Abstract
Background: Left ventricular outflow tract (LVOT) calcification is known to be associated with adverse outcomes after transcatheter aortic valve implantation (TAVI) in patients receiving first-generation transcatheter heart valves (THV).
Aims: The aim of the present study was to assess the prevalence of LVOT calcification as well as its impact on outcomes in a contemporary TAVI patient cohort.
Methods: This retrospective single-centre analysis includes 1,207 patients who underwent transfemoral TAVI between 2012 and 2018 and in whom adequate contrast-enhanced multislice computed tomography (MSCT) imaging for quantification of LVOT calcification was available.
Results: Significant LVOT calcification, defined as >10 mm3, was present in 37.4% (n=451) of the patient cohort. After applying propensity score matching there was no difference between patients without (w/o; n=358) and with (w; n=358) significant LVOT calcification with respect to baseline clinical characteristics. At 30 days, the composite of all-cause mortality and non-disabling/disabling stroke occurred more often in patients w LVOT calcification compared to those w/o (4.6 vs 10.1%, p=0.008). Moreover, the composite VARC-3 endpoint of device success at 30 days was in favour of patients w/o LVOT calcification (82.2% vs 73.4%, p=0.007). According to Kaplan-Meier analysis, all-cause mortality one year after TAVI was higher in patients w vs w/o LVOT calcification (12.9 vs 21.4 %, p=0.004).
Conclusions: In patients undergoing TAVI, the presence of significant LVOT calcification is common and associated with worse short-term clinical and functional outcomes as well as higher one-year mortality rates compared to patients w/o LVOT calcification.
Introduction
Transfemoral transcatheter aortic valve implantation (TAVI) has become the treatment of choice for the majority of patients with severe aortic stenosis (AS) and is approved by the U.S. Food and Drug Administration for patients across the complete surgical risk spectrum1. Hence, when weighing surgical aortic valve replacement (SAVR) against TAVI in AS patients in the Heart Team, there has been a paradigm shift in recent years away from mere surgical risk stratification towards an individualised assessment of clinical as well as anatomical eligibility for either one or the other treatment modality. Left ventricular outflow tract (LVOT) calcification is known to be associated with adverse outcomes after TAVI in patients receiving early-generation transcatheter heart valves (THV) as it is associated with a higher risk of significant paravalvular leakage (PVL) and aortic root injury234. Thus, the presence of significant LVOT calcification per se is considered an anatomical criterion unfavourable for TAVI. Current-generation THV, however, have been proven to reduce the rate of PVL and aortic root injury has become an extremely rare complication, even in highly calcified anatomies567. Accordingly, the aim of the present study was to assess the impact of LVOT calcification on outcomes after transfemoral TAVI using current-generation devices in a large, real-world, patient cohort.
Methods
STUDY POPULATION AND DATA ACQUISITION
Baseline and periprocedural variables were recorded from patients’ charts and entered into a dedicated database. Clinical follow-up was obtained from in-house data acquired as part of clinical routine, documentation from referring physicians, and hospital discharge letters. Clinical endpoints and periprocedural complications were defined in accordance with the updated Valve Academic Research Consortium (VARC)-3 definitions8. All patients provided informed consent for the procedure and data acquisition. Median follow-up was 1.10 (1.03, 1.19) years.
TAVI PROCEDURE AND DEVICES
The diagnosis of severe AS was made according to the current European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines9. The decision to proceed with TAVI was made by a dedicated Heart Team. Selection of THV type and size was based on the Heart Team's decision including clinical considerations as well as findings from preprocedural multislice computed tomography (MSCT) and transoesophageal echocardiography. In this analysis, only patients receiving current-generation THV through the transfemoral access site were included. During the observation period, balloon-expandable (SAPIEN 3; Edwards Lifesciences), self-expanding (ACURATE neo [Boston Scientific], Portico [Abbott Laboratories], CoreValve, Evolut R [Medtronic], ALLEGRA [New Valve Technology]) and mechanically expandable devices (Lotus/LOTUS Edge; Boston Scientific) and mechanically expandable devices (Lotus™/LOTUS Edge™; Boston Scientific) were used. Patients with significant LVOT calcification preferably received mechanically expandable and self-expanding devices due to the higher risk of annular rupture when using balloon-expendable devices in this patient subset2. The decision to perform predilatation or post-dilation was assessed by the operator team and was device dependent. The treatment of periprocedural complications was decided together with cardiac surgeons.
