Abstract
Background: Left ventricular outflow tract (LVOT) calcification has been associated with worse outcomes in patients undergoing transcatheter aortic valve implantation (TAVI) and may influence the selection of prosthetic valve type.
Aims: We aimed to evaluate the impact of LVOT calcification on outcomes after TAVI with a self-expanding valve (SEV) versus a balloon-expandable valve (BEV).
Methods: Patients of the SOLVE-TAVI trial, randomised to Edwards SAPIEN 3 or Medtronic Evolut R, were divided according to LVOT calcification into no/mild (≤1 calcium nodule extending <5 mm and covering <10% of the LVOT perimeter) and moderate/severe LVOT calcification groups. The primary endpoint was a composite of death, stroke, moderate/severe paravalvular regurgitation, permanent pacemaker implantation and annulus rupture at 30 days. Additional endpoints included all-cause and cardiovascular mortality at 1 year.
Results: Out of 416 eligible patients, moderate/severe LVOT calcification was present in 143 (34.4%). Moderate/severe LVOT calcification was associated with significantly longer fluoroscopy time and higher rates of pre- and post-dilation. Regardless of the LVOT calcification group, there was no significant difference in the primary endpoint associated with the valve type (no/mild LVOT calcification group: SEV 25.0% vs BEV 27.0%; hazard ratio [HR] 1.10, 95% confidence interval [95% CI]: 0.68-1.73; p=0.73 and moderate/severe LVOT calcification group: SEV 25.0% vs BEV 19.4%; HR 0.76, 95% CI: 0.38-1.61; p=0.49), no significant interaction between LVOT calcification and valve type (pint=0.29) and no differences between SEV vs BEV within LVOT calcification groups regarding 1-year all-cause and cardiovascular mortality.
Conclusions: Moderate/severe LVOT calcification was associated with longer fluoroscopy time and an increased need for pre- and post-dilation, but not with a higher incidence of early and mid-term adverse clinical outcomes, regardless of valve type. (ClinicalTrials.gov: NCT02737150)
Introduction
Transcatheter aortic valve implantation (TAVI) has become the method of choice for treating severe aortic valve stenosis in patients with intermediate and high surgical risk and is increasingly being used in patients at low risk123. However, some conditions and parameters currently remain a limitation and pose a challenge to the success of TAVI. Left ventricular outflow tract (LVOT) calcification is considered one of those challenges456. LVOT calcification is associated with a higher risk for annular rupture and paravalvular regurgitation (PVR)578. Both complications are associated with unfavourable early and late outcomes579. Despite recent developments in valve design, PVR remains a significant limitation of TAVI. Furthermore, it is unclear whether there is a risk difference between balloon-expandable and self-expanding valves (BEV or SEV) with regard to short- and long-term clinical outcomes in patients with LVOT calcification. Therefore, we investigated the impact of moderate and severe LVOT calcification on procedural, 30-day and 1-year outcomes post-TAVI comparing BEV with SEV in patients enrolled in the CompariSon of secOnd-generation seLf-expandable Versus Balloon-expandable Valves and gEneral Versus Local Anesthesia in Transcatheter Aortic Valve Implantation (SOLVE-TAVI) trial10.
Methods
Study population
The SOLVE-TAVI trial (ClinicalTrials.gov: NCT02737150) was an investigator-initiated multicentre study conducted at 7 German sites. The study design and main results were published elsewhere1011. In brief, the trial had a 2x2 factorial design to compare the second-generation SEV (Evolut R; Medtronic) to the latest-generation BEV (SAPIEN 3; Edwards Lifesciences) and to compare general anaesthesia to conscious sedation. The trial included 447 patients at high and intermediate risk for conventional surgical aortic valve replacement (SAVR).
Imaging analysis
Multidetector row computed tomography (MDCT) was performed with ECG-gating as per site-specific protocol. MDCT datasets of the heart and the ascending aorta were transferred to the study imaging core lab at the Heart Center Leipzig at the University of Leipzig and analysed independently using dedicated software (3mensio; Pie Medical Imaging).
The annular plane was defined by the 3 basal hinge points of the aortic cusps in the LVOT. LVOT parameters were determined 5 mm below the annular plane. The percent area oversizing was calculated as 100*(nominal transcatheter heart valve [THV] area–LVOT area)/nominal THV area. The nominal THV area was calculated as π*(nominal THV size/2)2. The annular area from systolic CT scans was used, if available.
