
Abstract
Aims: The impact of left ventricular outflow tract calcification (LVOT-CA) on SAPIEN 3 transcatheter aortic valve replacement (S3-TAVR) is not well understood. The aims of the present study were to determine optimal device sizing for S3-TAVR in patients with or without LVOT-CA and to evaluate the influence of residual paravalvular leak (PVL) on survival after S3-TAVR in these patients.
Methods and results: This study analysed 280 patients (LVOT-CA=144, no LVOT-CA=136) undergoing S3-TAVR. Optimal annular area sizing was defined as % annular area sizing related to lower rates of ≥mild PVL. Annular area sizing was determined as follows: (prosthesis area/CT annulus area–1)×100. Overall, ≥mild PVL was present in 25.7%. Receiver operating characteristic curve analysis for prediction of ≥mild PVL in patients with LVOT-CA showed that 7.2% annular area sizing was identified as the optimal threshold (area under the curve [AUC] 0.71). Conversely, annular area sizing for no LVOT-CA appeared unrelated to PVL (AUC 0.58). Aortic annular injury was seen in four patients (average 15.5% annular area oversizing), three of whom had LVOT-CA. Although there was no difference in one-year survival between patients with ≥mild PVL and without PVL (log-rank p=0.91), subgroup analysis demonstrated that patients with ≥moderate LVOT-CA who had ≥mild PVL had lower survival compared to patients with ≥mild PVL and none or mild LVOT-CA (log-rank p=0.010).
Conclusions: In the setting of LVOT-CA, an optimally sized S3 valve is required to reduce PVL and to increase survival following TAVR.
Abbreviations
AVC: aortic valvular complex
CA: calcification
CI: confidence interval
LVOT: left ventricular outflow tract
PVL: paravalvular leak
ROC: receiver operating characteristic
S3: SAPIEN 3
TAVR: transcatheter aortic valve replacement
THV: transcatheter heart valve
Introduction
Transcatheter aortic valve replacement (TAVR) with the third-generation balloon-expandable SAPIEN 3 (S3; Edwards Lifesciences, Irvine, CA, USA) is rapidly spreading as an alternative to older-generation balloon-expandable TAVR for patients with severe aortic valve stenosis1. The frequency and severity of paravalvular leak (PVL) vary according to the valve designs and are significantly lower following balloon-expandable S3-TAVR than following SAPIEN XT (Edwards Lifesciences) TAVR2,3. Left ventricular outflow tract (LVOT) calcification (CA) is frequently encountered in CT imaging before TAVR. Importantly, increased LVOT-CA, especially if located inferior to the annulus, has been shown to be a strong predictor of PVL and of aortic annular injury4-6. We therefore hypothesised that optimal annular area sizing may be different between patients with or without LVOT-CA. Additionally, the impact of mild PVL following S3-TAVR remains uncertain. Thus, the aims of the present study were to determine the optimal annular area sizing for S3-TAVR in patients with or without LVOT-CA and to evaluate the impact of PVL on survival after S3-TAVR in these patients.
Methods
STUDY POPULATION AND PROCEDURE
Between November 2013 and January 2016, a total 291 patients who had severe aortic stenosis (<1.0 cm2) underwent S3-TAVR and preprocedural contrast cardiac computed tomography (CT) at our institute. After excluding patients with poor CT imaging quality (11 patients), a total of 280 patients were included in the final analysis. To evaluate the association between LVOT-CA and optimal annular area sizing, patients were divided into two groups, according to the presence or the absence of LVOT-CA4,5. Optimal annular area sizing was defined as the percentage of annular area sizing related to reduced rates of ≥mild PVL.
Regions of the aortic valvular complex (AVC) were separated into aortic valve leaflet and LVOT regions4-6. Furthermore, the severity of LVOT-CA was assessed as follows: 1) mild: one nodule of calcium extending <5 mm and covering <10% of the perimeter of the annulus; 2) moderate: two nodules or one nodule extending >5 mm or covering >10% of the perimeter of the annulus; and 3) severe: multiple nodules or a single focus extending >1 cm in length, or covering >20% of the perimeter of the annulus (Figure 1)3,4. Clinical data, patient characteristics, echocardiographic data, and procedural variables were prospectively recorded.
