Abstract
Aims: Elevated gradients have been proposed to be associated with haemodynamic structural valve deterioration (SVD) after transcatheter aortic valve implantation (TAVI); however, data regarding their characterisation remain scarce. This study sought to investigate the prevalence and predictors of moderate or greater SVD and the prevalence of valve thrombosis during follow-up after TAVI with balloon-expandable valves.
Methods and results: A total of 691 patients undergoing transfemoral TAVI were enrolled. The primary endpoint was moderate or severe haemodynamic SVD during 12-month follow-up after TAVI, defined as (I) mean transvalvular gradient ≥20 mmHg or (II) mean transvalvular gradient increase ≥10 mmHg. The primary endpoint was observed in 10.3% after TAVI. Use of a 20 mm valve, valve-in-valve procedure and absence of oral anticoagulation (OAC) were independently associated with haemodynamic SVD, whereas valve-in-valve procedure and absence of OAC were the only significant variables after accounting for death as a competing event. Absence of OAC was significantly associated with haemodynamic SVD (RR 8.65; p=0.004) and death (RR 3.57; p=0.06), whereas valve-in-valve procedure was only associated with haemodynamic SVD (RR 52.76; p<0.001). Valve thrombosis was present in 0.87% (6/691) of the patients.
Conclusions: The prevalence of moderate or greater haemodynamic SVD during the first 12 months after TAVI is 10.3%. Procedural factors and pharmacotherapy seem to play a key role during manifestation. Future studies should focus on the underlying mechanisms.
Introduction
Transcatheter aortic valve implantation (TAVI) has been performed increasingly over the last decade and is currently recommended for patients with severe aortic stenosis who are considered at high or intermediate risk for conventional aortic valve replacement1. Results from contemporary randomised trials in low-risk TAVI patients will probably broaden the indication of use with this disruptive technology2,3.
Although the efficacy and safety of TAVI have been demonstrated in large, randomised trials4,5, data regarding long-term valve function are limited6,7,8. Standardised definitions of bioprosthetic structural valve deterioration (SVD) after TAVI have been published recently to enable objective evaluation of transcatheter heart valves (THV). Elevated gradients have been proposed to be associated with haemodynamic SVD9.
The objective of this study was to investigate the prevalence and predictors of moderate or greater haemodynamic SVD during follow-up in the first 12 months after TAVI with balloon-expandable valves in a large contemporary patient cohort and further assess the prevalence of valve thrombosis.
Methods
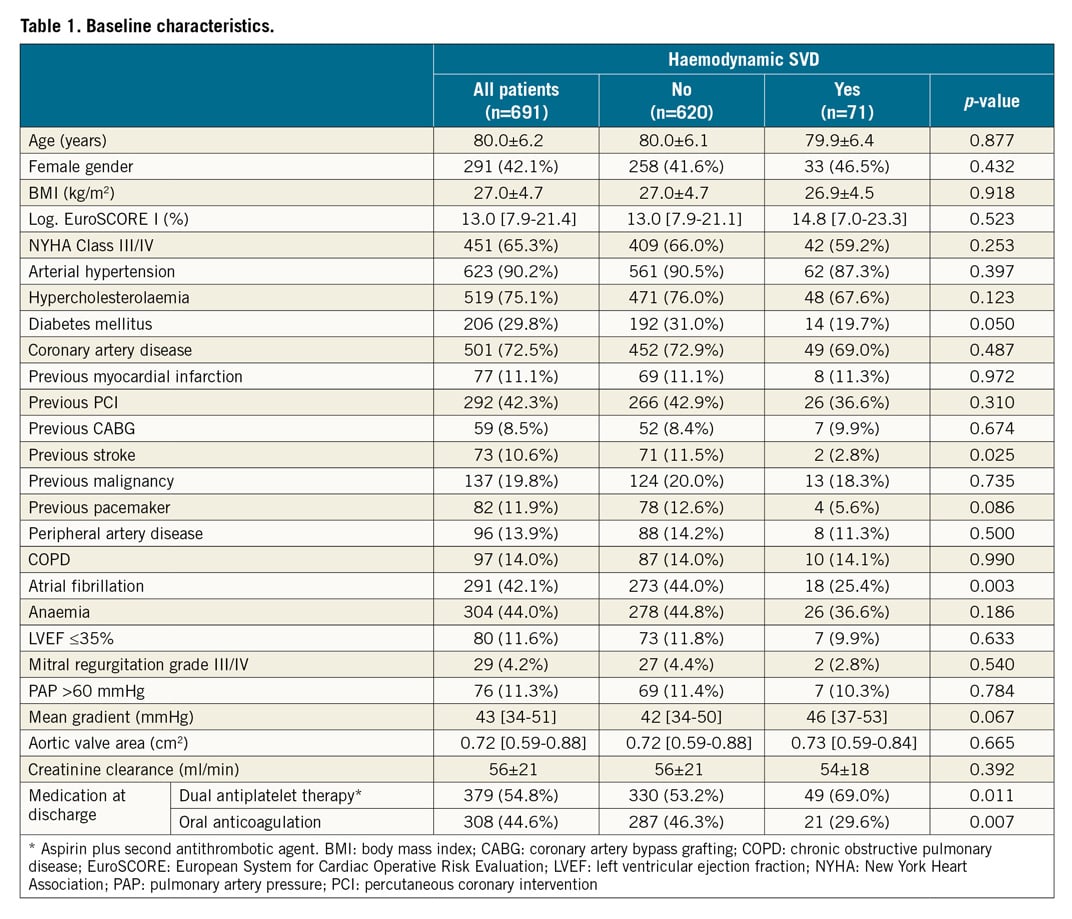
STUDY POPULATION AND PROCEDURES
