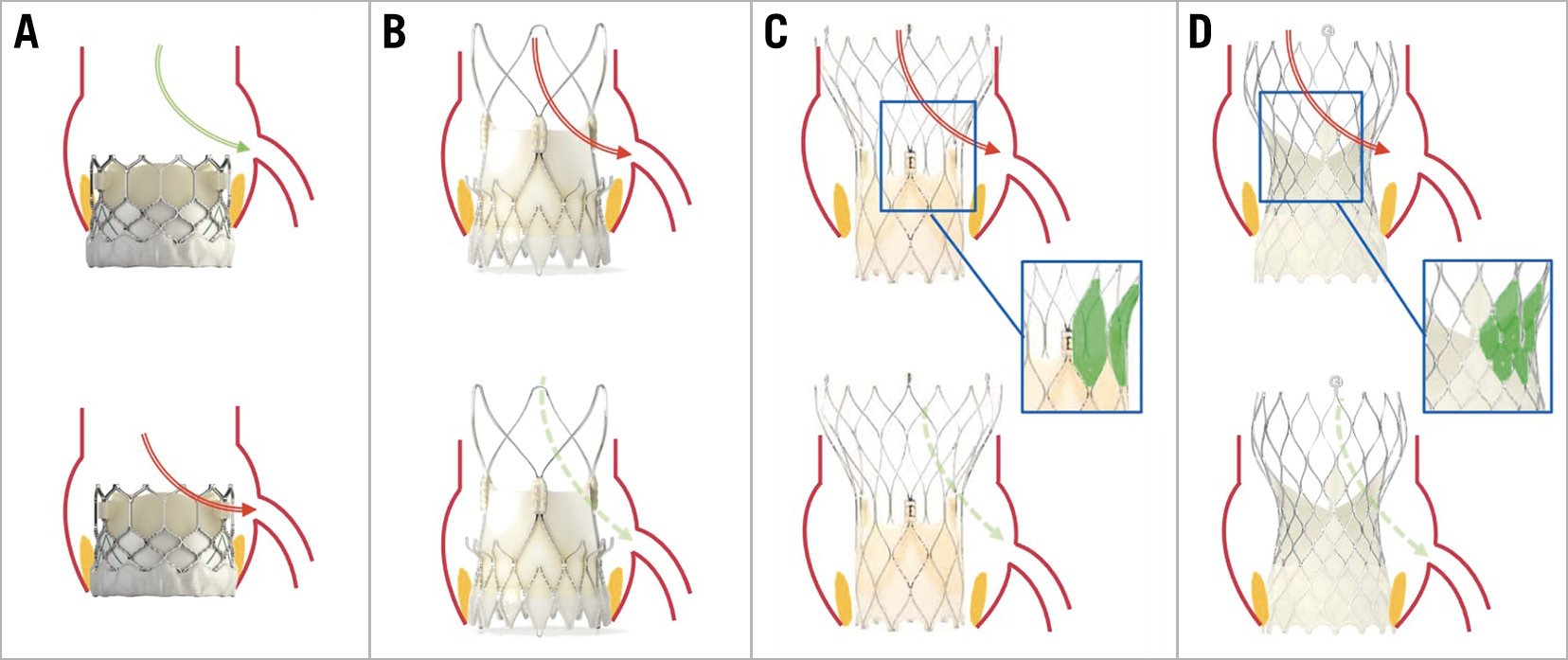
Figure 4. Coronary access according to valve design and implantation depth. Coronary engagement after previous SAPIEN 3 (A) implantation is commonly feasible with standard catheters, and the access route may be least impaired (green arrow); engagement across the stent frame (red arrow) may become necessary only in the case of high THV positioning or low coronary take-off. Potential access routes for the ACURATE neo valve (B) may be via the stabilisation arches (red arrow) or from outside the valve frame (dotted green arrow). The common access route in tall-frame valves ([C] Portico/Navitor; [D] Evolut) is across the uncovered stent struts above the leaflet plane (red arrows), whereas, especially in patients with a wide sinotubular junction or sinus of Valsalva, an access route from outside the valve frame (dotted green arrows) may be an alternative. The lower leaflet position and the larger cells of the Portico/Navitor THV stent frame may facilitate coronary catheterisation in comparison with the Evolut platform. Regardless of the THV type, correct commissural alignment of the prosthesis will usually facilitate coronary access. THV: transcatheter heart valve
Table 3. Feasibility of coronary access with different THV in available studies.