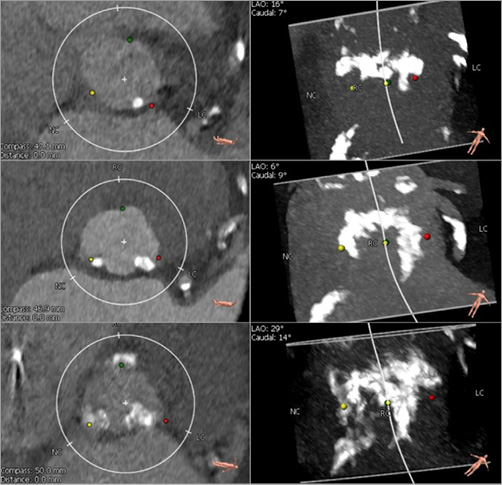
Figure 1. Severity of LVOT calcification. Definition of LVOT calcification was as follows: 1) mild: one nodule of calcium extending <5 mm and covering <10% of the perimeter of the annulus; 2) moderate: two nodules or one extending >5 mm or covering >10% of the perimeter of the annulus; and 3) severe: multiple nodules or a single focus extending >1 cm in length or covering >20% of the perimeter of the annulus. LVOT: left ventricular outflow tract
Transcatheter heart valve (THV) sizing and the degree of inflation volume was at the operator’s discretion, taking into account data from preprocedural CT. All CT AVC measurements were performed in our CT core laboratory and in line with the Society of Cardiovascular Computed Tomography expert consensus recommendations7. For reconstruction, mid-systolic data were used. Annular area sizing was determined as follows: (THV area/annulus area–1)×100. The LVOT area was defined as the cross-sectional region from 4 mm inferior to the annular plane. The LVOT cover index was measured as follows: (THV area/LVOT area–1)×100. The manufacturer’s recommended nominal inflation volumes of the deployment balloon for the 20, 23, 26, and 29 mm transfemoral NovaFlex delivery system (Edwards Lifesciences) are 11, 17, 23, and 33 ml, respectively. The nominal inflation volumes for the 23, 26, and 29 mm transapical Ascendra delivery system (Edwards Lifesciences) are 16, 20, and 30 ml, respectively. In patients with <0% undersizing, the interaction between the degree of inflation volume and ≥mild PVL was also evaluated. A recently validated 850 Hounsfield unit threshold was used to detect areas of calcium in the region of aortic valve leaflet6. Post-TAVR transthoracic echocardiography was performed before discharge. PVL was graded according to the VARC-2 guidelines8. The study complies with the Declaration of Helsinki. A locally appointed ethics committee approved the research protocol, and informed consent was obtained from all subjects.
STATISTICAL ANALYSIS
Continuous variables were tested for a normality of distribution using the Shapiro-Wilk test and reported and analysed appropriately thereafter. Categorical variables were compared by chi-square statistics or the Fisher’s exact test. Mann-Whitney U tests were used in case of abnormal distribution. A receiver operating characteristic (ROC) curve was plotted to determine a cut-off value for the degree of annular area sizing related to ≥mild PVL. Parameters for prediction (p<0.05) of ≥mild PVL were entered into a multivariable logistic regression model. Cumulative mortality was estimated by the Kaplan-Meier method, and differences were assessed with the log-rank test. All of the analyses were considered significant at a two-tailed p-value of less than 0.05. SPSS statistics software, Version 22.0 (IBM Corp., Armonk, NY, USA) was used to perform all statistical evaluation.
Results
PATIENTS AND PROCEDURAL CHARACTERISTICS
The study population included 280 patients who underwent TAVR for the treatment of severe aortic stenosis with the S3-THV. The LVOT-CA group included 144 patients, and the remaining 136 patients were included in the no LVOT-CA group. Baseline clinical and preprocedural characteristics are shown in Table 1. The prevalence of diabetes was higher in patients with no LVOT-CA (38.2% vs. 27.5%, p=0.02). Patients with LVOT-CA had a higher mean aortic valve gradient (44.9 [41.0-53.0] mmHg vs. 43.0 [40.0-47.0] mmHg, p=0.001). Patients with LVOT-CA had greater volumes of aortic valve calcium than patients without LVOT-CA and had a higher prevalence of mitral annular calcification (Table 1). Other baseline characteristics were similar between the groups.