Between January 2014 and April 2018, 872 consecutive patients with severe aortic stenosis or degenerated bioprosthetic aortic valves underwent transfemoral TAVI with balloon-expandable valves (SAPIEN 3; Edwards Lifesciences, Irvine, CA, USA) at the Department of Cardiovascular Diseases, German Heart Centre Munich, Germany. Patients with device failure according to the updated Valve Academic Research Consortium (VARC)-2 criteria were excluded from final analyses (n=78)10. Furthermore, patients with missing echocardiographic data during follow-up were excluded from final analyses (n=103). Finally, a total of 691 patients satisfied these criteria and were included in the final analysis. All patients were discussed by a multidisciplinary Heart Team and found eligible for transfemoral TAVI. All patients provided written informed consent. Post-procedural pharmacotherapy consisted of dual antiplatelet treatment with aspirin and clopidogrel for at least three months in patients without an indication for oral anticoagulation (OAC) or prior coronary intervention; when patients had an indication for OAC, single-therapy OAC was prescribed unless the patient also had an indication for antiplatelet treatment. Triple therapy was only prescribed in case of recent coronary intervention entailing reduced dose OAC.
DEFINITION OF ENDPOINTS AND FOLLOW-UP
The primary endpoint of this study was moderate or greater haemodynamic SVD during follow-up in the first 12 months after TAVI, defined as (I) mean transvalvular gradient ≥20 mmHg or (II) mean transvalvular gradient increase ≥10 mmHg compared with previous measurements after TAVI. Moreover, crude rates of both moderate and severe haemodynamic SVD as well as bioprosthetic valve failure (BVF) were reported individually. Additionally, elevated transvalvular gradients were investigated by transoesophageal echocardiography and/or multislice computed tomography (CT) at the discretion of the treating physician to rule out valve thrombosis.
Data collection involved demographic information, procedural data, as well as clinical and echocardiographic assessment. Adverse events were recorded throughout the follow-up period. All clinical endpoints, procedural data and in-hospital complications were categorised according to the updated VARC-2 criteria. Transthoracic echocardiography was performed at baseline, at discharge, at least once during 12-month follow-up after TAVI and yearly thereafter (up to four years).
STATISTICAL ANALYSIS
Categorical variables are expressed as frequencies and proportions and were compared using the chi-square or Fisher’s exact test, as appropriate. Continuous variables are presented as mean with standard deviation (SD) or median with interquartile range (IQR) and compared using the Student’s t-test or Mann-Whitney U test, respectively.