MSCT IMAGE ACQUISITION
The amount of aortic valve and LVOT calcification was measured quantitatively from contrast-enhanced MSCT using 3men-sio Structural Heart software (Pie Medical Imaging), as previously described410. The LVOT zone was defined as the region from the annular plane to 5 mm below (Figure 1). In accordance with previous data, the presence of calcium volume >10 mm3 in the LVOT region was defined as significant LVOT calcification4. To discriminate between calcification and contrast medium in the region of the valve leaflet and LVOT a threshold of 550 Hounsfield units (HU) was applied. Occasionally, the threshold had to be adjusted based on visual estimation, resulting in a mean threshold of 556±66 HU in the overall study population.
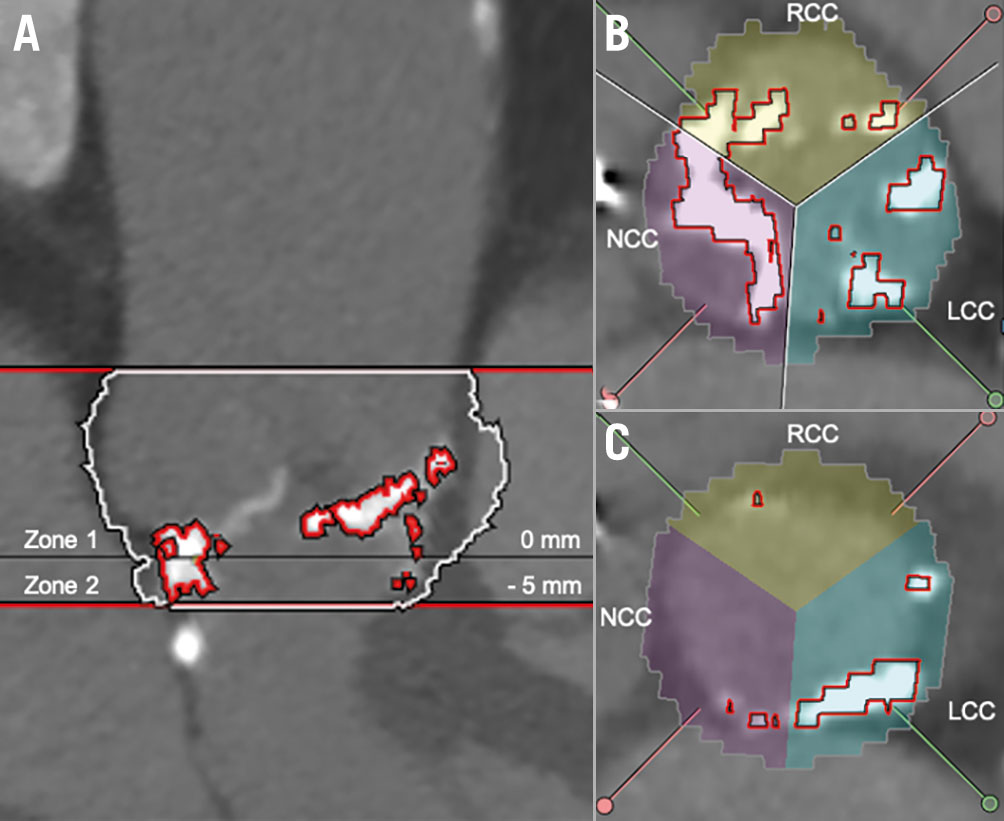
Figure 1. LVOT calcification quantification based on contrast-enhanced MSCT. A) Aortic valve (AV) complex calcification quantification: Zone 1 (=annular plane, basal plane to the coronary ostia) and Zone 2 (=LVOT, basal plane to 5 mm deep in the LVOT). B) Distribution of calcification in Zone 1 (annular plane) according to AV leaflets in annular plane. C) Distribution of calcification in Zone 2 (LVOT) according to AV leaflets. LCC: left coronary cusp; NCC: non-coronary cusp; RCC: right coronary cusp
STUDY ENDPOINTS
The primary study endpoint was defined as the composite of all-cause mortality and non-disabling/disabling stroke 30 days after the procedure. Secondary endpoints were defined in accordance with VARC-3 early safety at 30 days (freedom from all-cause mortality and/or all stroke and/or VARC type 2-4 bleeding and/or acute kidney injury >stage 2 and/or major access site complication and/or coronary artery obstruction requiring coronary intervention and/or valvular reintervention and/or a need for permanent pacemaker implantation), VARC-3 device success at 30 days (defined as “technical success” at exit from procedure room (freedom from procedural mortality and single device without malpositioning and freedom from conversion to surgery and freedom from major access site complication] and freedom from 30-day mortality and p mean <20 mmHg and PVL
STATISTICAL ANALYSIS
For statistical analysis, propensity score matching including the following variables was prepared: age, body mass index (BMI), chronic obstructive pulmonary disease, diabetes, Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) (%), left ventricular ejection fraction <55%, gender, New York Heart Association (NYHA) stage, mean pressure gradient (p mean, aortic valve), coronary artery disease, stroke, atrial fibrillation, glomerular filtration rate (GFR), pulmonary artery pressure (PAP) <31 mmHg and coronary artery bypass graft (CABG). The nearest neighbour method with the Mahalanobis distance measure and a calliper of 0.25 was used. The average absolute standardised difference before matching was 0.1 and 0.07 after matching. Data from 358 patients in each group were analysed after the propensity score matching was carried out.