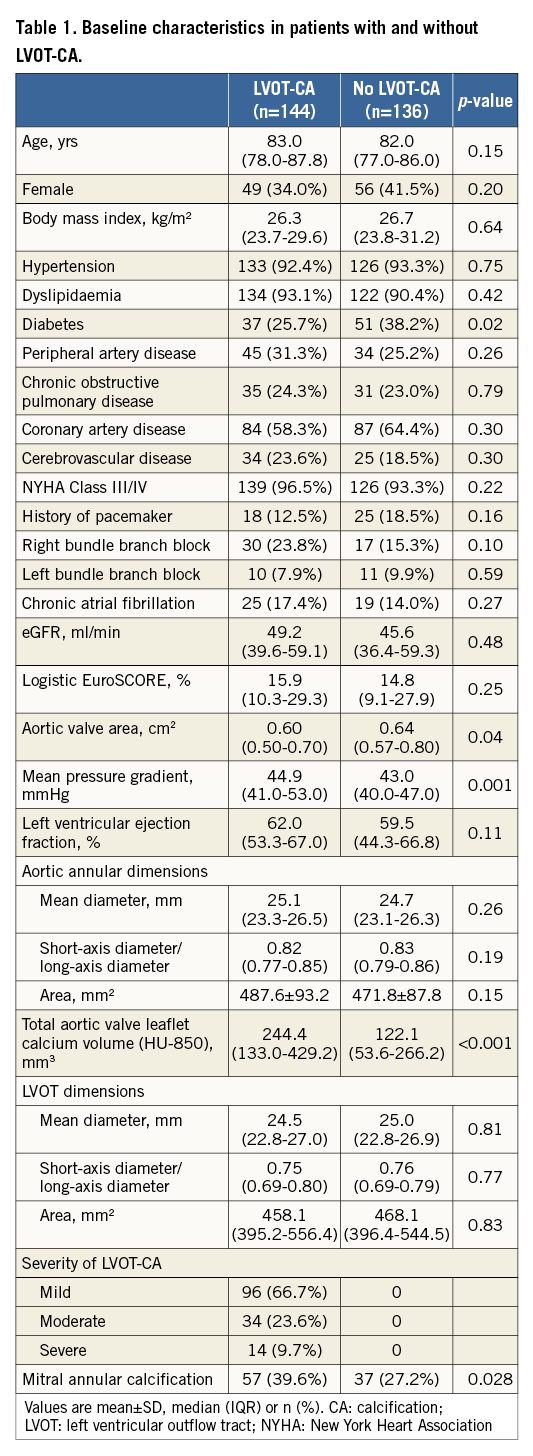
Procedural outcomes are shown in Table 2. Patients with LVOT-CA had a trend towards a lower degree of annular area sizing (7.0% [2.3-13.9%] in LVOT-CA patients vs. 9.9% [2.7-15.6%] in patients without LVOT-CA, p=0.08). The frequency of <0% undersizing was comparable in both groups. Predilatation was performed more frequently in patients with LVOT-CA (51.4% vs. 29.4%, p<0.001). Overall, four patients had aortic annular injury following TAVR (mean annular area sizing of 15.5%). Three of the latter patients had LVOT-CA (Figure 2A, Figure 2B). The LVOT-CA group had a significantly higher rate of post-TAVR pacemaker implantation compared to the no LVOT-CA group (23.0% vs. 6.4%, p<0.001). There was no difference in the degree of annular area sizing between patients who required a pacemaker and those who did not require a pacemaker (8.9% [3.9-14.9] vs. 8.3% [2.2-15.6], p=0.78).