For dichotomous analysis, patients were divided into strata with and without haemodynamic SVD. Cox proportional hazards analysis was performed to determine factors associated with the primary endpoint. Possible covariables were selected by clinical relevance and were as follows: female gender, New York Heart Association (NYHA) Class III/IV, previous myocardial infarction, previous stroke, previous malignancy, previous pacemaker implantation, peripheral artery disease, anaemia, left ventricular ejection fraction (LVEF) <35%, mitral regurgitation grade III/IV, valve-in-valve procedure, use of a 20 mm valve, predilatation, post-dilatation and OAC at discharge. Corresponding hazard ratios (HR) were computed.
Additionally, multivariable competing risk regression (CRR) was performed using the R package “cmprsk” (R Foundation for Statistical Computing, Vienna, Austria). Similar to conventional multivariable regression analysis, this method aims to find predictors of the primary endpoint while adjusting for statistically relevant cofactors and accounting for death as a competing event. Due to the limited number of events (haemodynamic SVD and/or death), risk regression models were constrained to the following predictor variables to avoid model overfitting: age, use of a 20 mm valve, previous malignancy, valve-in-valve procedure and OAC. Additionally, cumulative incidence function (CIF) estimates from competing risk data were calculated for valve-in-valve procedures and OAC.
A two-sided p-value <0.05 was considered statistically significant. Statistical analyses were performed using JMP, Version 13 (SAS Institute Inc., Cary, NC, USA), R Version 3.3.2 (R Foundation for Statistical Computing) and IBM SPSS Statistics, Version 24.0 for Macintosh (IBM Corp., Armonk, NY, USA).
Results
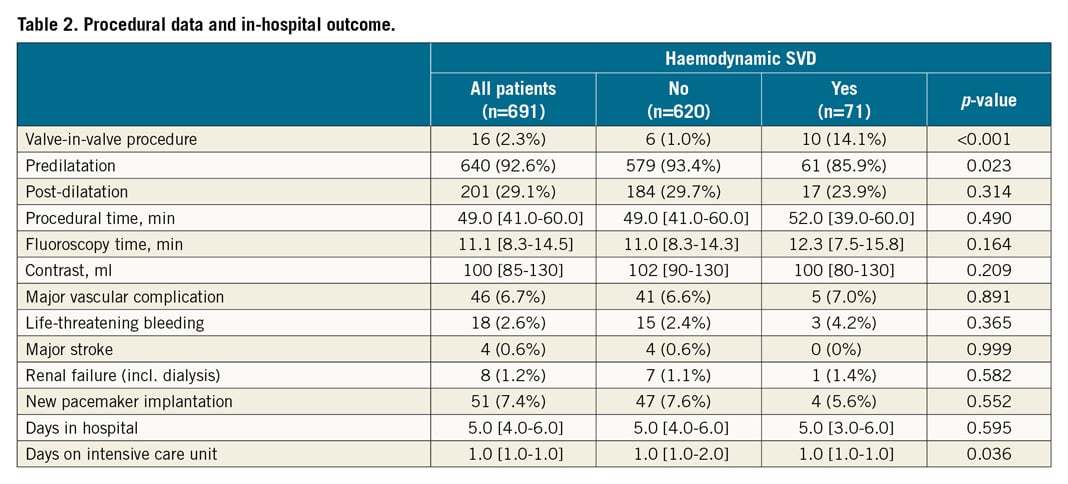
Baseline characteristics of the entire population are shown in Table 1. A total of 691 patients were included in this analysis (mean age 80.0±6.2 years, 42% female). The population was a contemporary and unselected cohort of patients with a median logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) I of 13.0% [7.9-21.4]. Procedural data and in-hospital outcomes are provided in Table 2.
HAEMODYNAMIC SVD AFTER TAVI
The prevalence of moderate or greater haemodynamic SVD during the first 12 months after TAVI was 10.3% (71/691). There was incremental prevalence observed over time during the first 12 months after TAVI: 1.9% (13/691) at 30-day and 10.3% (71/691) at 12-month follow-up. Moderate haemodynamic SVD was observed in 9.4% (65/691), whereas severe haemodynamic SVD was observed in only 0.9% (6/691). Moreover, BVF was observed in 1.88% (13/691).