Continuous variables are shown as mean±standard deviation or as median and 25th and 75th percentile. Binary variables are shown as absolute numbers and percentages. Differences to total numbers (n) were due to missing values, the calculation of proportions did not include missing values in the denominator. For two group comparisons, the Mann-Whitney test was used for continuous variables, and the χ2 test for binary ones. Survival curves were produced using the Kaplan-Meier method and the log-rank test was used to test for survival curve differences. For overall tests, p<0.05 was considered statistically significant. All statistical analyses were performed using R version 4.0.3 (R Foundation for Statistical Computing).
Results
PATIENT BASELINE CHARACTERISTICS
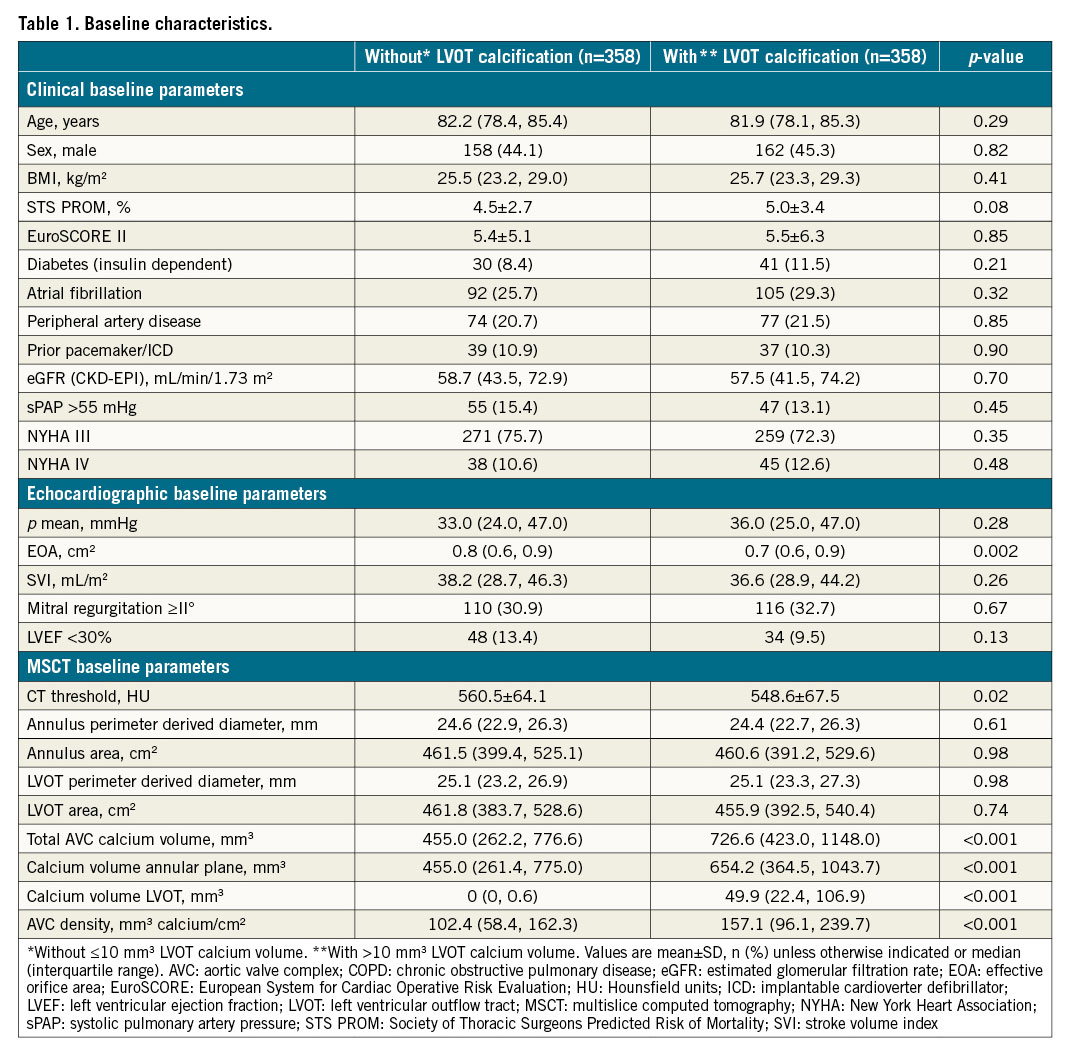
A total of 1,761 patients with severe AS receiving TAVI between 2012 and 2018 through the transfemoral access were identified. Of these, 152 patients with valve-in-valve procedures and 23 patients with concomitant procedures (e.g., transcatheter mitral valve repair or percutaneous coronary intervention) were excluded. Furthermore, 379 patients had to be excluded because of missing or poor MSCT imaging quality, leading to a total of 1,207 eligible study patients (Central illustration). Significant LVOT calcification was found in 37.4% (n=451) of all patients. After applying 1:1 propensity score (PS) matching, 358 patients with (w; >10 mm3 calcium volume) versus 358 without (w/o; ≤10 mm3 calcium volume) LVOT