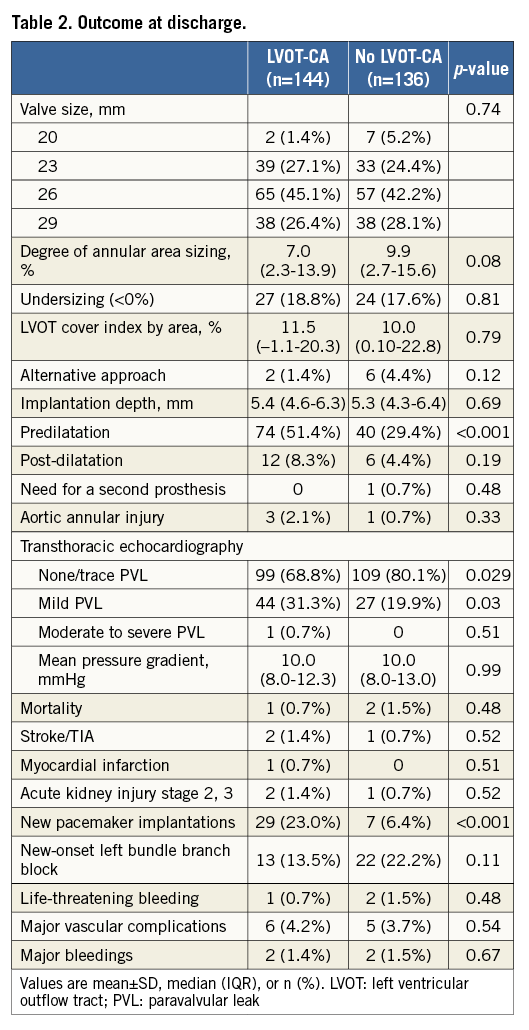
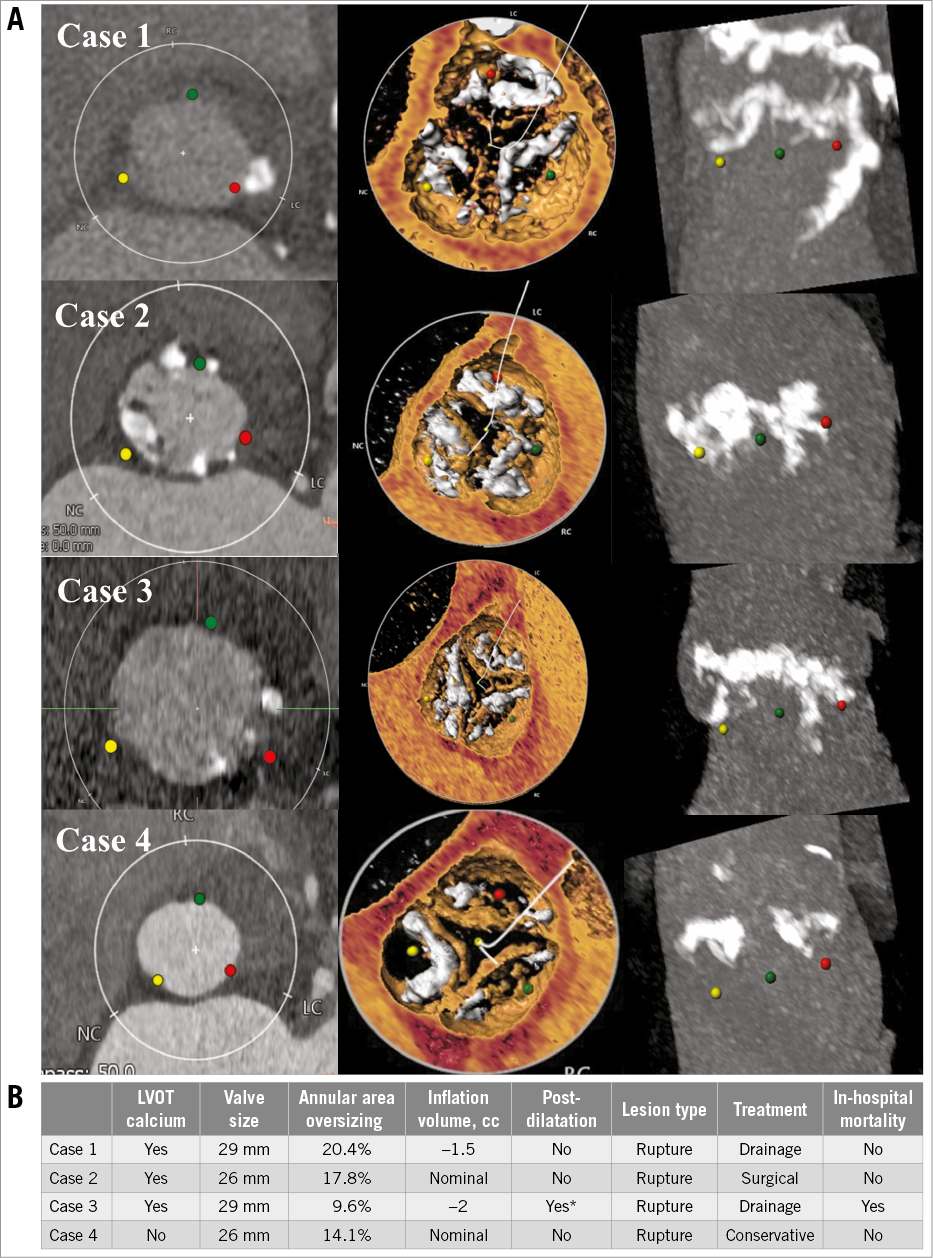
Figure 2. Aortic root and sizing characteristics, procedural variables, and outcomes in patients with aortic annular injury. A) MDCT reconstruction of the aortic root in four different patients who were undergoing evaluation pre-TAVR. B) Sizing characteristics, procedural variables, and outcomes in patients with annular injury. Of the four cases of aortic annular injury, three of the patients had LVOT calcification. * Transoesophageal echocardiography revealed moderate PVL after implantation. A second inflation (post-dilatation with Commander valve delivery system [Edwards Lifesciences]) was performed to the nominal inflation volume. LVOT: left ventricular outflow tract; PVL: paravalvular leak
PARAVALVULAR LEAK
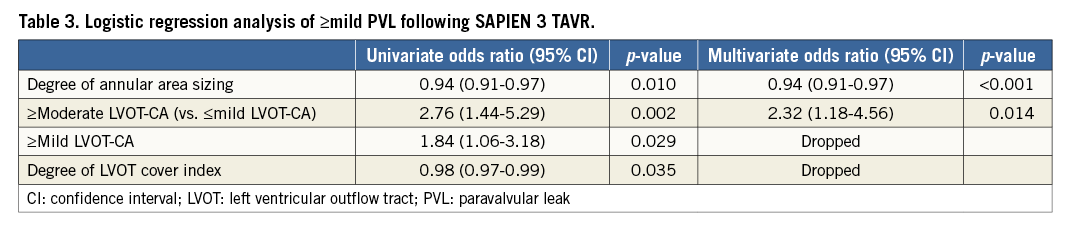
Overall, ≥mild PVL was present in 25.7% of the patients following TAVR. Only one patient had moderate PVL (LVOT-CA present, –9.0% undersizing). Lower degrees of annular area sizing and LVOT decreased cover index were associated with ≥mild PVL (annular area sizing: 4.4% [–1.0-10.8] vs. 9.7% [3.2-16.1], p=0.001; LVOT cover index: 4.1% [–5.8-20.1] vs. 12.1% [1.5-21.5], p=0.027). Although there was no difference in aortic valve calcium volume between patients with or without PVL (p=0.31), patients with ≥mild PVL had a higher prevalence of