Baseline characteristics according to the primary endpoint are shown in Table 1. Patients with haemodynamic SVD (n=71) had a lower rate of atrial fibrillation (p=0.003) and previous strokes (p=0.03) and were less likely to be treated with oral anticoagulants (p=0.007). Regarding procedural data, valve-in-valve procedures (p<0.001) were more frequently performed, whereas predilatation (p=0.023) was less frequently performed in patients with elevated gradients (Table 2).
Cox proportional hazards analysis revealed that use of a 20 mm valve (hazard ratio [HR] 9.43; p<0.001) and valve-in-valve procedure (HR 9.92; p<0.001) were independent predictors of haemodynamic SVD during follow-up after TAVI, whereas OAC (HR 0.46; p=0.003) was a protective factor of haemodynamic SVD. Based on these observations, crude rates of haemodynamic SVD were further analysed in patients with and without OAC (Figure 1).
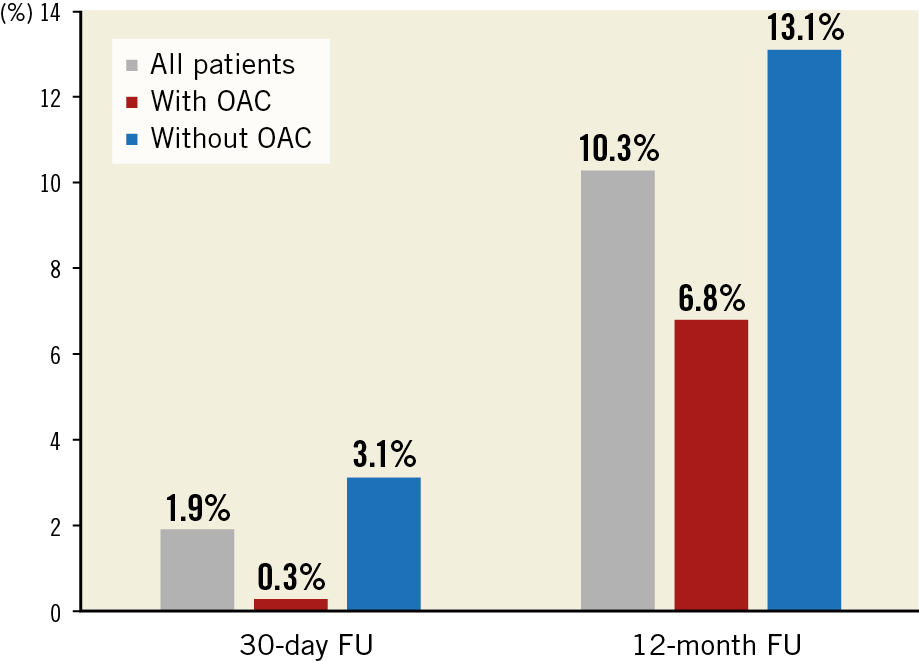
Figure 1. Crude rates of haemodynamic structural valve deterioration during follow-up after TAVI in patients with and without oral anticoagulation.
The crude mortality rate 12 months after TAVI was 4.3% (30/691). After CRR accounting for death as a competing event, valve-in-valve procedure (relative risk [RR] 13.01; p<0.001) and OAC (RR 0.39; p<0.001) were the only significant predictor and protective factor of haemodynamic SVD, respectively. According to CIF estimates, absebce of OAC was significantly associated with both haemodynamic SVD (RR 8.65; p=0.004) and death (RR 3.57; p=0.06), whereas valve-in-valve procedure was only significantly associated with haemodynamic SVD (RR 52.76; p<0.001) but not with death (RR 0.89; p=0.35) (Figure 2).