LVOT calcification was defined according to earlier studies12 and divided into 4 categories: (1) none; (2) mild: the presence of 1 nodule of calcification extending <5 mm in any dimension and covering <10% of the perimeter of the LVOT; (3) moderate: the presence of 2 nodules of calcification or 1 extending >5 mm in any direction or covering >10% of the perimeter of the LVOT; and (4) severe: the presence of multiple nodules of calcification of single focus extending >10 mm in length or covering >20% of the perimeter of the LVOT12. LVOT and annular calcification was also determined quantitatively. A cut-off of 550 Hounsfield units (HU) was used if the luminal attenuation was between 200 and 500 HU, and cut-off of 300 HU if the luminal attenuation was below 200 HU. If the luminal attenuation was above 550 HU, the cut-off was set at 50 HU higher than luminal attenuation13.
The operators performing the LVOT calcification analysis were blinded to clinical events and also to randomised strategy at the time of the image analysis.
Study endpoints
The primary endpoint for this specific LVOT calcification analysis was defined as the composite of all-cause mortality, stroke, moderate or severe PVR, permanent pacemaker (PPM) implantation and annulus rupture at 30-day follow-up. In addition to the initial combined clinical endpoint of the SOLVE-TAVI trial1014, annulus rupture has been included as LVOT calcification is a risk factor for annulus rupture. Secondary endpoints included device time in minutes, total procedure time in minutes, overall and cardiovascular mortality, device success, early safety, clinical efficacy as well as time-related safety at 30 days according to Valve Academic Research Consortium 2 (VARC-2) criteria15. Furthermore, overall and cardiovascular mortality, clinical efficacy and time-related safety according to VARC-2 criteria at 1 year were also reported. PVR was evaluated by echocardiography at 30 days, was graded semi-quantitatively according to VARC-2 criteria and was centrally analysed by an independent core laboratory.
Statistical analysis
Baseline clinical and procedural characteristics were stratified according to the dichotomised LVOT calcification (none or mild vs moderate or severe) and implanted valve. Categorical variables were reported as absolute and relative frequencies and compared using the χ2 or Fisher’s exact test where appropriate. The denominator of proportions may differ because of missing values, which were not imputed. Ordinal variables as well as not normally distributed continuous variables were reported as median and interquartile range and were compared by the Median test or Mann-Whitney U test. Normal distribution was assessed by the Shapiro-Wilk test, and normally distributed variables were compared by the one-way Analysis of Variance (ANOVA). Survival was reported as a Kaplan-Meier percentage and the number of events and compared using the log-rank test. Cox proportional hazards regression models assessed the impact of the randomised valve implanted in the none/mild vs moderate/severe LVOT calcification groups, expressed as an adjusted hazard ratio (HR) and associated 2-sided 95% confidence interval (CI). The consistency of the randomised treatment effect between the none/mild vs moderate/severe LVOT calcification groups was assessed via a formal treatment by the LVOT calcification interaction test. All endpoints are reported according to the randomised valve type assigned (intention-to-treat analysis). A p-value of <0.05 was considered significant for 2-sided testing, with the Bonferroni-Holm correction applied where appropriate. All statistical tests were performed using SPSS statistics software version 24 (IBM).
Results
Patient population
Out of the 447 patients randomised in the SOLVE-TAVI trial, 7 patients withdrew consent prior to the study procedure, 2 died prior to the study procedure, 1 patient was diagnosed with having only moderate aortic stenosis (AS) after randomisation and received conservative treatment, 2 patients received other valve prostheses, no MDCT data were available in 17 patients and in 2 patients semiquantitative calcification scoring could not be performed due to excessive motion artefacts, leaving 416 patients with data available for the present analysis. The patients’ clinical characteristics are presented in Table 1. Moderate/severe LVOT calcification was detected in 143 (34.4%) of the patients. In these patients, 76 (53.1%) received SEV and 67 (46.9%) BEV (Table 1). There were no differences in baseline clinical characteristics between the two groups except for a lower rate of female sex and higher baseline peak pressure gradient in patients with moderate/severe compared to no/mild LVOT calcification. Similarly, no differences were found in baseline clinical characteristics across the 4 groups: none/mild LVOT calcification SEV, none/mild LVOT calcification BEV, moderate/severe LVOT calcification SEV and moderate/severe LVOT calcification BEV (Supplementary Table 1). Data on annular and LVOT dimensions as well as calcification quantitation are reported in Supplementary Table 2.