| Study author, year | Valve type (n) | ACS | RCA CA success | RCA CA selective | LCA CA success | LCA CA selective | PCI, n; success, % |
|---|---|---|---|---|---|---|---|
| Blumenstein et al. 201555 | SAPIEN XT (n=19) CoreValve (n=10) ACURATE (n=4) Other (n=2) |
13.3% | 94.3% | 77.1% | 97.1% | 79.4% | n=8; 100% |
| Boukantar et al. 201766 | CoreValve (n=16) | 43.8% | 58% | 16% | 75% | 44% | n=7; 85.7% |
| Htun et al. 201767 | CoreValve (n=28) | 90.0% | 100% | 90% | 100% | 97% | n=29; 100% |
| Zivelonghi et al. 201754 | Evolut R (n=25) SAPIEN 3 (n=41) |
0% | 100% | 94% | 98% | 97% | n=17; 100% |
| Tanaka et al. 201961 | CoreValve/Evolut (n=41) | 56.5% | 50% | 31.3% | 87.5% | 57.1% | n=30; 93.3% |
| Ferreira-Neto et al. 201953 | SAPIEN XT (n=28) | 64.3% | 100% | 81.5% | 100% | 82.6% | n=13; 100% |
| Couture et al. 202097 | Evolut R/PRO (n=10) | 10.0% | NA | 60% | NA | 40% | n=2; 50% |
| Nai Fovino et al. 202052 | SAPIEN XT/3 (n=36) CoreValve/Evolut R/Pro (n=8) Jena (n=2) Lotus (n=2) |
35.0% | 100% IA vs 75% SA | 94% IA vs 25% SA |
100% IA vs 100% SA |
97% IA vs 50% SA |
n=26; 96.2% |
| Barbanti et al. 202051 | SAPIEN (n=96) Evolut (n=123) ACURATE (n=72) Portico (n=9) |
0% | 96.0% | 88.0% | 95.3% | 68.3% | n=0; 0% |
| Kim et al. 202198 | SAPIEN (n=201) ACURATE (n=62) CoreValve/Evolut (n=140) Portico (n=16) Other (n=30) |
100% | 98.3% | 71.6% | 99.3% | 79.3% | n=243; 91.4% |
| ACS: acute coronary syndrome; IA: intra-annular; CA: coronary access; LCA: left coronary artery; PCI: percutaneous coronary intervention; RCA: right coronary artery; SA: supra-annular; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve | |||||||
COMMISSURAL ALIGNMENT WITH DIFFERENT TYPES OF TRANSCATHETER HEART VALVES
Alignment of the THV commissures with the native aortic valve commissures is essential to avoid overlap with the coronary ostia and optimally preserve coronary access.
The JenaValve and J-Valve (JenaValve Technology) are based on leaflet rather than annular anchoring, and commissural alignment is both necessary and inevitable as part of the implantation process76.
Commissural alignment should always be pursued when implanting a tall-frame THV. It has been shown to improve the success rate of selective coronary angiography (CA) after TAVI, as well as reducing the contrast volume and procedure time77. However, for most of the THVs currently used, alignment of the commissures can only be approximated and requires specific preprocedural planning and procedural techniques. Even with commissural alignment techniques, failure to achieve coronary access post-TAVI remains more frequent for THVs with a supra-annular leaflet position.
Commissural alignment also has implications for redo-TAVI, Specifically, without commissural alignment, the use of leaflet modification techniques such as BASILICA (Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction) to prevent coronary obstruction will not be possible78.
There are three general overarching principles to improve commissural alignment during TAVI79:
1. Preprocedurally, to identify by CT scan a patient-specific fluoroscopic projection (C-arm angulation) in which the native aortic valve commissures can be identified – specifically, the right and left (RL) coronary cusp overlap view or the 3-cusp coplanar view (Figure 5).
2. To know the THV-specific fluoroscopic markers which enable identification of the prosthetic neocommissures when the bioprosthesis is still crimped prior to deployment (Figure 6).
3. To know the possible manoeuvres of THV orientation during deployment, so that one of the neocommissures are placed where the right coronary cusp and left coronary cusp are overlapping (Figure 7).
Radiopaque markers with different THV devices:
A) Edwards SAPIEN iterations and Myval (Meril Life).
After valve deployment, neocommissures are identified by 3 “double lines” between the hexagonal crowns at the top cells. However, there is no way of identifying the commissures before deployment; hence, predictable commissural alignment with these THVs is not possible. Moreover, crimping the SAPIEN 3 THV at specific orientations seems to have no impact on commissural alignment80.
B) CoreValve/Evolut (Medtronic) iterations.
