
Abstract
Aims: Paravalvular leak (PVL) remains an important issue in TAVI. The Edwards SAPIEN 3 (S3) valve has reduced PVL but in up to one third of patients mild leak remains. Our study aimed to identify predictors of mild PVL after TAVI with the S3 valve.
Methods and results: From October 2015 to May 2017, 122 consecutive patients underwent S3 TAVI for symptomatic severe aortic stenosis. Thirty-three patients with mild PVL on transthoracic echocardiography at 30-day follow-up were compared to 89 with none/trace PVL. Thirty-day mortality was 2.5% (n=3), with zero stroke and major vascular complications. There were no differences between the two groups in patient characteristics, annular and left ventricular outflow tract (LVOT) sizing, distribution and severity of annular calcification, valve implantation technique, post-dilatation and implant depth. Mild PVL was associated with higher annular eccentricity (p=0.04) and moderate-severe LVOT calcification (p=0.03). Independent predictors of mild PVL were LVOT eccentricity (OR 1.05 per % ellipticity, 95% CI: 1.02-1.09, p=0.005), discordant sizing (OR 3.08, 95% CI: 1.20-7.90, p=0.02) and three-leaflet calcification (OR 13.3, 95% CI: 2.66-66.7, p=0.002).
Conclusions: LVOT eccentricity and discordant sizing predict PVL after S3 TAVI. Further studies are needed to understand their mechanism and significance.
Abbreviations
LBBB: left bundle branch block
LVOT: left ventricular outflow tract
MDCT: multidetector computed tomography
PVL: paravalvular leak
ROC: receiver operating characteristic
S3: SAPIEN 3
TAVI: transcatheter aortic valve implantation
TEE: transoesophageal echocardiography
TTE: transthoracic echocardiography
TVT: transcatheter valve therapy
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve implantation (TAVI) has become the preferred treatment in patients with symptomatic severe aortic stenosis with high or prohibitive surgical risks1. More recently, TAVI was also approved in intermediate-risk patients2,3, and randomised trials in low-risk patients are underway. Yet, paravalvular leak (PVL) remains a concern even with newer-generation valves. The incidence of moderate or greater (≥3+) PVL in the latest-generation balloon-expandable Edwards SAPIEN 3 (S3) transcatheter valve (Edwards Lifesciences, Irvine, CA, USA) is reduced compared to its predecessors3-5. However, at least 30% of patients in the PARTNER (Placement of AoRtic TraNscathetER valve) II S3 trial developed mild (2+) PVL4-6. As TAVI expands to lower-risk patients, the longer-term impact of PVL may become relevant7,8. Our study therefore aimed to identify predictors of mild PVL in S3 TAVI.
Methods
PATIENT POPULATION
From October 2015 to May 2017, 122 consecutive patients underwent S3 TAVI for symptomatic severe aortic stenosis. Patient data were prospectively collected in the American College of Cardiology/Society of Thoracic Surgeons (ACC/STS) US Transcatheter Valve Therapy (TVT) registry and retrospectively analysed. Our study was approved by the Institutional Review Board and patient consent was waived.
PREPROCEDURAL ASSESSMENT
All patients underwent transthoracic echocardiography (TTE) and multidetector computed tomography (MDCT) to evaluate aortic root dimensions, morphology and calcification, except two with severe kidney disease who underwent non-contrast CT and transoesophageal echocardiography (TEE).