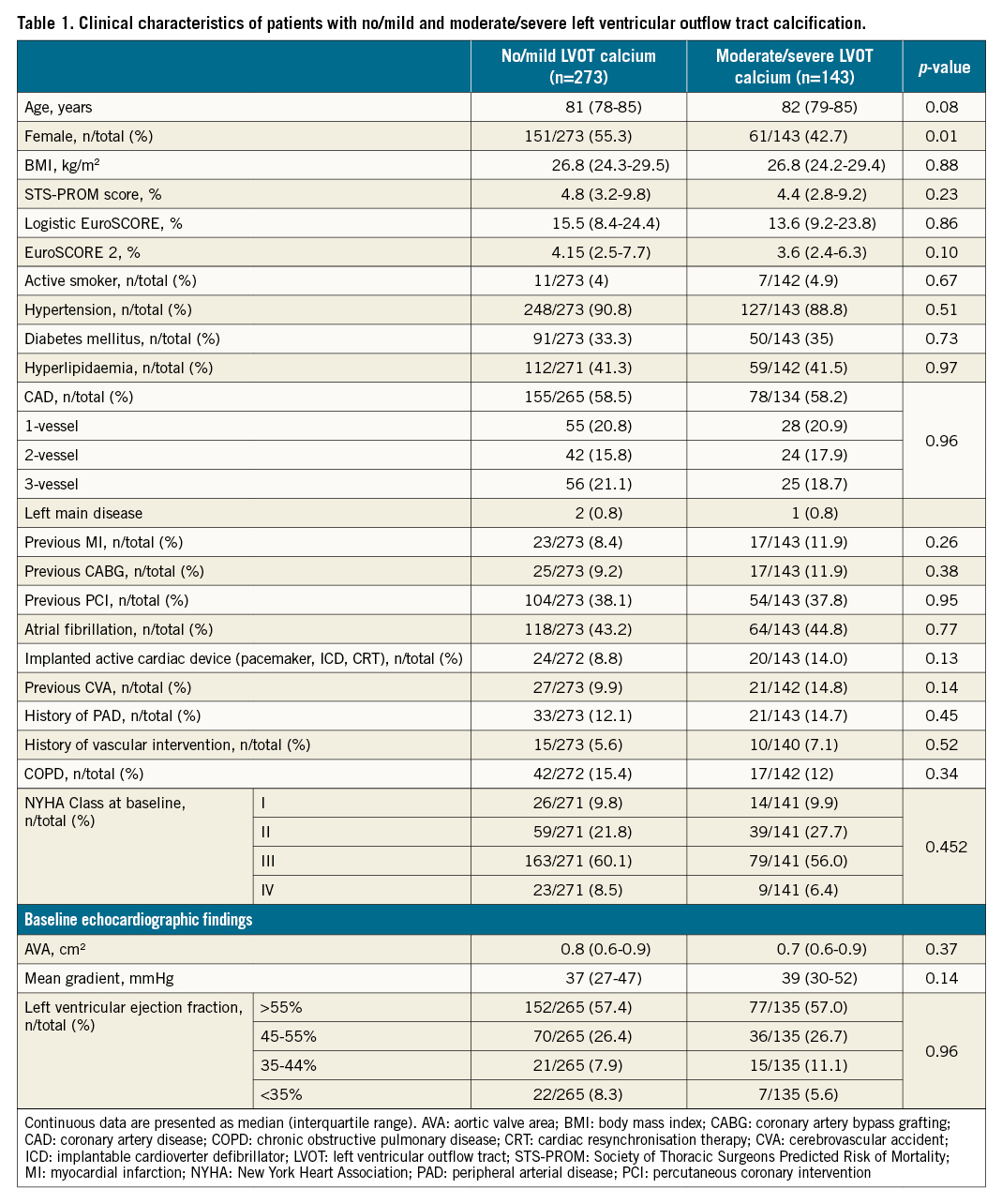
Procedural characteristics
Procedural characteristics are presented in Table 2 and Supplementary Table 3. Fluoroscopy time was 11 minutes (interquartile range 8-15) in no/mild and 12 minutes (interquartile range 9-16) in moderate/severe LVOT calcification (p=0.01), and significantly higher rates of pre- and post-dilation were observed in patients with moderate/severe LVOT calcification compared to those with no/mild LVOT calcification (Table 2). There were no differences in device time and procedure time, volume of contrast and procedural success when comparing both LVOT calcification groups (Table 2). Significantly higher contrast media doses were administered in SEV implantation when compared to BEV in both no/mild and moderate/severe LVOT calcification patients (Supplementary Table 3). There were no significant differences in the rate of pre- and post-dilation when comparing SEV with BEV within the no/mild and moderate/severe LVOT calcification groups.