LVOT-CA, especially those with moderate or severe LVOT-CA (29.2% vs. 13.0%, p=0.002). Using ROC curve analysis for the prediction of ≥mild PVL, 7.2% annular area oversizing was identified as the best threshold in patients with LVOT-CA (area under the curve 0.71, 95% confidence interval [CI]: 0.61-0.80, p<0.001, sensitivity 73.8%, specificity 57.9%) (Figure 3A). Overall, 50.7% (73 patients) of the patients had a degree of annular area sizing lower than 7.2%. Conversely, for patients with no LVOT-CA, the degree of annular area sizing appeared unrelated to PVL ≥mild [9.0% (0.5-13.8) in PVL vs. 10.7% (2.8-17.3) in no PVL, p=0.21] (area under the curve 0.58, p=0.21) (Figure 3B). In patients with >20% annular area sizing, mild PVL was observed in 11.1% of the patients with LVOT-CA, and in 11.8% of the patients without LVOT-CA. In the setting of <0% undersizing, regardless of LVOT-CA, increased additional inflation volume was found to be associated with decreased ≥mild PVL: this ranged from 77.8% (nominal volume) to 25.0% (over 10% additional volume) for LVOT-CA and from 30.0% (nominal volume) to 14.8% (over 10% additional volume) for no LVOT-CA (Figure 4). In a logistic regression model, independent predictors of ≥mild PVL included lower degree of annular area sizing (odds ratio [OR] 0.94, 95% CI: 0.91-0.97, p<0.001) and ≥moderate LVOT-CA (OR 2.32, 95% CI: 1.18-4.56, p=0.014) (Table 3).