After valve deployment, the C-paddle on the Evolut corresponds to one commissure, but this marker cannot be seen under fluoroscopy before deployment. However, as per the manufacturer’s recommendations, the valve is routinely loaded with a prespecified angle of 90° between the C-paddle and the radiopaque “hat” marker of the capsule, which thus becomes a “surrogate” marker. Commissural alignment is defined as favourable if the hat marker is positioned in the centre-front (CF) orientation when the valve is deployed in RL cusp overlap view (putting the C-paddle to the right of the screen after complete deployment). This usually corresponds to the hat marker at the outer curve (OC) in left anterior oblique three-cusp view, or CF in the RL cusp overlap view818283. During the introduction of the delivery system, orientation of the flush port at 3 o’clock will provide an approximate initial alignment.
C) ACURATE neo/neo2. The 3 commissural posts are easily identifiable on the fluoroscopic view once the valve is deployed and are in line with 3 “free stent struts”. Favourable commissural alignment is reached if the valve is deployed with one isolated post and the corresponding free stent strut, which are visible under fluoroscopy, facing the right side of the fluoroscopic screen in RL cusp overlap view. Orientation of the flush port of the delivery catheter at 6 o’clock provides initial approximate commissural alignment79.
D) Portico/Navitor THV. As above, the Portico and Navitor THVs have 3 fluorogenic commissural posts, one of which should be isolated and aligned to the right of the screen in the RL cusp overlap view during deployment; although, it should be noted that visualisation of the commissural posts on fluoroscopy can be difficult. No general rules on how to insert the delivery system can be made79.
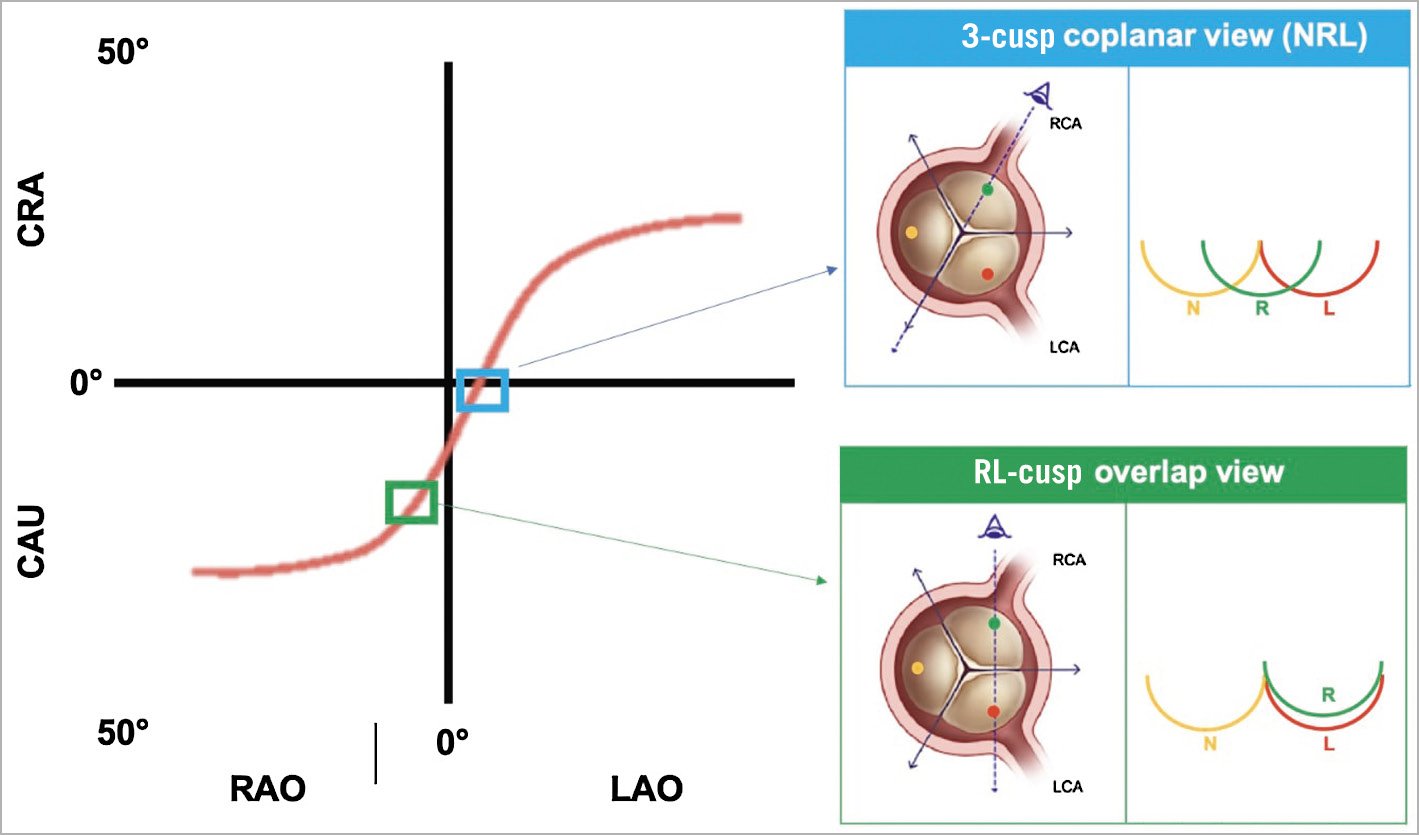
Figure 5. The S-curve of different fluoroscopic working views for transcatheter aortic valve deployment. The S-curve identifies infinite patient-specific fluoroscopic projections in which the 3 aortic cusps are aligned on the same plane. Among them, there are 3 projections where the 3 cusps are equidistant (NRL, LNR and RLN) and 3 other projections where 2 of 3 cusps are overlapping (RL cusp overlap, LN cusp overlap, NR cusp overlap). The most widely used implanting views are the 3-cusp coplanar view NRL and the RL cusp overlap view. CAU: caudal; CRA: cranial; L: left coronary cusp; LAO: left anterior oblique; LCA: left coronary artery; N: non-coronary cusp; R: right coronary cusp; RAO: right anterior oblique; RCA: right coronary artery
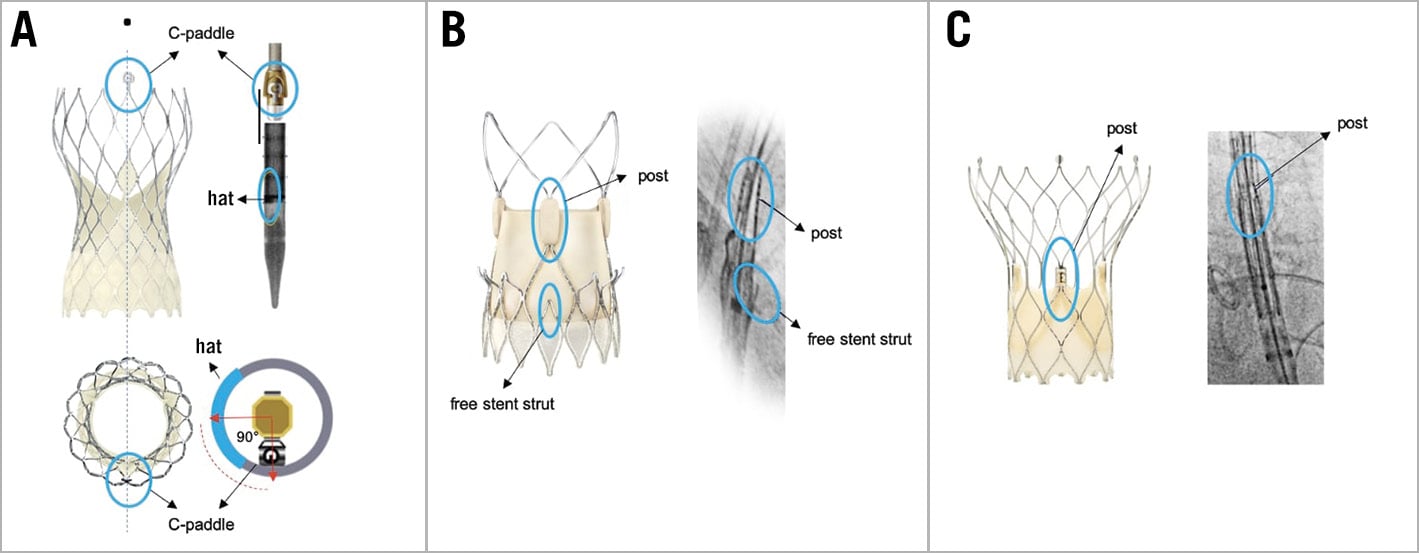
Figure 6. THV-specific fluoroscopic markers of the THV commissural posts. THV-specific fluoroscopic markers identifying prosthetic neocommissures in different types of expanded and crimped THVs. A) CoreValve Evolut platform; B) ACURATE neo2 platform; C) Portico/Navitor platform. THV: transcatheter heart valve. Adapted with permission from79.