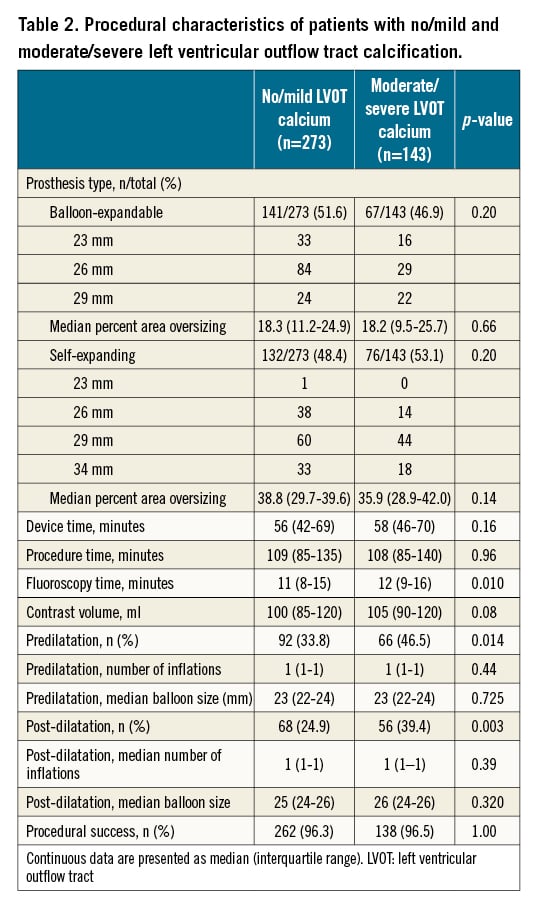
Outcomes comparing LVOT calcification groups
Clinical outcomes at 30 days and 1 year are presented in Table 3. At 30 days there was no significant difference in the primary composite endpoint (no/mild LVOT calcification 26.0% vs moderate/severe LVOT calcification 22.4%, hazard ratio [HR] 0.84, 95% confidence interval [CI]: 0.55–1.27; p=0.40). There were also no significant differences in any of the secondary endpoints including device success, early safety, clinical efficacy and time-related safety according to VARC-2 criteria, as well as total and cardiovascular mortality (Table 3).
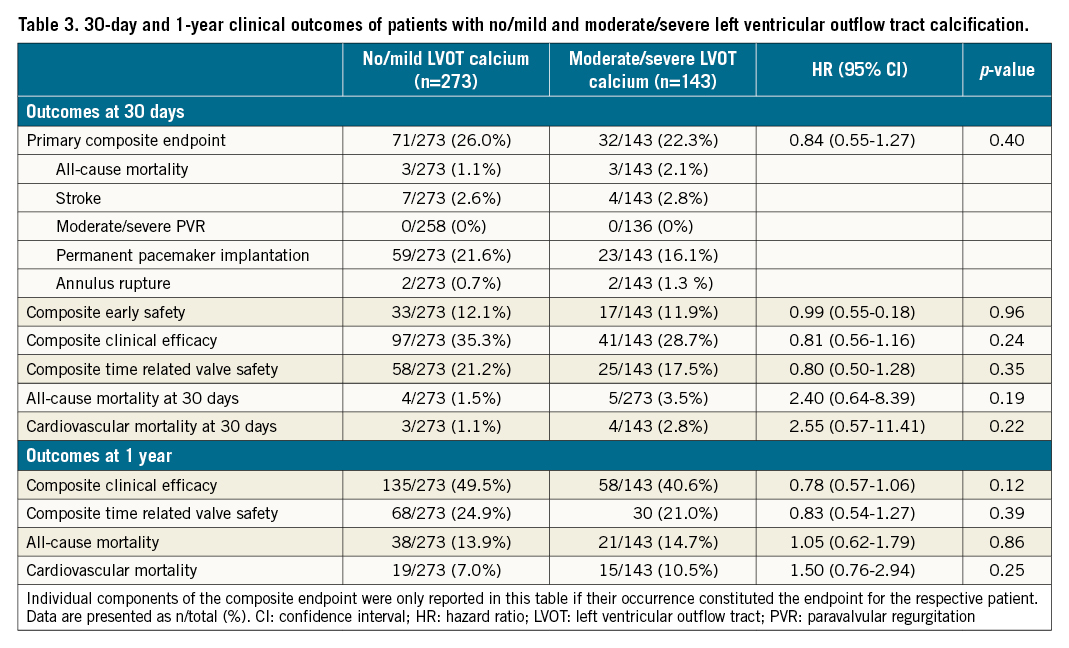
Similarly, at 1 year, there were no significant differences in clinical efficacy, time-related valve safety, and all-cause and cardiovascular mortality (Table 3).