calcification remained for comparison. Clinical, echocardiographic and MSCT baseline parameters of the PS matched cohort are summarised in Table 1. There was no significant difference with respect to age, gender, or relevant comorbidities between groups. Accordingly, there was no significant difference regarding estimated surgical risk by using either the STS PROM score or the EuroSCORE II. Effective orifice area (EOA) measured by echocardiography was significantly smaller in patients with significant LVOT calcification. Furthermore, patients with significant LVOT calcification showed a greater amount of calcification in the THV landing zone (annular plane and LVOT) according to MSCT.
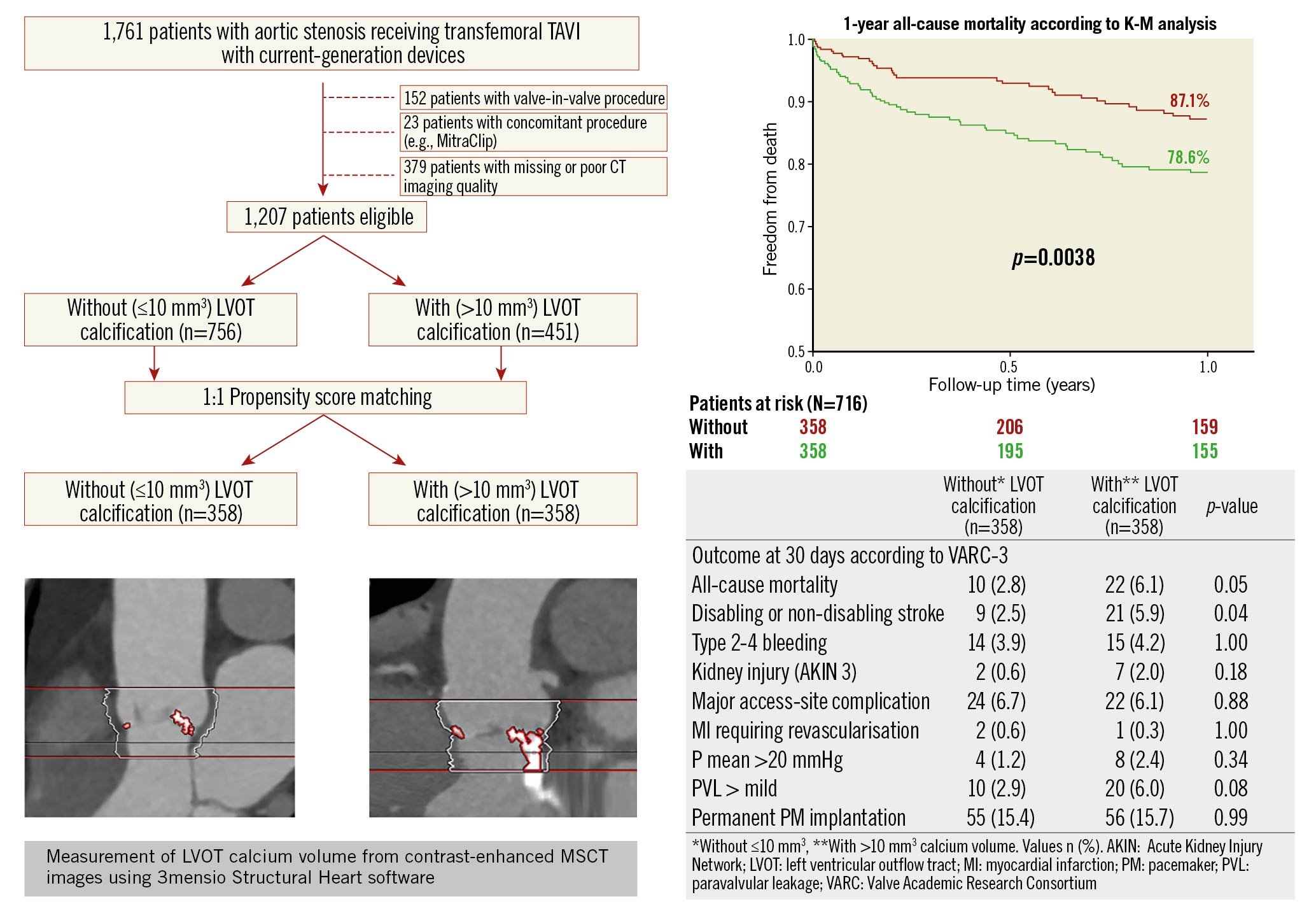
Central illustration. Study flow diagram and one-year all-cause mortality and functional outcomes at 30 days in patients with and without LVOT calcification. A total of 1,207 patients with adequate MSCT data treated with transfemoral TAVI was included in this cohort; 451 patients with relevant LVOT calcification were identified. After propensity score matching, a total of 358 patients with/without LVOT calcification were compared. One-year mortality rate according to Kaplan-Meier analysis, red line indicates without (≤10 mm3) and green line indicates with (>10 mm3) LVOT calcification. Outcomes at 30 days post-TAVI according to VARC-3.
PROCEDURAL DATA AND COMPLICATIONS
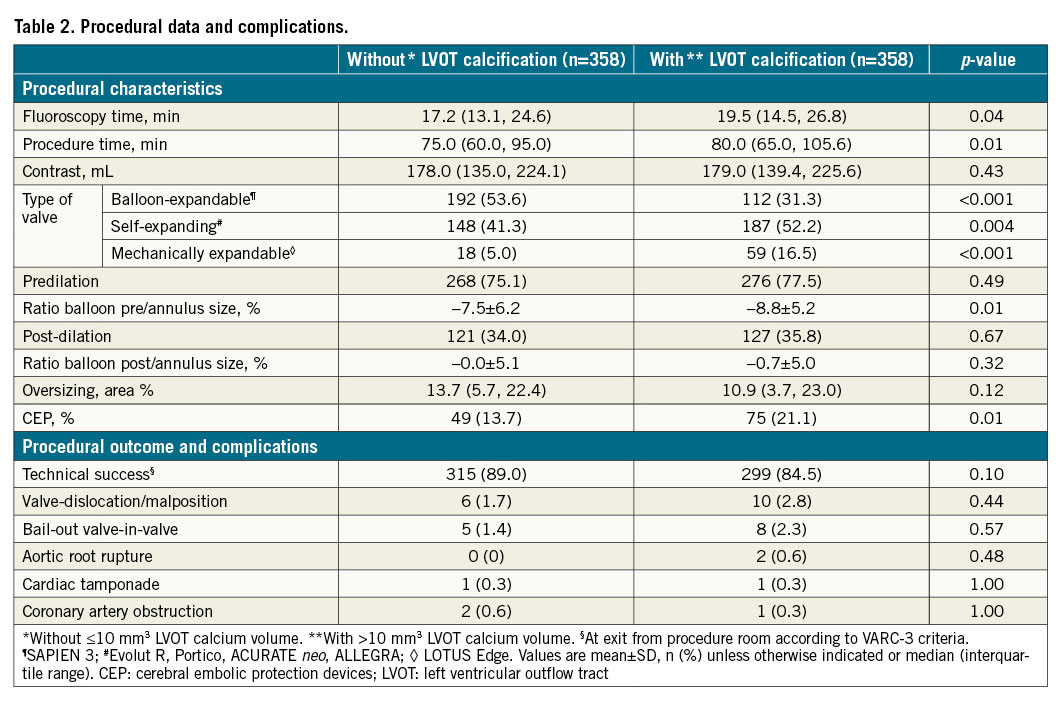