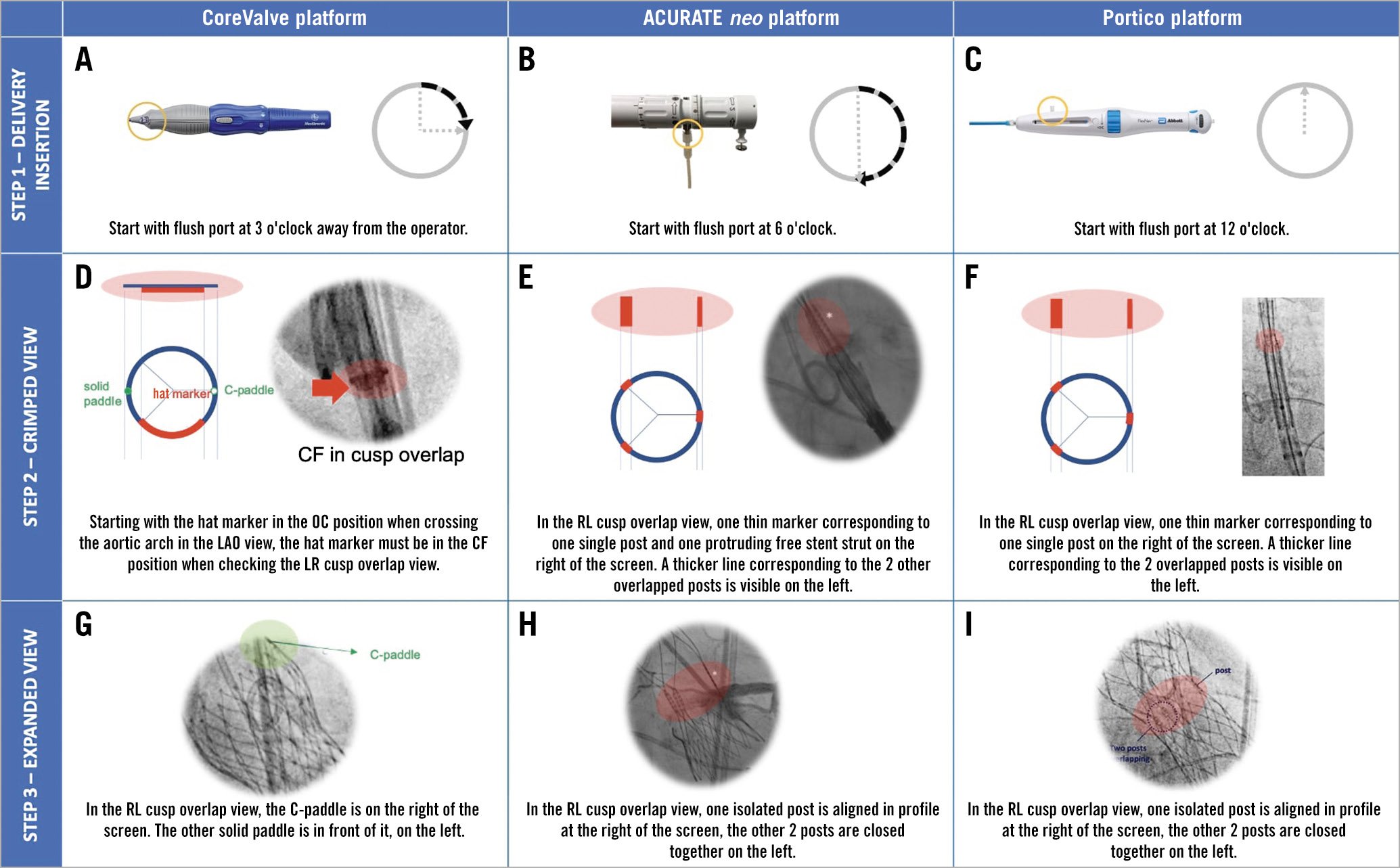
Figure 7. Implantation steps to obtain commissural alignment with different type of THVs. The figure shows the suggested rotation of the delivery system at the time of insertion (A, B, C), the expected fluoroscopic view of radiopaque markers in the RL cusp overlap view when the THV is still crimped (D, E, F) and the final fluoroscopic view after valve deployment in the same RL cusp overlap view (G, H, I) when implanting different THVs pursuing commissural alignment. If, before valve deployment, the fluoroscopic view is different from expected in the cusp overlap view, the operator should slowly torque the delivery catheter clockwise in order to obtain the desired orientation. Even if a gentle torque could be attempted with the THV at the level of the aortic valve, with the CoreValve platform, it is advisable to perform this manoeuvre only in the descending aorta to facilitate torquing force transmission through the delivery system. CF: central front; LAO: left anterior oblique; OC: outer curve; THV: transcatheter heart valve. Adapted with permission from79.
TIPS AND TRICKS FOR CORONARY ACCESS AFTER TAVI WITH DIFFERENT VALVE TYPES
Coronary access after TAVI may be challenging, particularly in patients treated before the adoption of commissural alignment techniques.