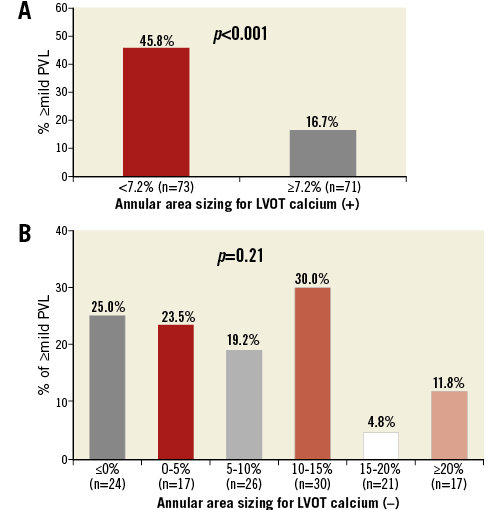
Figure 3. Incidence of ≥mild PVL according to annular area sizing. A) Using ROC curve for prediction of ≥mild PVL, 7.2% sizing by area was identified to be the best threshold in patients with LVOT calcium. An optimally oversized SAPIEN 3 device had lower rates of ≥mild PVL in these patients. B) Conversely, regardless of an increased area sizing, patients without LVOT calcification had little change in rates of PVL. LVOT: left ventricular outflow tract; PVL: paravalvular leak
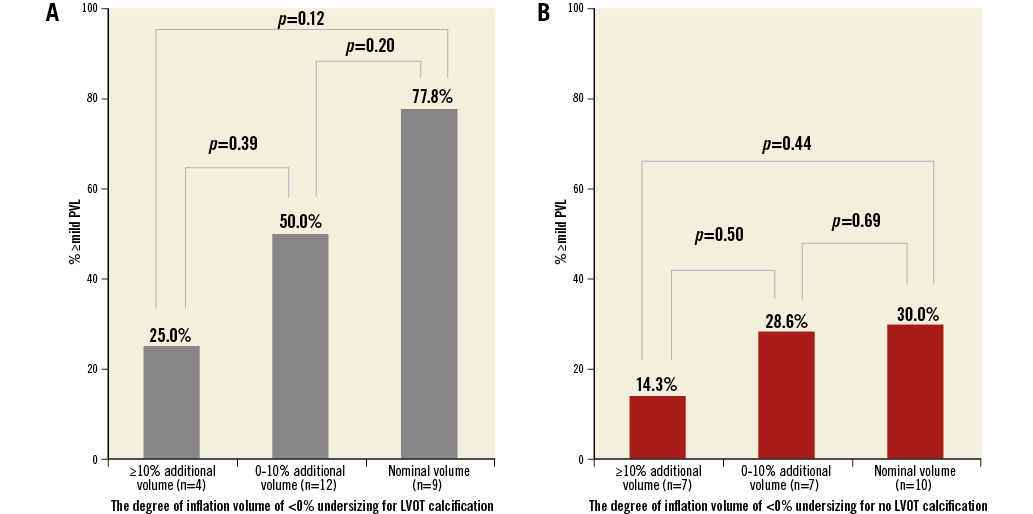
Figure 4. The relationship between degree of inflation volume and ≥mild PVL rates in patients with <0% undersizing. A) Degree of inflation volume of <0% undersizing for LVOT calcification. B) Degree of inflation volume of <0% undersizing for no LVOT calcification. Increased inflation volume was associated with decreased ≥mild PVL rates after S3-TAVR. The degree of additional inflation volume was calculated in the following way: (inflation volume-nominal volume/nominal volume)×100. Patients with underinflation volume were excluded. LVOT: left ventricular outflow tract; PVL: paravalvular leak
ONE-YEAR SURVIVAL FOR PATIENTS WITH ≥MILD PVL VS. PATIENTS WITH NO PVL AFTER TAVR
At a median follow-up of 465 days (interquartile range 265-680 days), a total of 20 patients had died. Five of these patients had ≥mild PVL after TAVR while 15 patients had no PVL. Kaplan-Meier analysis of cumulative survival between these two groups on the basis of ≥mild PVL is shown in Figure 5A. One-year survival was 92.8% in patients with no PVL versus 93.1% for patients with ≥mild PVL following TAVR (p=0.91). After subdividing the ≥mild PVL group according to LVOT-CA, patients with ≥mild PVL who had ≥moderate LVOT-CA had decreased survival at one year compared to patients with ≥mild PVL who had no or mild LVOT-CA (81.0% vs. 98.0%, p=0.010) (Figure 5B). On the other hand, in patients without PVL, there was no difference in survival between ≥moderate LVOT-CA and no or mild LVOT-CA (Figure 5B).