MDCT ANALYSIS OF THE AORTIC ROOT
Aortic root analysis was performed at the end-systolic phase using 3mensio valve software, version 8.1 (Pie Medical Imaging, Maastricht, the Netherlands) to determine the largest dimensions at the annulus and left ventricular outflow tract (LVOT) (5 mm below the annulus, within the landing zone of most S3 implantations). Annular and LVOT eccentricities were defined as (1-minimal diameter/maximal diameter)×100%. Locations and severity of annular, LVOT and leaflet calcifications were semiquantitatively evaluated (Figure 1)9. Prosthesis sizing was according to manufacturer recommendations. S3 sizing to LVOT was calculated as ([prosthesis area/LVOT area]–1)×100%. In cases where the anatomy lay between two sizes, balloon valvuloplasty with aortography was used to finalise valve size10. Annular sizing was compared to LVOT sizing and defined as “concordant” if the S3 was either oversized or undersized to both planes, and “discordant” if the S3 was oversized to one plane and undersized to the other. We derived four sizing combinations as:
– Concordant-undersized: annulus-undersized/LVOT-undersized
– Concordant-oversized: annulus-oversized/LVOT-oversized
– Discordant-undersized: annulus-undersized/LVOT-oversized
– Discordant-oversized: annulus-oversized/LVOT-undersized
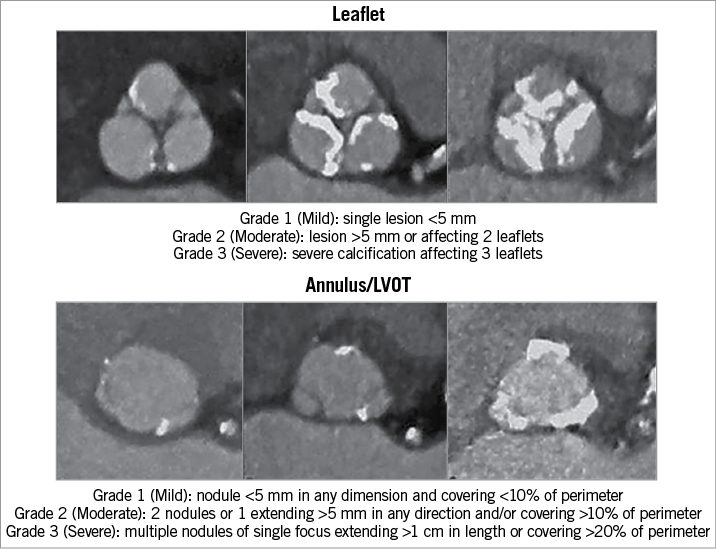
Figure 1. Semiquantitative evaluation of aortic root calcification. Semiquantitative evaluation of aortic valve leaflet, annulus and left ventricular outflow tract calcifications performed in our study, as described by Barbanti and colleagues9.
S3 IMPLANTATION TECHNIQUE AND FOLLOW-UP
The S3 was implanted using standard techniques. In brief, balloon valvuloplasty using an Edwards balloon with aortography was performed in all cases10. Balloon volume was based on percent oversizing and aortic root calcification. Valve positioning was based on the top of the S3 frame relative to the sinotubular junction (STJ) and position of the bottom of the centre marker. When feasible, the marker bottom was positioned at or above the annulus (>0 mm) for a more aortic implantation. Valve deployment was preceded by aortography with a slow inflation technique. PVL severity was determined by TTE using the Valve Academic Research Consortium 2 (VARC-2) criteria11, invasive haemodynamics and aortography. Post-dilatation was performed if PVL was mild or greater, with consideration of risk factors for annular injury.
The procedure was performed under conscious sedation by default with TTE evaluation by experienced echocardiographic sonographers. Patients at high procedural risk underwent general anaesthesia with TEE. S3 implant depth was analysed as previously described12. Thirty-day follow-up was 100% complete. PVL was graded by an expert echocardiographer (T. Dutta), blinded to procedure and valve selection. Outcomes were reported using the VARC-2 and US TVT Registry definitions.
STATISTICAL ANALYSIS
The clinical, anatomic, and procedural characteristics of patients with mild or greater PVL on TTE at 30 days were compared to those with none/trace PVL. Continuous variables were reported as means with standard deviations, while categorical variables were reported as proportions. Differences were detected using the t-test for continuous variables and chi-squared or Fisher’s exact test for categorical variables. Univariate predictors of PVL were identified. In multivariate analysis, all univariate predictors with p<0.10 were considered, and only those with p<0.05 were included in the final model. Based on the number of events in our patient cohort, no more than three variables were included in the final model. To evaluate increasing odds associated with our multivariate predictors in a binary fashion, we assessed continuous variables included in the final multivariate model at incremental cut-offs via the c-statistic derived from the receiver operating characteristic (ROC) curve in logistic regression modelling. All statistical tests were two-tailed with p<0.05 considered significant. Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
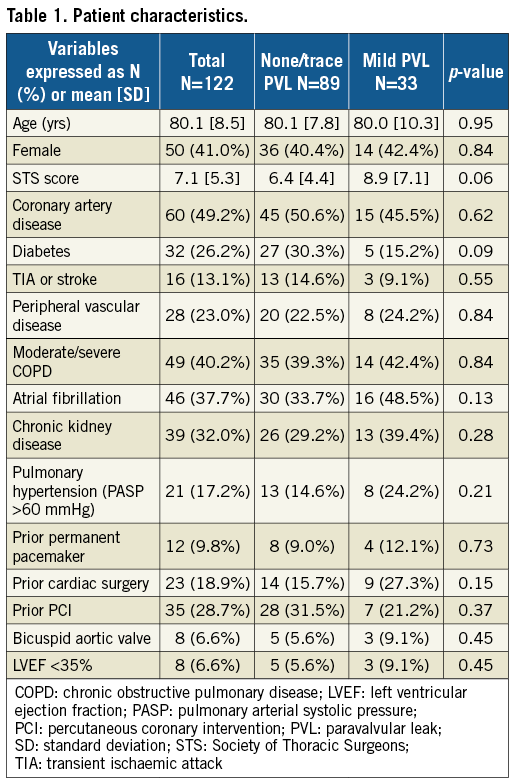
Patient characteristics are listed in Table 1. Mild PVL was seen in 27.0% of patients and none/trace in 73.0%. Mean age was 80.1±8.5 years and 41.0% were female. The STS score averaged 7.1±5.3%. There were no differences in patient characteristics between the two groups.