A prospective MSCT post-TAVI theoretical study suggested that 10-20% of patients present an increased risk of compromised coronary access84.
The challenge of engaging the coronary ostia varies according to the aortic root and STJ anatomy, sinus sizes, coronary take-off height and position, THV design and height, and commissural alignment8586. Several strategies may be considered:
⢠Usually, implantation of a low-frame THV with an intra-annular leaflet position in a subcoronary position does not require any modification to the coronary cannulation technique.
⢠Preprocedural planning is especially important when THVs are implanted in a partially supracoronary position, most often seen when the implant position is high, and/or in patients with a shallow sinus of Valsalva and a low coronary take-off height5487. This is observed specifically with tall-frame self-expanding valves but also when a balloon-expandable valve is implanted with the top edge of the frame above a low STJ. Use of the left radial or transfemoral approach might facilitate traversing the stent frame. Operators may need to adapt their catheter choice for coronary cannulation according to specific anatomies and the aortic root/THV relation. Selective intubation is usually best achieved with smaller catheter sizes, such as Judkins Left 3.5 or 4.0, Judkins Right 4.0, Amplatz 2.0 (Cordis), Ikari 1.0 (or 1.5) (Terumo) or 3DRC (Medtronic) catheters, or mammary catheters628688.
⢠In cases with difficult access, the use of intracoronary guidewires facilitates selective CA, as demonstrated in a prospective consecutive registry that performed FFR after TAVI54. If PCI is needed, catheter extensions can improve support in case of non-selective cannulation89.
⢠The guide should be disengaged from the ostium, preferably over a wire, avoiding excessive force, as it can kink the catheter or dislodge the THV. If necessary, the use of an angioplasty balloon may help.
Consensus points:
1) Coronary access after TAVI will be become increasingly common given the expansion of TAVI to younger and lower-risk patients and the high prevalence of CAD among patients undergoing TAVI.
2) Although the reported success rate of coronary access after TAVI remains high, we advise that the feasibility of coronary access, based on pre-TAVI imaging evaluation, should be considered in the decision-making process when choosing a transcatheter valve during index TAVI, in patients with previously treated CAD or with moderate CAD that may require revascularisation in the future.
3) Given the reduction in the physical barrier of a commissural post facing a coronary orifice when commissural (or coronary) alignment is achieved in TAVI, is advisable to aim for commissural (or coronary) alignment in all TAVI cases, particularly in patients where lifetime management of CAD and aortic valve reintervention would be important. Doing so would increase the feasibility of coronary access and redo-TAVI.
4) Specific tips and tricks on coronary access after TAVI are reviewed elsewhere86.
5) Non-selective aortic root angiography might be a good starting point to identify the relationship between the THV and coronary artery origins, particularly in the absence of post-TAVI CT imaging.
Coronary access after TAVI-in-TAVI
With the expansion of TAVI towards lower-risk and younger subjects, an increasing proportion of patients is likely to outlive THV durability and need redo-TAVI.
The implantation of a second THV will cause the leaflets of the first bioprosthesis to be displaced, creating a neoskirt extending from the inflow part of the stent frame to the top of the commissural posts699091929394 (Figure 3). Notably, the height of the neoskirt will also be influenced by the THV implantation depth. Thus, coronary access impairment after TAVI-in-TAVI will depend on 1) the coronary take-off height in relation to the neoskirt, 2) the distance between the THV stent frame and the aortic wall above the coronary ostia.
Accordingly, the first THV choice will determine coronary access impairment after TAVI-in-TAVI, as different prostheses have different neoskirt heights. In a highly selected cohort of patients undergoing TAVI-in-TAVI in tertiary care centres, with THVs with both supra- and intra-annular leaflet positions, the incidence of coronary obstruction after TAVI-in-TAVI was as low as 0.9%95. However, this incidence is expected to be significantly higher in an unselected TAVI-in-TAVI population.
Figure 8 depicts possible coronary access scenarios after TAVI-in-TAVI.
A) Coronary ostia are located above the neoskirt. This scenario is more likely with a short stent frame with an intra-annular leaflet position as compared to a tall stent frame with a supra-annular leaflet position. No issues with coronary access are expected with the implantation of a short stent frame THV or an aligned tall-frame THV at the time of TAVI-in-TAVI.
B) Coronary ostia are located below the neoskirt, but the sinotubular junction is wide. If there is enough space to navigate a coronary catheter between the aortic wall and the THV stent frame (>2 mm), coronary access after TAVI-in-TAVI will be possible, albeit difficult, if two THVs with a small-cell stent frame design are implanted and not perfectly aligned.