Procedural data is provided in Table 2. Fluoroscopy time and procedure time were both longer in patients with significant LVOT calcification. Rate of predilation and post-dilation were evenly distributed between the groups. The ratio between balloon size and annulus size in predilation was significantly lower in patients with LVOT calcification, which indicates more undersizing of the valvuloplasty balloon in patients with LVOT calcification. The ratio between balloon and annulus size post-dilatation was similar in both groups. The rate of VARC-3 technical success was higher in patients without compared to patients with LVOT calcification, albeit that the results did not reach a level of statistical significance. Furthermore, procedural complications such as aortic root rupture, valve malpositioning or the need for a second THV were numerically more frequent in patients with significant LVOT calcification. Coronary artery obstruction requiring coronary interventions and valvular reintervention did not show significant differences. Distribution of THV types showed higher use of balloon-expandable valves (i.e., SAPIEN 3) in patients without significant LVOT calcification compared to patients with significant LVOT calcification. In contrast, self-expanding (e.g., ACURATE neo, Evolut R, Portico, ALLEGRA) and mechanically expandable valves (i.e., LOTUS Edge) were used more frequently in patients with LVOT calcification. The usage of cerebral embolic protection devices (CEP [SENTINEL; Boston Scientific]) was significantly higher in patients with LVOT calcification.