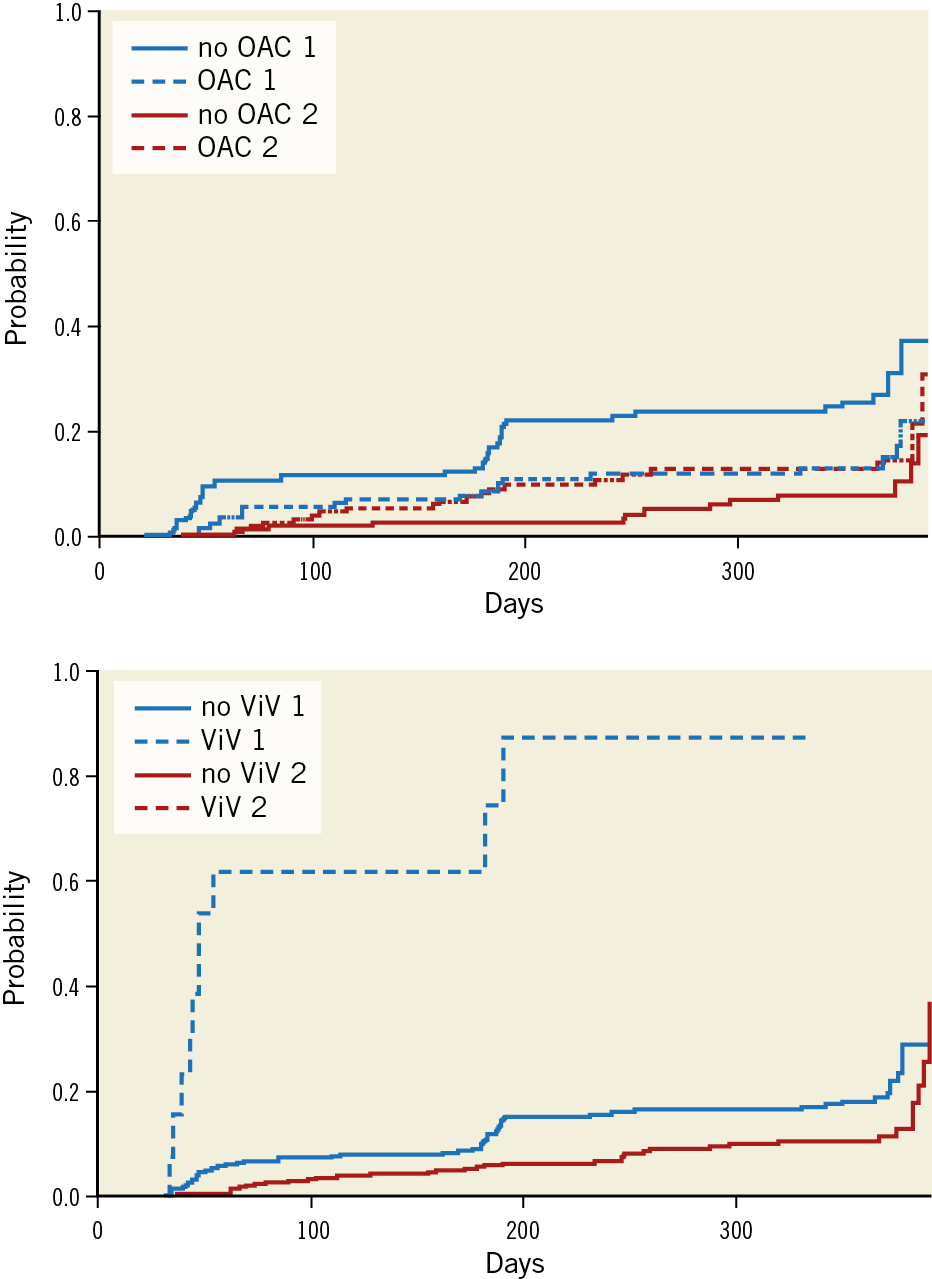
Figure 2. Cumulative incidence function estimates from competing risk data for oral anticoagulation and valve-in-valve (ViV) procedures. 1: haemodynamic SVD, 2: death. Note that CIF for death in cases with and without ViV are superimposed.
Long-term echocardiographic follow-up was available in a subgroup of patients, namely 107 patients at two-year follow-up and 48 and 31 patients at three- and four-year follow-up, respectively. Haemodynamic SVD was observed in 9.3% (10/107) at two years, 10.4% (5/48) at three years and 6.5% (2/31) at four-year follow-up.