C) Coronary ostia are located below the neoskirt in the setting of a small sinotubular junction. If the distance between the stent frame and the aortic wall is <2 mm, the implantation of a second THV will make coronary access impossible. If the displaced leaflets of the first THV are in contact with the aortic wall, TAVI-in-TAVI is likely to cause sinus sequestration (i.e., impaired blood flow below the STJ level because the displaced leaflets are in contact with the aortic wall) and possibly coronary obstruction. This scenario is more likely if a high stent frame with supra-annular leaflets is implanted at the time of the first TAVI.
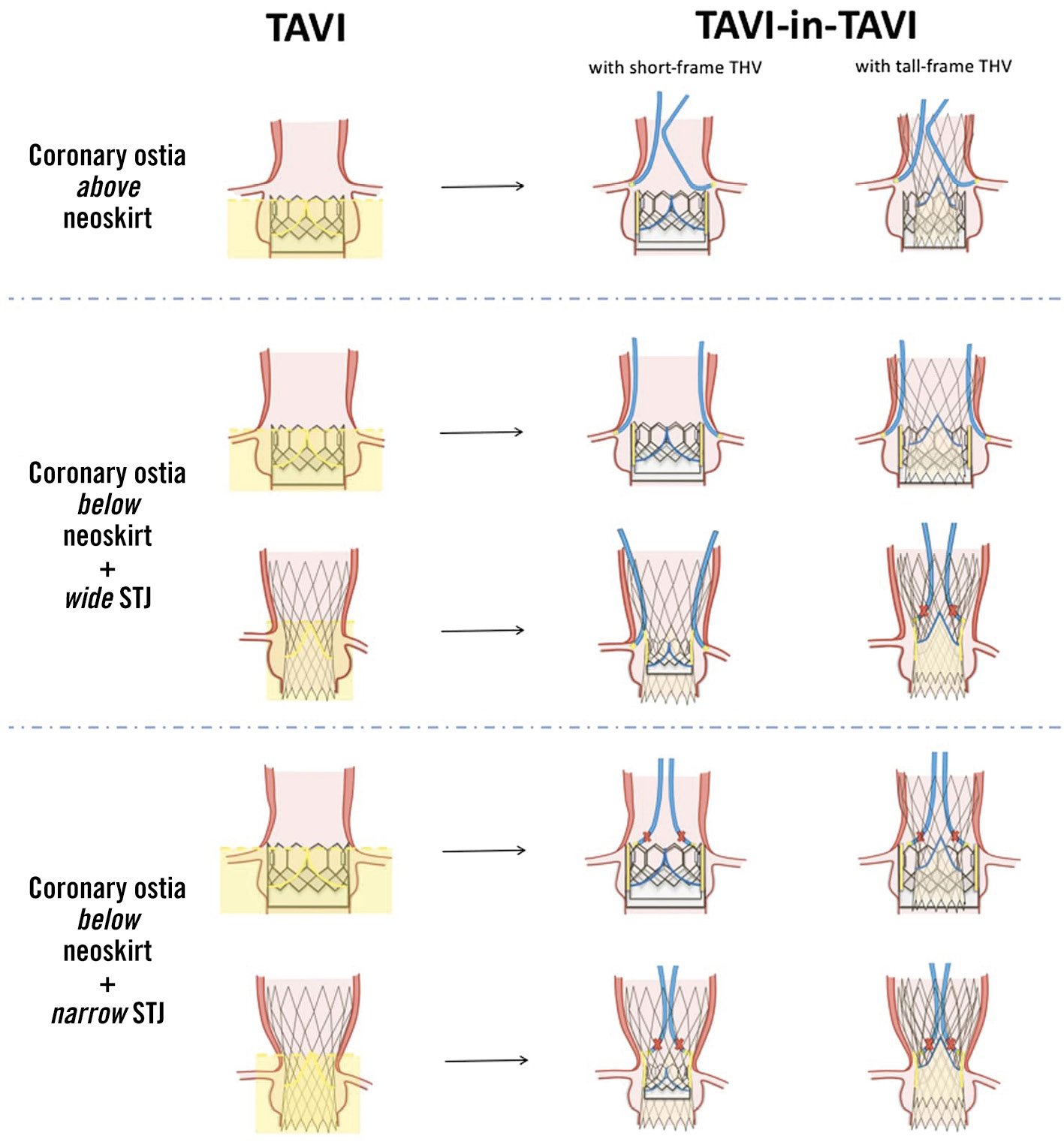
Figure 8. Coronary access after TAVI-in-TAVI with different combinations of SAPIEN and CoreValve/Evolut transcatheter heart valves, depending on aortic root anatomy. STJ: sinotubular junction; TAVI: transcatheter aortic valve implantation. Adapted with permission from90.