CLINICAL OUTCOMES
The primary endpoint, defined as the composite of all-cause mortality and non-disabling/disabling stroke at 30 days after TAVI, occurred more frequently in patients with significant LVOT calcification compared to those without. Moreover, the VARC-3 device success endpoint was in favour of patients without significant LVOT calcification. The updated VARC-3 early safety endpoint at 30 days after TAVI did not show significant differences between patients with and without LVOT calcification (Figure 2). All-cause mortality 30 days after TAVI was higher in patients with LVOT calcification with borderline significance (w/o, 2.8 vs w, 6.1%, p=0.05). Furthermore, the rate of all stroke was significantly higher in patients with LVOT calcification (w/o, 2.5 vs w, 5.9 %, p=0.04) (Central illustration), whereas the timepoint of stroke was not different between the groups (Supplementary Table 1). The rate of permanent pacemaker implantation post-TAVI was similar in both groups.
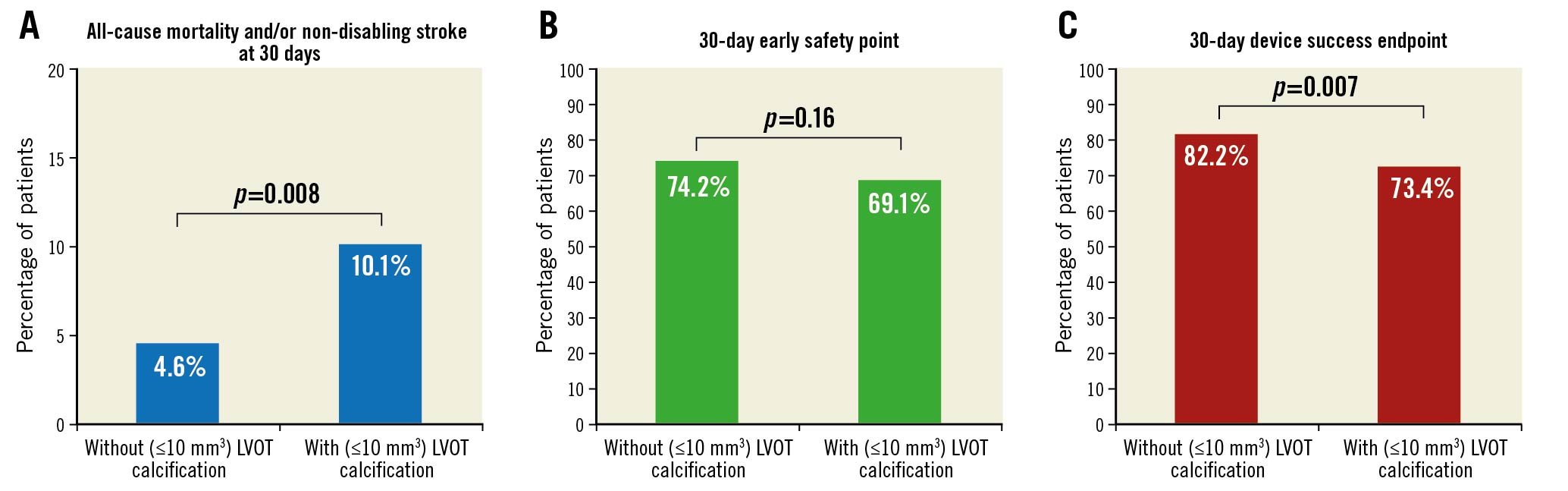
Figure 2. Primary and secondary composite endpoints. A) Primary composite endpoint of all-cause mortality and/or non-disabling/disabling stroke at 30 days. B) VARC-3 early safety composite endpoint of freedom from all-cause mortality and/or all stroke and/or VARC type 2-4 bleeding and/or acute kidney injury >stage 2 and/or major access site complication and/or coronary artery obstruction requiring coronary intervention and/or valvular reintervention and/or need for permanent pacemaker implantation at 30 days. C) VARC-3 device success endpoint defined as “technical success” at exit from procedure room (freedom from procedural mortality and single device without malpositioning and freedom from conversion to surgery and freedom from major access-site complication) and freedom from 30-day mortality and p mean <20 mmHg and PVL
During a median follow-up period of 1.05 (0.99, 1.12) years, 141 patients (19.7%) in the matched cohort died, 58 patients (16.2%) without LVOT calcification and 83 (23.2%) with LVOT calcification (p=0.02). According to Kaplan-Meier analysis, all-cause mortality one year after TAVI was significantly higher in patients with versus those without significant LVOT calcification (12.9 vs 21.4 %, p=0.004) (Central illustration). Detailed patient characteristics and further categorisation of LVOT calcification and its impact on clinical outcomes are given in Supplementary Table 1 and Supplementary Table 2.