Outcomes comparing randomised valve types within LVOT calcification groups
There was no significant difference in the primary endpoint at 30 days when comparing SEV and BEV in either none/mild or moderate/severe LVOT calcification (HR 1.09, 95% CI: 0.68-1.73; p=0.73 and HR 0.78, 95% CI: 0.383-1.569; p=0.48, respectively). There was no significant interaction between the degree of LVOT calcification and valve type (pint=0.29) (Table 4, Central illustration). Similarly, there were no differences between groups regarding the secondary endpoints including device success, early safety, clinical efficacy, overall and cardiovascular mortality, with no significant interaction between the degree of LVOT calcification and valve type (Table 4, Central illustration).
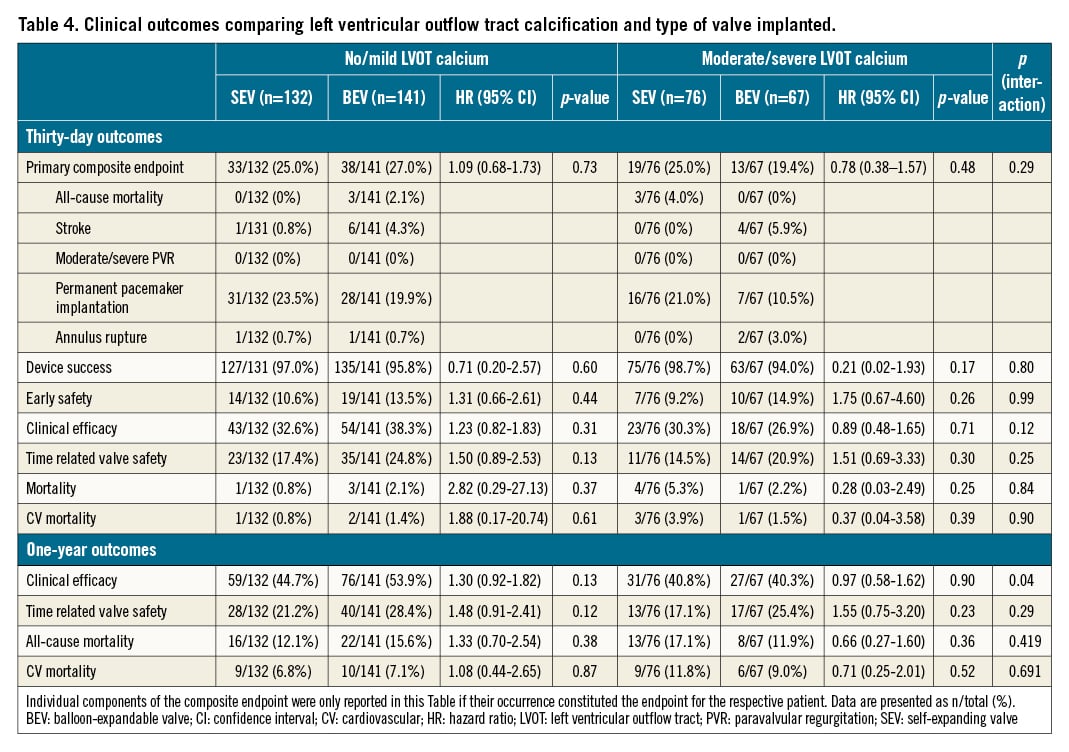
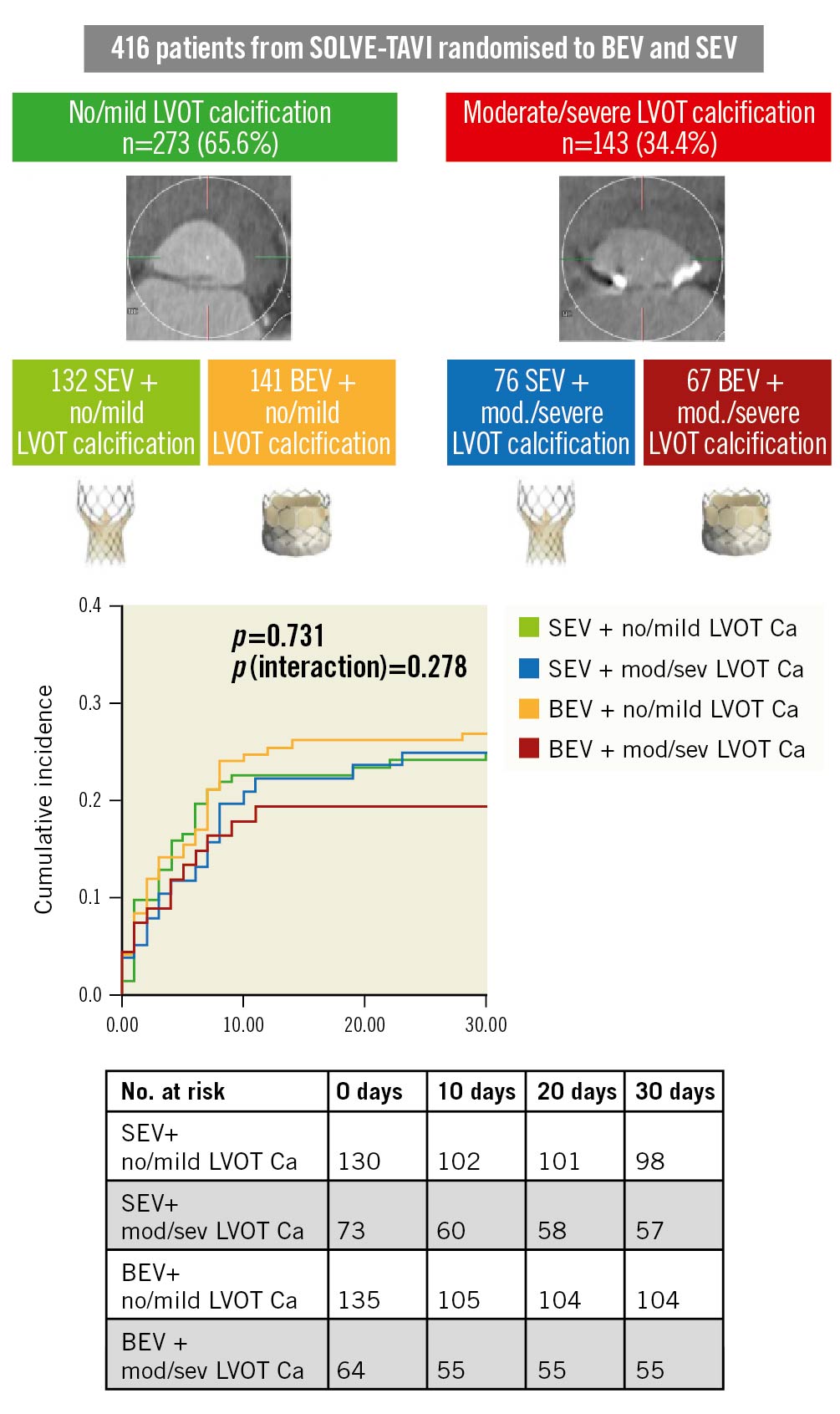
Central illustration. .Clinical outcomes after TAVI according to prosthesis type and LVOT calcification. LVOT calcification was analysed in 416 patients from the SOLVE-TAVI trial, which randomised patients to TAVI with either BEV or SEV and either general anaesthesia or conscious sedation. There was no significant difference in the incidence of a composite endpoint of all-cause mortality, stroke, moderate or severe paravalvular regurgitation, permanent pacemaker implantation and annulus rupture. There was no interaction between prosthesis type and LVOT calcification. BEV: balloon-expandable valve; Ca: calcification; LVOT: left ventricular outflow tract; SEV: self-expanding valve; TAVI: transcatheter aortic valve implantation
At 1 year, there was no significant difference in the composite clinical efficacy endpoint, time-related valve safety, all-cause and cardiovascular mortality comparing SEV with BEV within the LVOT calcification groups (Table 4), with no interaction between valve type and degree of LVOT calcification (Table 4). Of note, a p-value of 0.041 for the interaction of valve type and LVOT calcification in 1-year clinical efficacy lost significance after adjustment for multiple testing.