Coronary access after TAVI in degenerated surgical aortic valves
Factors affecting the feasibility of coronary access after valve-in-valve (ViV) TAVI are largely similar to those related to TAVI-in-TAVI. The height of the neoskirt is determined by the length of the degenerated surgical aortic bioprosthesis’ leaflets. Thus, coronary access after ViV-TAVI is expected to be more challenging with supra-annular versus intra-annular degenerated surgical aortic valves and stentless bioprosthesis or stented with externally mounted leaflets versus stented with internally mounted leaflets. Moreover, coronary access after ViV-TAVI is more difficult in the setting of a small aortic root with low coronary ostia. As for TAVI in the native aortic valve, the choice of an intra-annular THV and the commissural alignment of a supra-annular THV will reduce the risk of coronary access impairment after ViV-TAVI.
Risk of coronary obstruction
A detailed description of coronary protection techniques during TAVI is beyond the scope of this document. Briefly, different techniques may be adopted to mitigate the risk of coronary obstruction during TAVI in the native aortic valve. Coronary protection with a guidewire with/without an undeployed stent is advisable when coronary ostia are low and the presence of a bulky leaflet is expected to interfere with coronary perfusion after the valve is deployed. The same technique can be adopted in ViV-TAVI or redo-TAVI if the neoskirt is above one or both coronary ostia and the distance between the valve frame and the coronary ostium is <4 mm. After THV implantation, coronary perfusion should be assessed and, if impaired, chimney stenting should be performed. Leaflet modification techniques such as BASILICA78 may also be adopted in cases at high risk of coronary obstruction; however, neocommissural alignment of the index THV with the native aortic valve should be preprocedurally assessed, as the effectiveness of such a procedure will be reduced in case of severe misalignment of the index THV artery (yet, if the risk is sinus sequestration, BASILICA may suffice in providing adequate flow into the sinus for coronary perfusion even with a misaligned THV). Finally, with regard to redo-TAVI, a low implantation of a THV with a short stent frame inside a degenerated tall-frame THV will leave a degree of leaflet overhang, lowering the height of the neoskirt without impacting on the hydrodynamic performance of the second THV96.
Conclusions
Since CAD and AS often coexist, the evaluation and management of CAD in TAVI candidates is of paramount importance, particularly with the extension of the procedure to younger and lower-risk patients. Invasive coronary angiography remains the mainstay for CAD diagnosis, although CTCA might be considered for initial screening, particularly in patients at low risk for CAD. The role of coronary invasive physiology assessment needs to be further clarified. In patients undergoing TAVI, PCI should be performed in the setting of severe CAD with the involvement of proximal vessel segments or in patients with angina, preferably before THV implantation, and in particular if a THV with supra-annular leaflets is selected. The THV choice affects future coronary access after TAVI. Commissural alignment techniques should be routinely adopted to optimise coronary access.
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Conflict of interest statement
G. Tarantini reports lecture fees from Medtronic, Edwards Lifesciences, Abbott Vascular, and Boston Scientific. H. Eltchaninoff reports lecture fees from Edwards Lifesciences. D. Blackman reports consulting and lecture fees from Medtronic, Edwards Lifesciences, Abbott Vascular, and Boston Scientific. N. Bonaros reports lecture fees from Edwards Lifesciences and Medtronic. N. Karam reports consulting and lecture fees from Medtronic, Edwards Lifesciences, and Abbott Vascular. D. Mylotte reports consulting fees from Medtronic, Boston Scientific, and MicroPort. P. Carrilho-Ferreira reports lecture fees from Biotronik and Medtronic. N. Van Mieghem reports consulting fees from Biotronik, Boston Scientific, Abbott Vascular, Medtronic, PulseCath BV, and Abiomed; and research grants from Abbott Vascular, Edwards Lifesciences, Boston Scientific, Abiomed, Medtronic, PulseCath BV, Amgen, and Daiichi Sankyo. W-K. Kim reports lecture fees from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Meril Life Sciences. G. Tang is a physician proctor, physician advisory board member and consultant for Medtronic; physician advisory board member and consultant for Abbott Structural Heart; physician advisory board member for JenaValve; consultant for NeoChord; and has received speaker honoraria from Siemens Healthineers. O. De Backer received institutional research grants and consulting fees from Abbott, Boston Scientific, and Medtronic. L. Sondergaard received consultant fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic, and SMT. The other authors have no conflicts of interest to declare.The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; and consultancy fees paid to his institution from Boehringer.