ANATOMIC CHARACTERISTICS
There were no differences in annular and LVOT dimensions between the PVL groups (Table 2). However, the mild PVL group had greater annular (29.2% vs. 25.7%, p=0.04) and LVOT eccentricities (43.4% vs. 36.1%, p=0.008). Increasing annular and LVOT eccentricity was associated with more mild PVL (Figure 2), but mild PVL was not associated with decreasing annular or LVOT sizing (Figure 3). There were no differences in mild PVL among valve sizes with increasing annular and LVOT eccentricities (Supplementary Figure 1). The mild PVL group was associated with more three-leaflet calcification (90.9% vs. 61.8%, p=0.002), severe leaflet calcification (36.4% vs. 16.9%, p=0.02) and moderate-severe LVOT calcification (36.4% vs. 18.0%, p=0.03).
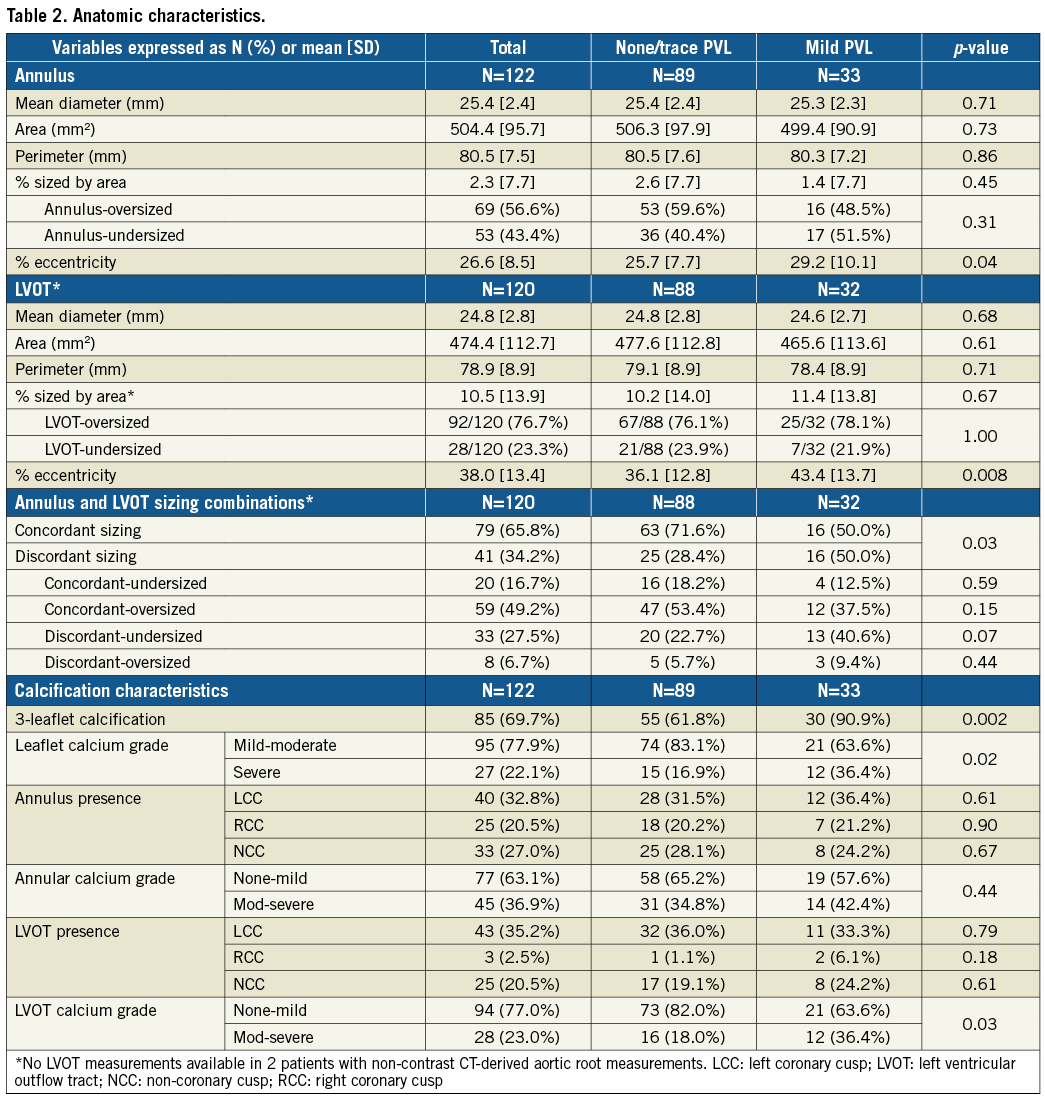
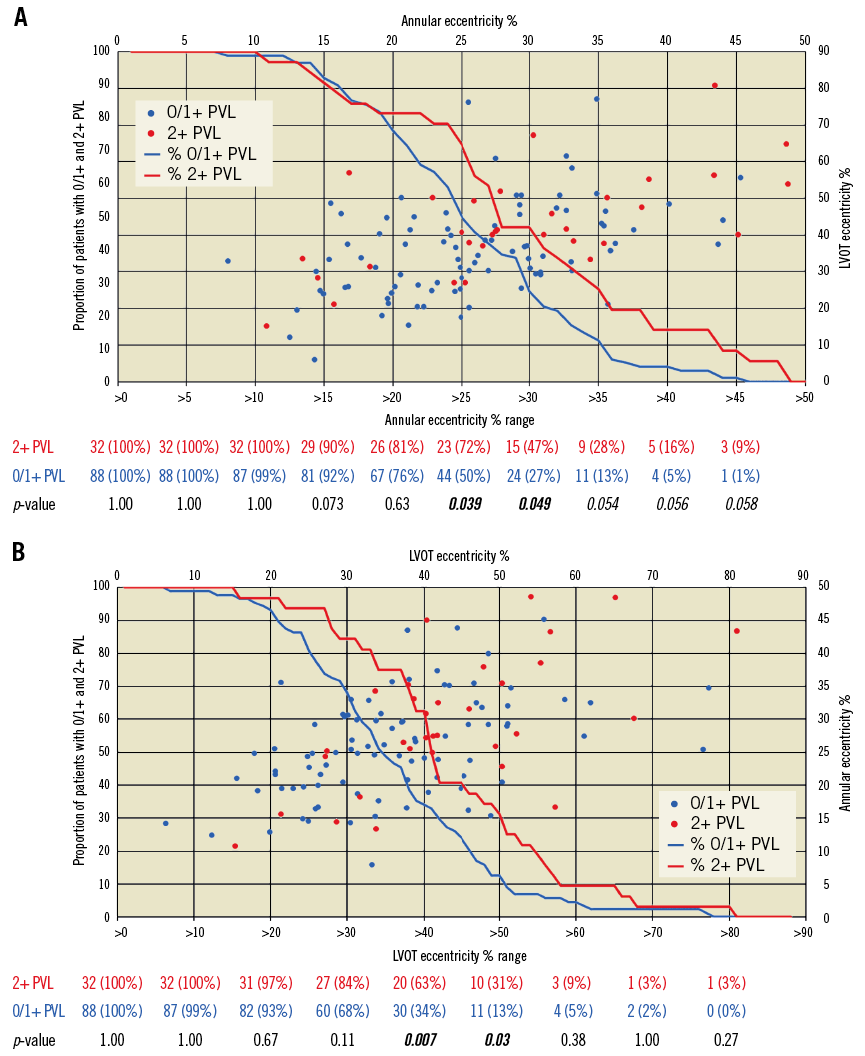