Discussion
The present study compared outcomes in patients who underwent transfemoral TAVI using current-generation devices with versus without the presence of significant LVOT calcification in a propensity score-matched fashion. The main findings of the study are as follows. 1) Significant LVOT calcification (>10 mm3) was present in more than one third of the overall patient cohort. 2) Patients with significant LVOT calcification were more often treated with self-expanding or mechanically expandable THVs and less frequently with balloon-expandable THVs compared to those without significant LVOT calcification. 3) 30-day clinical outcome was inferior in patients with versus without significant LVOT calcification regarding the composite of all-cause mortality and stroke as well as device success according to VARC-3 definition. 4) The rate of one-year all-cause mortality was significantly higher in patients with versus without significant LVOT calcification.
Previous studies have already demonstrated an adverse impact of LVOT calcification on procedural and clinical outcomes459. Such patients are at higher risk of significant PVL, bail-out valve-in-valve treatment, and annular rupture, as well as long-term mortality111213. However, these data mainly refer to early-generation devices. Newer-generation TAVI devices have been designed to reduce the rate of significant PVL. Moreover, most of the current non-balloon-expandable devices provide resheathability, which allows device repositioning and controlled valve deployment, thereby minimising the risk of THV malpositioning. In addition, due to thorough preprocedural planning, including MSCT-based assessment of annular and LVOT geometry, the risk of annular rupture with balloon-expandable devices in patients with LVOT calcification has become extremely low. In fact, as demonstrated by Nomura et al, compared to early-generation devices, newer-generation TAVI devices yield better procedural as well as short-term clinical outcomes in patients with LVOT calcification5. Yet, as we demonstrate in the present study, even by using current-generation TAVI devices in these patients, they still suffer from worse short-term functional and clinical outcome as well as higher mortality one year after the procedure compared to TAVI patients without LVOT calcification.
Especially the increased rate of periprocedural cerebrovascular accidents in these patients, which was more than twofold higher compared to patients without LVOT calcification, raises concern. Others have also found a numerically higher rate of stroke or transient ischaemic attack (TIA) in patients with LVOT calcification undergoing TAVI compared to those without1112. It remains unclear, whether this finding is a device-related effect, e.g., due to the higher use of repositionable devices among patients with significant LVOT calcification, or related to patients’ anatomy, such as the higher burden of calcification in the device landing zone, which is thought to be associated with a higher risk of stroke613. As a consequence, TAVI candidates with significant LVOT calcification might be a patient subset that benefits from the use of CEP. Interestingly, in the current study, the use of CEP was actually higher in patients with LVOT calcification. Whether consistent use of CEP among all patients with LVOT calcification would have prevented further cerebral embolic events is uncertain. This hypothesis definitely warrants further investigation.