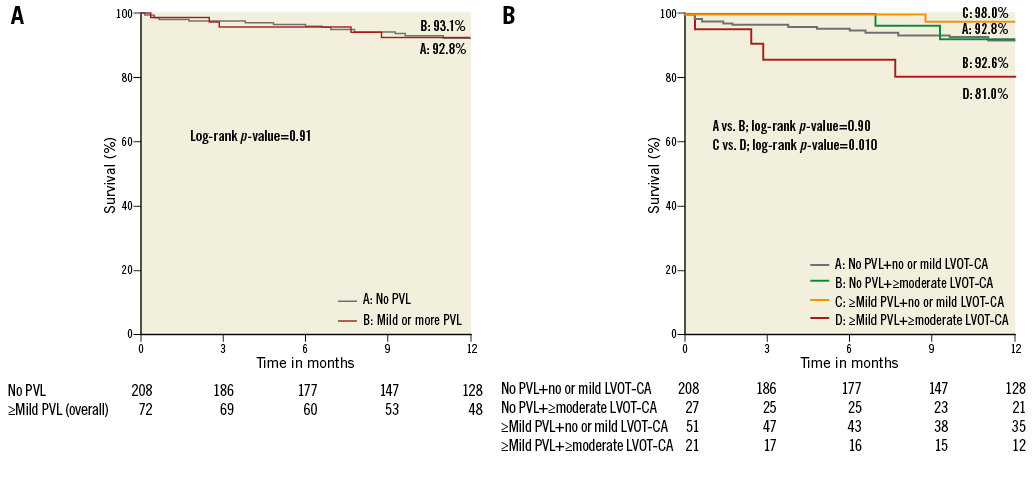
Figure 5. Kaplan-Meier survival curves according to PVL and LVOT-CA. One-year survival was similar between patients with or without ≥mild PVL (A). Patients with ≥moderate LVOT-CA who had ≥mild PVL had lower survival compared to patients with ≥mild PVL and no or mild LVOT-CA (B). LVOT-CA: left ventricular outflow tract calcification; PVL: paravalvular leak
Discussion
LVOT-CA was previously found to be associated with increased PVL rates or risk of aortic annular injury4-6. Nonetheless, there are scarce data available regarding the influence of annular area sizing on PVL rates following S3-TAVR in patients with LVOT-CA.
In a logistic regression model, higher grades of LVOT-CA and a lower degree of annular area sizing were found to be significant predictors of ≥mild PVL. The degree of annular area sizing was found to be unrelated to PVL in patients without LVOT-CA. Prior studies have demonstrated that leaflet calcification, or LVOT-CA, was predictive of PVL with old-generation balloon-expandable valves5,6. In the present study, only the region of LVOT-CA was found to be an independent predictor of PVL. Contrary to previous reports5,6,9, there was no trend for higher PVL rates among patients with increased leaflet calcification. A previous study published by our group, using MDCT post TAVR, reported that outward expansion of the S3-THV is greater than that of the SAPIEN XT THV, especially at the level of the native aortic annulus and LVOT10. John et al demonstrated that interaction between the calcified wall and device frame induces gaps, which in turn may cause several diastolic PVL jets11. Therefore, unlike in the SAPIEN XT THV, if there are severely calcified valve leaflets but with no LVOT-CA, the more expanded annulus or inflow frame of the S3-THV essentially covers the potential areas of PVL. Moreover, the covered skirt feature of this device, designed to reduce PVL, particularly contributed in patients with no LVOT-CA. In the setting of LVOT-CA, there may be a space that remains between the LVOT wall and the THV frame. Seiffert et al demonstrated that increased LVOT-CA predicts PVL jet volume5. Furthermore, it is conceivable that there is a contrecoup effect, resulting in the device being directed away from the most calcified area. Consequently, the covered skirt feature may have diminished efficacy, especially with suboptimally oversized S3-THV for high-grade LVOT-CA. With regard to valve depth, high implantation was not related to PVL, consistent with the findings of a previous study12. Interestingly, patients with ≥20% annular area oversizing had similar PVL rates (approximately 11%) regardless of LVOT-CA. A possible reason for the latter may be that the upper limit of expansion of the S3 frame may be under 20% in terms of annular area oversizing, and greater annular area oversizing may cause acute THV frame recoil.