The findings of the 30-day and 1-year endpoints did not change when the analysis was performed in the per protocol population (data not shown).
Discussion
The major findings of the present analysis are the following: first, moderate to severe LVOT calcification occurred in over one-third of patients with severe AS at intermediate to high surgical risk. Second, there was no significant difference in the composite primary endpoint of 30-day all-cause mortality, stroke, moderate or severe PVR, PPM implantation and annulus rupture between patients with no/mild and moderate/severe LVOT calcification. Third, there was no difference in the primary and secondary outcomes comparing the types of valve implanted in the respective degrees of LVOT calcification and no significant interaction between LVOT calcification and valve choice.
Research conducted mainly with previous versions of TAVI prostheses has outlined that LVOT calcification constitutes a challenge for adequate valve selection and the subsequent implantation. The implantation becomes even more challenging with certain LVOT calcification locations and dimensions913. LVOT calcification has been associated with a higher risk for annulus rupture and residual significant PVR in SAPIEN/SAPIEN XT (Edwards Lifesciences), Lotus (Boston Scientific) and CoreValve (Medtronic) prostheses57816. Annular rupture in patients with LVOT calcification appears to be a problem especially after BEV implantation91216. On the other hand, it has been shown in older-generation devices that the implantation of an SEV in moderate or severe LVOT calcification increases the risk for significant PVR with a subsequent increased risk of mortality1718. Post-dilation to mitigate the degree of PVR further increases the risk of annular rupture16. Furthermore, LVOT calcification adds challenges to the correct positioning of an SEV19. The partial deployment followed by recapturing of SEV has been linked to increased debris embolisation and subsequent stroke20. Additionally, pacemaker implantation has also been linked to calcification load in the upper LVOT21.
Recently, Okuno et al investigated the outcomes of TAVI patients divided into no/mild vs moderate/severe LVOT calcification16. The authors found that moderate/severe LVOT calcification appeared in almost one quarter of patients undergoing TAVI16, which was a lower rate compared to ours. LVOT calcification was associated with longer fluoroscopy time, higher rates of pre- and post-dilation, and a significantly higher rate of procedural complications (e.g., bailout valve-in-valve, annular rupture and moderate/severe PVR). However, when separately examining the same 1,000 patients receiving second-generation prostheses, the authors did not find any significant differences in procedural or clinical short-term outcomes16. Albeit these works provide some orientation concerning valve design choice in calcified LVOT, valve choice was not randomised, resulting in an uneven distribution of valve types among LVOT anatomies16.
Moreover, severe LVOT calcification has been an exclusion criterion for enrolment in several randomised clinical trials23. In the NOTION trial, in which severe LVOT calcification was not a reason for exclusion,22 ≥moderate PVR was considerably more frequent than in the PARTNER 3 and Evolut low-risk trials2223. Hence, the selection of one valve design over another remains a largely subjective decision based on the experience of the centre and operator7.
No randomised trials are currently investigating the comparative efficacy and safety of different transcatheter heart valve systems in patients with LVOT calcification. Only in the CHOICE trial, where the first-generation SEV (Core Valve) and BEV (SAPIEN XT) were compared, subgroup analyses indicated that there was no interaction between the degree of LVOT calcification and valve type23. Of note, in no/mild LVOT calcification, BEV were associated with a significantly higher rate of device success than SEV, while for those with moderate/severe LVOT calcification, both BEV and SEV had similar success rates23. The numerically higher rate of pacemaker implantation in the none/mild LVOT calcification group as compared to the moderate/severe LVOT calcification group might appear surprising. Of note, patients from the moderate/severe LVOT calcification group had higher rate of history of pacemaker or cardioverter defibrillator implantation compared to the none/mild LVOT calcification group, with a similar rate of pacemaker implantation in both groups after the procedure.
There might be several reasons for the different outcomes obtained in the studies assessing first- or older-generation devices compared to our data. First, the present findings are unique as they originate from a study randomising valve type, thereby eliminating operator preference for a certain valve design as a confounding factor. Secondly, the technical advances of the valve prostheses, such as a sealing skirt to reduce PVR24, improved delivery systems24 and optimised inflow portions25 might have reduced the incidence of complications, especially in patients with calcified LVOT26.