Figure 2. Incidence of mild paravalvular leak with annular and LVOT eccentricity. Analysis of relationships between annular (A) and LVOT (B) eccentricity in association with none/trace (0/1+) and mild (2+) paravalvular leak. Increasing annular and LVOT eccentricity was associated with more mild PVL (A & B). LVOT: left ventricular outflow tract; PVL: paravalvular leak
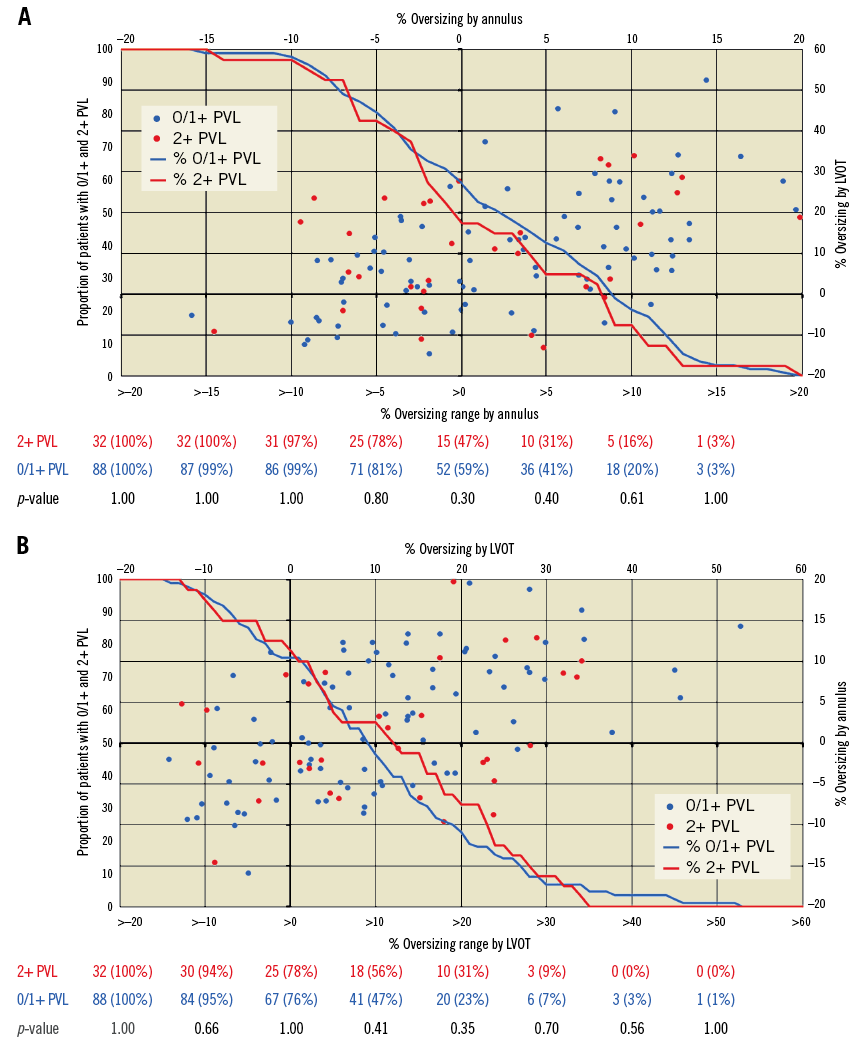
Figure 3. Incidence of mild paravalvular leak with annular and LVOT prosthesis sizing. Analysis of relationships between SAPIEN 3 sizing to annulus (A) and LVOT (B) in association with none/trace (0/1+) and mild (2+) paravalvular leak. Mild PVL was not associated with increasing annular or LVOT sizing (A & B). LVOT: left ventricular outflow tract; PVL: paravalvular leak
Figure 4 shows the distribution of PVL in concordant-sized, discordant-sized and the four annulus-LVOT sizing combinations. Although 43.4% were annulus-undersized (of which 20 [37.7%] were concordant-undersized and 33 [62.3%] discordant-undersized), our sizing criteria were all within manufacturer recommendations and targeted to <20% to annulus to reduce risks of annular injury and heart block. There were no differences in annular undersizing (p=0.31) between groups. Annulus-undersized patients had more balloon overfilling (71.7% vs. 27.5%, p<0.001) and post-dilatation (49.1% vs. 31.9%, p=0.06) than annulus-oversized, although no differences in balloon overfilling (p=0.31) and post-dilatation (p=0.40) were seen between groups. The mild PVL group had more discordant sizing (50.0% vs. 28.4%, p=0.03), and there were more mild PVL with discordant sizing overall (39.0% in discordant-sized vs. 20.3% in concordant-sized), and within the annulus-undersized subgroup (39.4% in discordant-undersized vs. 20.0% in concordant-undersized).