The present study did not have sufficient power to show the interaction between annular area sizing and the risk of aortic annular injury due to the limited number of this catastrophic event. However, aortic annular injury occurred mostly in patients with lower than 20% annular area oversizing. Barbanti et al demonstrated that >20% annular area oversizing and LVOT-CA predicted an increased risk of aortic annular injury during earlier-generation balloon-expandable TAVR4. Our findings indicate that excessive annular area oversizing of S3-THV that is related to annular injury may have a lower threshold (15%) following S3-TAVR. S3-THV has a flared inflow morphology, whereas the prior-generation device, SAPIEN XT, has relatively constrained inflow morphology post TAVR10. Conversely, LVOT dimensions are usually smaller than the annulus dimensions if LVOT-CA is present; therefore it is acceptable that aortic annular injury may be caused by <20% annular area oversizing, unlike in the SAPIEN XT. This hypothesis will need further validation in a larger multicentre cohort.
One-year survival was similar between patients with and without ≥mild PVL following S3-TAVR, parallel to the recently published report from the PARTNER II S3 trial1. Interestingly, when subdividing these patients according to LVOT-CA, we found that patients with ≥moderate LVOT-CA and ≥mild PVL following TAVR had lower survival compared to patients with no or mild LVOT-CA who had ≥mild PVL. Increased LVOT-CA was significantly associated with the severity of PVL5. Therefore, the potential mechanism explaining the results of the present study is probably that mild PVL in patients with high grades of LVOT-CA might progress rapidly to moderate or severe PVL due to suboptimally oversized S3-THV or recoil. Our results therefore indicate that optimal annular sizing and minimisation of PVL following TAVR are extremely important in the subgroup of patients with moderate or higher LVOT-CA.
According to the present analysis, in patients with high grades of LVOT-CA, even mild PVL should be avoided. Aortic annular injury was mostly observed with >14% annular area oversizing (average of 15.5%), although the number of aortic annular injuries was low (four cases). Nonetheless, the results of the present study imply that annular area oversizing of between 7% and 15% may be optimal for S3-TAVR in patients with LVOT-CA. In the setting of no LVOT-CA, –5% undersizing is perhaps the desirable target, as suggested by Webb et al3. Our findings also demonstrate that over 10% additional inflation volume decreased ≥mild PVL compared to nominal inflation volume, particularly in the presence of LVOT-CA. If there is a low risk for aortic annular injury, the addition of ≥10% volume in the delivery balloon for undersized (<0%) cases should be considered, particularly in patients with LVOT-CA.
In the present study, more than half of the study subjects had LVOT-CA. Atherosclerosis contributes to the development of calcification; however, the prevalence of diabetes was lower in the LVOT-CA group. A combination of other factors (e.g., age) may have contributed to higher grades of calcification among these patients.
Finally, the REPRISE II study reported promising results for the next-generation self-expanding Lotus™ valve (Boston Scientific, Marlborough, MA, USA) in TAVR13. Head-to-head comparisons with S3-TAVR will be needed to determine the influence of LVOT-CA on annular injury, PVL and mortality in these patients.
Study limitations
Several limitations of the present study should be acknowledged. The study represents a retrospective, single-centre experience. In addition, the findings are subject to selection bias and confounding factors. PVL was not assessed by an echocardiography core laboratory. Given the limited number of patients with ≥moderate PVL, we were not able to use this as an outcome related to mortality.
Conclusions
The degree of annular area sizing in patients with ≥moderate LVOT-CA was associated with increased rates of ≥mild PVL following S3-TAVR. Furthermore, ≥mild PVL in patients with moderate to severe LVOT-CA was associated with increased one-year mortality. Therefore, optimal annular area sizing is essential in order to reduce PVL, especially in patients with moderate to severe LVOT-CA.
| Impact on daily practice Even mild PVL might be associated with increased mortality following S3-TAVR, if high grades of LVOT-CA are present. Therefore, optimally oversized S3-TAVR should be considered to reduce PVL in patients with LVOT-CA. |
Conflict of interest statement
H. Jilaihawi is a consultant for Edwards Lifesciences, St. Jude Medical, and Venus MedTech. R. Makkar has received grant support from Edwards Lifesciences and St. Jude Medical, is a consultant for Abbott Vascular, Cordis, and Medtronic, and holds equity in Entourage Medical. The other authors have no conflicts of interest to declare.