Nonetheless, rates of predilation were rather high. Although the current, minimalist approach has been shown to be feasible without an increase in adverse outcomes2728, this has not been assessed in patients with moderate or severe LVOT calcification. Additionally, the need for post-dilation was higher than earlier reported1627, especially in the BEV group. Post-dilation was significantly more frequent in the moderate/severe LVOT calcification group, and especially in patients treated with SEV, although post-dilation rates in BEV-treated patients were higher than previously reported as well. Post-dilation is frequently used to ameliorate paravalvular leaks (PVL) after implantation, however, reasons for post-dilation were not reported to data management. This might confound the assessment of PVL frequency in the moderate/severe LVOT calcification group, but as our primary endpoint includes potentially adverse effects of post-dilation, such as annular rupture, PPM or stroke29, estimation of the overall clinical effect of moderate/severe LVOT calcification should be accurate.
What clinical consequences can be drawn from our findings? It can generally be assumed that both next-generation valve designs are safe to use in moderately or severely calcified annuli. However, implantation seems to be more challenging, as reflected by slightly longer fluoroscopy times and higher numbers of pre- and post-dilation in our study cohort, and, as the overall incidence of procedural complications in our cohort was low, an increased risk for annular rupture and PVR could not be entirely excluded. Hence, a high degree of caution should nonetheless be exercised concerning the placement of the valve prosthesis, and interventions in patients with calcified LVOT should be reserved for the most experienced of operators.
Limitations
Several limitations have to be considered when interpreting the results of the present analysis. First, as this was a post hoc analysis of an equivalence trial, our findings should be interpreted as hypothesis-generating rather than conclusive. Second, the study investigated only patients at intermediate to high surgical risk and utilised new-generation valves. Nevertheless, both valve designs have already undergone a novel iteration to mitigate the risk of PVR, especially in calcified LVOT (Evolut Pro and Evolut Pro+ [both Medtronic] and SAPIEN 3 Ultra [Edwards Lifesciences]). Using the newest-generation devices or other prostheses , such as the Acurate neo (Boston Scientific)30, might change the findings. Our results cannot necessarily be extrapolated to low surgical risk patients or those who underwent implantation of the newest valve designs. Third, the incidence of the relevant endpoints, e.g., moderate PVR, annulus rupture or stroke, was low overall, resulting in a loss of statistical power after division of the study population into 2 or 4 groups. Finally, the implantation depth, which might well modify the effect of LVOT calcification, was not routinely captured in the SOLVE-TAVI trial.
Conclusions
In conclusion, in AS patients at intermediate to high risk for surgery, moderate or severe LVOT calcification is frequent and associated with longer fluoroscopy time as well as an increased need for pre- and post-dilation. Moderate or severe LVOT calcification did not lead to a higher incidence of early and mid-term adverse clinical outcomes. Furthermore, we could not detect differences between BEV and SEV. Further research is warranted to identify the ideal valve type for patients with heavily calcified LVOT.
Impact on daily practice
Previous evidence suggests that left ventricular outflow tract (LVOT) calcification predicts adverse outcomes after transcatheter aortic valve implantation (TAVI). In our analysis of data from the randomised SOLVE-TAVI trial, a composite endpoint comprising death, stroke, moderate or severe paravalvular regurgitation, pacemaker implantation and annulus rupture was not significantly more frequent in patients with moderately or severely calcified LVOT, neither with second-generation balloon-expandable nor with self-expanding prostheses. However, fluoroscopy time and use of pre- and post-dilation was higher in those patients, indicating increased procedural complexity.
As the overall incidence of adverse events was relatively low, further research to identify the optimal prosthesis type for patients with calcified LVOT is needed. Nonetheless, TAVI in patients with calcified LVOT should be reserved for the most experienced of operators.
Funding
This work was supported by the German Heart Research Foundation (DSHF).
Conflict of interest statement
D. Holzhey is advisor/proctor for Edwards Lifesciences and Medtronic. U. Landmesser has received lecture and advisory honoraria from Abbott and Boston Scientific (outside the submitted work). M.A. Borger received consulting fees and honoraria paid to his hospital on his behalf from Edwards Lifesciences, Medtronic, CryoLife, and Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