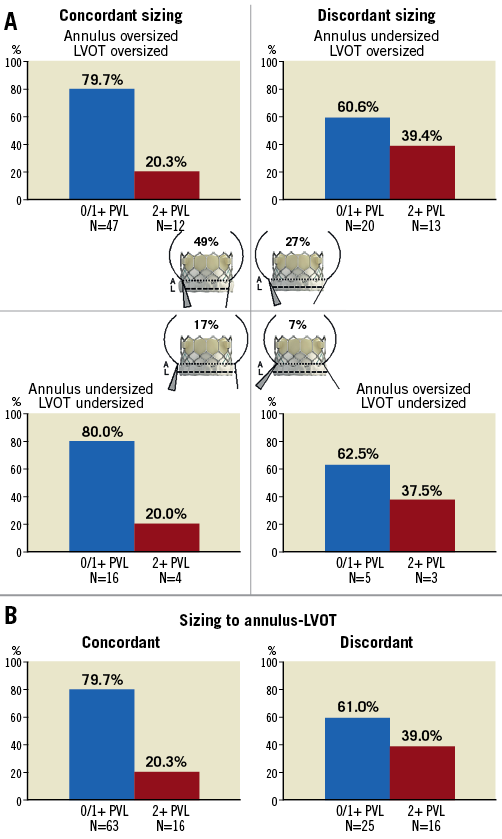
Figure 4. Incidence of mild paravalvular leak (PVL) across annular and LVOT sizing composites. Distribution of patients with none/trace vs. mild PVL after SAPIEN 3 TAVI across four different annulus and LVOT sizing combinations (A) and between concordant and discordant sizing (B). Discordant sizing was predictive of mild PVL. 0/1+: none/trace; 2+: mild; LVOT: left ventricular outflow tract
PROCEDURAL CHARACTERISTICS
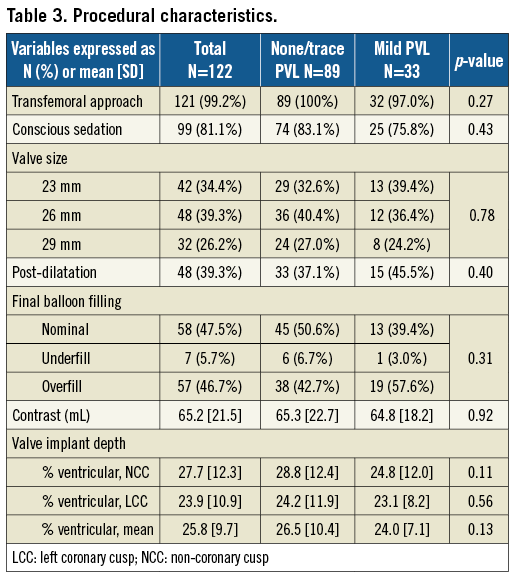
All procedures were successful, with the transfemoral approach in 121 patients and left axillary in one (Table 3); 81.1% had conscious sedation, with two conversions to general anaesthesia due to patient anxiety and haemodynamic instability. No differences in S3 size distribution, balloon filling, contrast use and implant depth were observed.
OUTCOMES
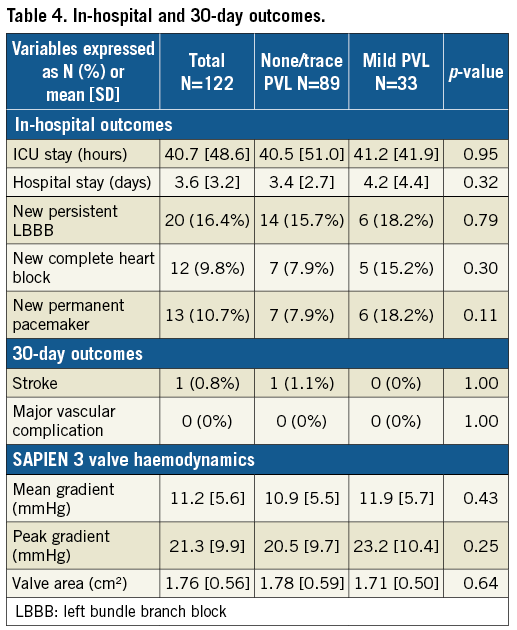
There were no differences in ICU and hospital length of stay (Table 4). One in-hospital mortality occurred in a patient who presented with cardiogenic shock but died of persistent renal failure and withdrawal of care. There were two 30-day mortalities, one from primary cardiac failure with a baseline ejection fraction of 15%, and one from intracranial haemorrhage after a fall. No stroke or vascular complication occurred, and 20 patients (16.4%) developed new left bundle branch block (LBBB). Of the 13 patients (10.7%) who required a new permanent pacemaker, nine had pre-existing right bundle branch block (RBBB). Mean follow-up was 10.7±5.8 months (range 0.1-20.8). All surviving patients had NYHA class improvements.
PREDICTORS OF MILD PARAVALVULAR LEAK
There was no moderate or greater PVL in our cohort. Mild PVL was associated with increasing annular eccentricity (OR 1.05, 95% CI: 1.001-1.10, p=0.04) and moderate-severe LVOT calcification (OR 2.61, 95% CI: 1.07-6.36, p=0.03) (Table 5). The area under the ROC curve for the multivariate model was robust with a 0.80 c-statistic. Independent predictors of mild PVL were three-leaflet calcification (OR 13.3, 95% CI: 2.7-66.7, p=0.002), LVOT eccentricity (OR 1.05 per % LVOT eccentricity, 95% CI: 1.02-1.09, p=0.005), and discordant sizing (OR 3.08, 95% CI: 1.20-7.90, p=0.02).