In the present study, the rate of more-than-mild PVL was numerically, but not significantly, higher in TAVI patients with versus without LVOT calcification. Actually, with regard to previous data on TAVI patients with LVOT calcification, we observed a comparably low rate of more-than-mild PVL (6% in the present study versus 8%, 11%, and 18%, respectively)51114. This finding nicely demonstrates that the main focus of device iteration from early- to current-generation THVs primarily addressed the improvement of paraprosthetic sealing and device positioning to reduce the rate of PVL. The prime example of this approach was the advent of the mechanically expandable LOTUS Edge valve, a fully retrievable, repositionable device that included a paraprosthetic skirt for optimal annular sealing. This device diminished the rate of significant PVL71516. Accordingly, mechanically expandable devices were favourably used by many TAVI operators, particularly in patients with LVOT calcification11. Still, to the best of our knowledge, there is so far no evidence that the reduction of PVL by using this device truly translated into superior clinical outcomes. Just recently, the LOTUS Edge valve has been recalled by the manufacturer due to safety concerns regarding the delivery system17. Thus, this THV concept will not be available at any time in the near future. However, it underlines the fact that the adverse impact of LVOT calcification on outcome in TAVI patients cannot be overcome only by reducing the rate of PVL but will also require an easy-to-use device with a reliable mechanism of valve deployment. It remains to be seen whether the newest-generation devices, such as the SAPIEN 3 Ultra (Edwards Lifesciences), Evolut PRO+ (Medtronic), Navitor (Abbott Laboratories) or ACURATE neo2 (Boston Scientific) will be able to find the proper balance between efficacy and safety in TAVI patients with LVOT calcification.
As shown in the current study, patients with significant LVOT calcification experience worse functional and clinical outcome after TAVI compared to those without LVOT calcification. With the current trend of expending TAVI for AS treatment towards low-risk patients with longer life expectancy, this finding is of crucial importance because current evidence regarding comparisons between TAVI and SAVR is mainly based on data excluding patients with significant LVOT calcification13181920. Thus, as with certain other anatomic features in AS patients, e.g., small aortic annuli or bicuspid aortic valves, the particular use of TAVI in patients with LVOT calcification should be evaluated against SAVR in a dedicated prospective randomised controlled study, especially for those patients at lower surgical risk. Until then, when assessing treatment options in the Heart Team in younger, surgical low-risk AS patients with significant LVOT calcification, unfavourable outcome after TAVI will have to be anticipated, even when using current-generation devices, and SAVR should remain the preferred treatment modality for these patients.
Limitations
The following limitations of this study should be acknowledged. First, in this study, measurements from contrast-enhanced MSCT were used, which provides a less accurate quantification of calcification load compared to non-contrast MSCT21. Second, we cannot provide sufficient data on the quality of LVOT calcification as well as that for mitral annular calcification. Third, we cannot rule out that the uneven distribution of THV types used in patients with versus without significant LVOT calcification introduces bias to the analysis. However, device selection was performed by an experienced Heart Team and guided by anatomic criteria of which the presence of significant LVOT calcification was one criterion (apart from, e.g., annular size, annular calcification load, annulus angulation and access vessel size). Fourth, as mentioned above, the newest generation of THV devices were not included in this analysis. Fifth, further limitations of the current study relate to its retrospective, single-centre study design.
Conclusions
Significant LVOT calcification is present in a substantial proportion of TAVI patients and is associated with worse short-term clinical and functional outcomes as well as higher one-year mortality compared to patients without significant LVOT calcification.
Impact on daily practice
This study emphasises the importance of patient selection by the Heart Team with regard to unfavourable outcome after TAVI in patients with significant LVOT calcification, especially for those patients at lower surgical risk. Efforts should be made to optimise TAVI devices further in order to improve outcomes among TAVI patients with LVOT calcification.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
N. Schofer has received travel compensation from Abbott, Edwards Lifesciences and St. Jude Medical, as well as speaker honoraria and travel compensation from Boston Scientific. L. Conradi is an advisory board member for Abbott, Medtronic and Boston Scientific and has received personal honoraria from Edwards Lifesciences. M. Seiffert has received travel support from Abbott Vascular, Biotronik, Boston Scientific, Edwards Lifesciences, Nicolai Medizintechnik, and OrbusNeich Medical and speaker honoraria from Abiomed, Amgen, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Philips, Medtronic, and Shockwave Medical, and consulting honoraria from Boston Scientific and Shockwave Medical. D. Westermann has received speaker honoraria from AstraZeneca, Bayer, Belin-Chemie and Novartis. S. Blankenberg reports grants and personal fees from Abbott Diagnostics, Thermo Fisher, and Bayer, grants from Siemens and Singulex, personal fees from Abbott, AstraZeneca, Amgen, Medtronic, Pfizer, Roche, Novartis and Siemens Diagnostics, is an advisory board member for Bayer, Novartis and Thermo Fisher, and is board member of DGK (German Cardiac Society), UHZ Medical Director, and Faculty member UHZ. J. Schirmer has received speaker honoraria from Edwards Lifesciences and received travel compensation from Boston Scientific. S. Ludwig has received travel compensation from Edwards Lifesciences. All reported honoraria and travel compensations are outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