BIOPROSTHETIC VALVE THROMBOSIS
Bioprosthetic valve thrombosis was present in 0.87% (6/691) of the entire cohort and 8.5% (6/71) of patients with haemodynamic SVD during follow-up after TAVI (Figure 3, Moving image 1, Moving image 2). The clinical characteristics of patients with valve thrombosis are shown in detail in Supplementary Table 1. At detection time, median transvalvular gradient was 28 mmHg (IQR 25-47). All patients were on single or dual antiplatelet therapy. OAC with either phenprocoumon (international normalised ratio [INR] target range 2.0-3.0) or apixaban (5 mg twice daily) was initiated in all cases after valve thrombosis was diagnosed; valve thrombosis was successfully resolved in all cases (Figure 4).
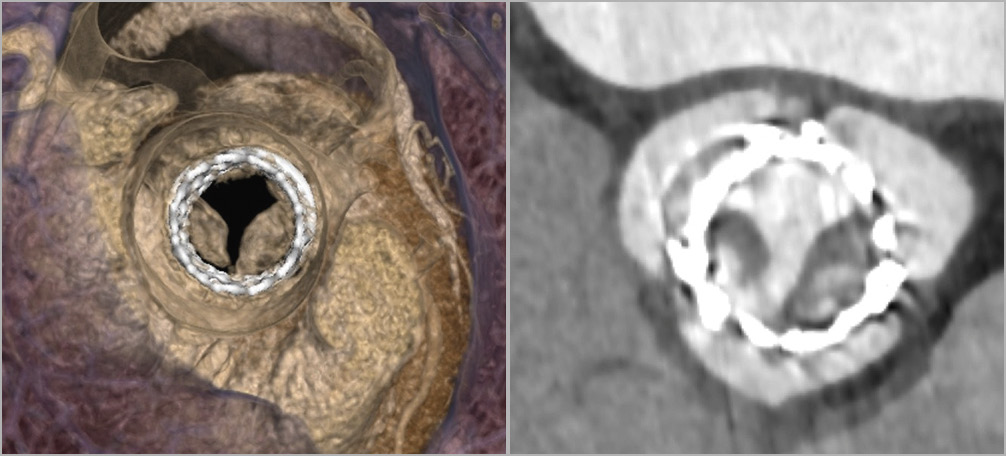
Figure 3. Computed tomography images showing a case of valve thrombosis after valve-in-valve TAVI.
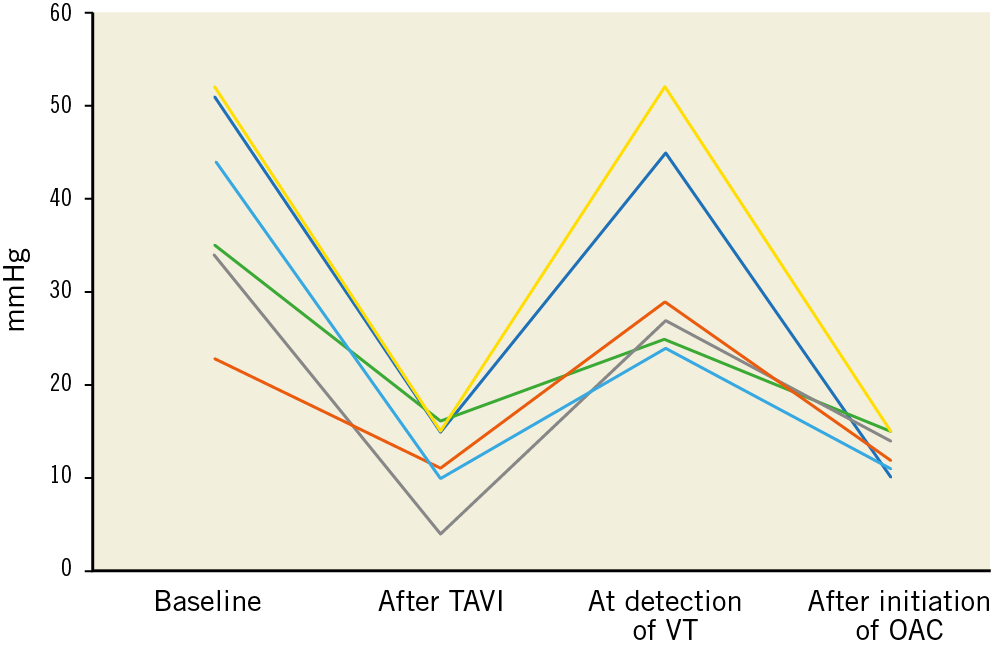
Figure 4. Mean transvalvular gradients of patients with valve thrombosis at baseline, after TAVI and during follow-up after TAVI.