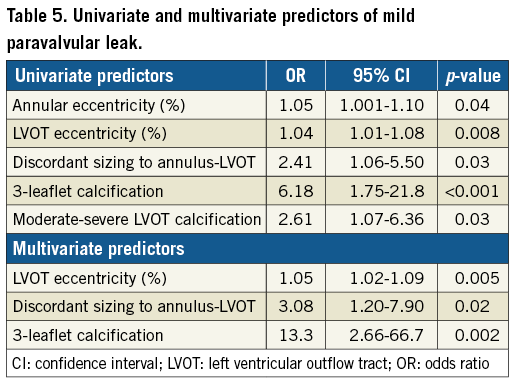
We identified LVOT eccentricity >40% as the most predictive binary variable for the associated continuous variable based on the most robust area under the ROC curve in univariate modelling (0.63). Composed of this variable and discordant sizing and three-leaflet calcification, we created a composite variable. Compared to having 0 or 1 of these predictors, a patient with two predictors had a 7.1-fold higher odds, and with all three had an 11.6-fold higher odds of developing mild PVL (p<0.001).
Discussion
Our main findings are: 1) mild PVL was seen in 27.0% of our S3 TAVI, with no differences in valve sizing, balloon filling, implantation technique, post-dilatation and implant depth; 2) annular and LVOT eccentricities, three-leaflet and moderate-severe LVOT calcification and discordant sizing were associated with PVL; 3) three-leaflet calcification, LVOT eccentricity and discordant sizing were independent predictors of PVL.
PVL remains an ongoing issue in TAVI. Mild PVL predicted mortality6,7,13 with the SAPIEN/XT but not the S35. More recent PARTNER 2A intermediate-risk data showed no higher two-year mortality with mild PVL in SAPIEN/XT2. Nonetheless, the longer-term impact of mild PVL on left ventricular remodelling and survival remains unknown in lower-risk patients. PVL predictors include valve undersizing, implant depth and aortic root calcification6,14-17. In the S3, less oversizing and annular/LVOT calcification were associated with PVL15-18. Compared to the PARTNER II intermediate-risk trial17, our averaged annular oversizing was less at 2.3±7.7% with fewer patients (55.8%) oversized. Yet, our mild PVL rate was only 27.0% with none moderate/severe, consistent with those reported in S3 studies3-5,15-18,20. This could be due to our higher balloon overfilling and post-dilatation rates4. Similar to other studies14,17,18, three-leaflet and moderate-severe LVOT calcification were associated with PVL, although our evaluation of calcification was limited by its semiquantitative nature.
LVOT ECCENTRICITY: INDEPENDENT PREDICTOR OF PVL
Eccentricities at both annulus and LVOT were associated with PVL, and increasing LVOT eccentricity was an independent predictor of PVL. This result has not been reported and was somewhat surprising given that balloon-expandable valves are known to circularise the annulus and LVOT19. It is conceivable that, in more elliptical LVOT, the S3 while stretching the LVOT short axis may not foreshorten the long axis sufficiently to seal against the LVOT. The long axis of the LVOT typically spans from non-coronary to left coronary sinuses, with a significant portion comprised of the aortomitral curtain. In undersized LVOT, the aortomitral curtain segment of the LVOT, being fibrous in nature, may not seal as well against the S3 frame after circularisation, compared to the muscular portion of the LVOT, which would provide better sealing. This hypothesis may be supported by Khalique and colleagues, who found that most of the PVL were located at non-coronary and left coronary portions of the annulus14.
SIZING MISMATCH OF ANNULUS-LVOT: CONCEPT OF THE SAPIEN 3 SEAL ZONE
Unlike previous reports6,15-17, annular undersizing did not predict PVL in our study, but discordant sizing did. This is the first study to have examined LVOT anatomy in predicting PVL in balloon-expandable TAVI. We conceptualised a seal zone at and below the annulus to explain the impact of discordant sizing on PVL (Figure 5). It is conceivable that patients with discordant sizing have a more heterogeneous landing surface with a shorter seal zone (Figure 4A, Figure 5), that may seal less optimally against the S3 frame after expansion. Concordant sizing provides a more homogenous landing surface with a longer seal zone that maintains contact more optimally with the S3 frame (Figure 5). TEE to localise PVL and MDCT to examine valve geometry in relation to aortic root anatomy may help to evaluate the above hypothesis.
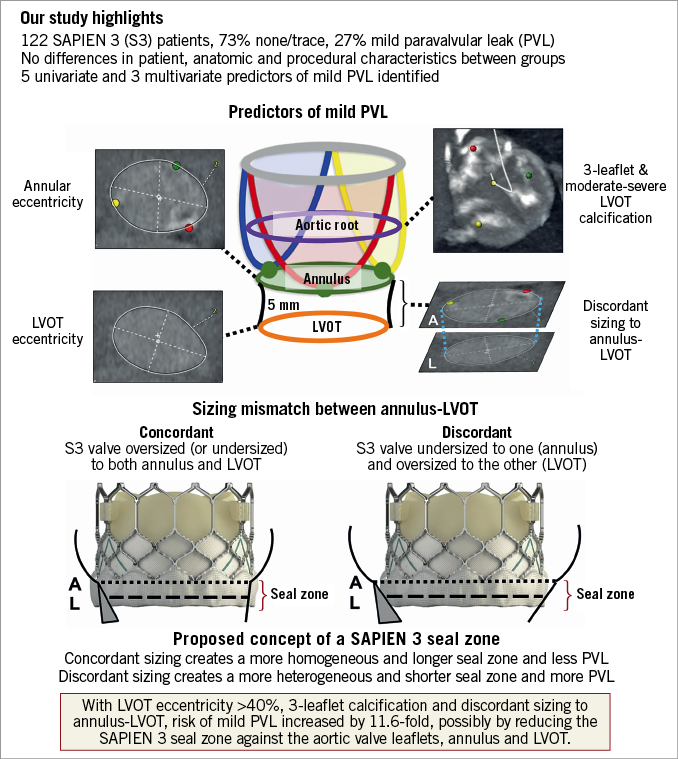
Figure 5. Predictors of paravalvular leak after SAPIEN 3 TAVI.
Previous studies have recommended 1-8% oversizing as optimal to minimise PVL in S3 TAVI15,17,18. Fifty-three percent (53%) of our 122 patients who were annulus-undersized had more balloon overfilling and post-dilatation, and those with concordant undersizing developed less PVL. It is conceivable that, when both annulus and LVOT are undersized, balloon overfilling and post-dilatation can more uniformly expand the S3 frame, to create a longer seal zone to minimise PVL. Interestingly, valve size had no effect on concordant versus discordant sizing, and PVL occurred more frequently in discordant sizing across all valve sizes (Supplementary Figure 2).
The clinical impact of discordant sizing resulting in a shorter, more heterogeneous seal zone in S3 TAVI remains to be seen. Our group is currently evaluating different LVOT morphologies and their relationship to discordant sizing to quantify LVOT-annular mismatch and further the present study. Of note, there was no difference in the ratio of annulus to LVOT area between the none/trace and mild PVL groups (1.07±0.11 vs. 1.10±0.14, p=0.26), but discordant sizing was an independent predictor of PVL.
ESCALATING RISK OF PVL WITH MULTIPLE PREDICTORS
When LVOT eccentricity was analysed as a binary variable, having more than one independent predictor increased the odds of PVL after S3 TAVI. LVOT eccentricity >40%, three-leaflet calcification and discordant sizing increase the risk of PVL by 11.6-fold. The clinical implication of this large risk magnitude is unclear. What we suggest is that, during case planning, if two to three of the above risk factors are present, we would probably expect PVL after S3 implantation. In the absence of high-risk root anatomy, post-dilatation to reduce PVL may be beneficial, but residual mild PVL may be expected, given the underlying anatomic risk factors. Future innovations (e.g., SAPIEN Ultra) may address this limitation.
Limitations
Our study has limitations. It was retrospective and the sample size was relatively small. However, we feel confident of our multivariate analysis with a c-statistic of 0.80. Unlike a previous study14, calcification was evaluated semiquantitatively. PVL severity was not core lab evaluated and PVL location was not determined. There was no moderate or severe PVL (which has been associated with mortality20), and the longer-term impact of mild PVL remains unknown. Further studies are needed to validate our predictive model in an independent cohort.
Conclusions
As TAVI expands to lower-risk patients, the longer-term impact of mild PVL may become important. We identified LVOT eccentricity and discordant sizing as novel predictors of PVL in S3 implantation. Further studies should aim to understand their mechanism and significance, to minimise PVL in TAVI with balloon-expandable valves.
| Impact on daily practice Paravalvular leak remains an important issue in TAVI. The Edwards SAPIEN 3 valve has reduced PVL but >30% remain with mild PVL. The longer-term impact of mild PVL may be relevant as TAVI expands to lower-risk patients. In this retrospective analysis, mild PVL was associated with higher annular eccentricity and moderate-severe LVOT calcification. Independent predictors of mild PVL were LVOT eccentricity, discordant sizing and three-leaflet calcification. This study calls for a more routine evaluation of the LVOT anatomy, annular and LVOT eccentricity, and annulus-LVOT sizing mismatch when considering TAVI with balloon-expandable valves. |
Conflict of interest statement
G. Tang is a physician proctor for Edwards Lifesciences and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Mild paravalvular leak across valve sizes.
Supplementary Figure 2. Paravalvular leak according to concordant vs. discordant sizing across SAPIEN 3 valve sizes.
To read the full content of this article, please download the PDF.