Discussion
The present study investigated the prevalence of moderate or greater haemodynamic SVD during follow-up after TAVI and also assessed the prevalence of valve thrombosis. To our knowledge, this is the first study using CRR to account for the probability of death as a competing event. The results can be summarised as follows. The prevalence of moderate or greater haemodynamic SVD was 10.3% during 12-month follow-up after TAVI. Cox proportional hazards analysis revealed that haemodynamic SVD after TAVI was more frequent using a 20 mm valve or in case of valve-in-valve procedures and less frequent in case of OAC. Valve thrombosis was present in 0.87% of the entire cohort during follow-up after TAVI.
BIOPROSTHETIC STRUCTURAL VALVE DETERIORATION
Bioprostheses are prone to SVD. Experience from surgically implanted bioprostheses indicates onset of SVD six to eight years after implantation11. Heterogeneous definitions have been a major limitation in the past12. In these studies, diagnosis of SVD often involved the need for re-operation or clinically apparent symptoms, hence the prevalence of SVD has probably been underestimated.
Despite the widespread use of TAVI since its inception in 2007, long-term data beyond five years are still limited6,7,8. As we proceed into the period in which SVD has been observed with surgical bioprostheses, standardised definitions have now been proposed9. Haemodynamic SVD, which can be assessed by means of echocardiography, requires special attention. According to updated VARC-2 and European Association of Percutaneous Cardiovascular Interventions (EAPCI) criteria, we investigated moderate or greater haemodynamic SVD during the first 12 months after TAVI9,10.
PREVALENCE AND PREDICTORS OF HAEMODYNAMIC SVD
In this study, moderate or greater haemodynamic SVD was present in 10.3% of all patients treated with balloon-expandable valves. To date, available studies regarding haemodynamic SVD after TAVI are scarce with conflicting results. Early randomised TAVI studies and large registries have reported unchanged valve function up to five years after TAVI, although these data are generally limited by high mortality rates in this cohort of inoperable/high-risk patients6,7,13. In contrast, other authors have reported low rates of haemodynamic SVD in up to 5% of patients undergoing TAVI as well as a mild, but significant increase of gradients over time after TAVI14,15.
The clinical relevance of haemodynamic SVD after TAVI is unknown. An association with an increased risk of adverse cardiovascular events has not been reported to date16. Nevertheless, given the current trend to treat younger, lower-risk patients, identifying predictors associated with an increased risk for haemodynamic SVD is of utmost relevance; further research is needed to assess the clinical impact of haemodynamic SVD. Apparently, short-term follow-up after TAVI is warranted since most patients with haemodynamic SVD presented within the first year after TAVI14.
In the present Cox proportional hazards analysis, haemodynamic SVD was less frequently observed in case of treatment with oral anticoagulants after TAVI and more frequent using a 20 mm valve or in case of valve-in-valve TAVI procedures. The observed association of OAC and haemodynamic SVD is in line with previous studies that have already reported OAC providing protection from haemodynamic SVD14,15. This particular finding is of tremendous interest given the current uncertainty and low evidence level with regard to the optimal pharmacotherapy after TAVI.
Just recently, the authors of the FRANCE TAVI registry have shown for the first time that OAC at discharge is a significant and independent predictor of increased long-term mortality after TAVI17. As a higher operative risk might partly account for this observation, CRR with death as a competing event seems appropriate in these patients.Our analysis revealed that OAC is a significant protective factor of haemodynamic SVD after TAVI, whereas it is a predictor of death after TAVI.
The observational character of our and other available studies and the given collinearity of several variables further support the urgent need for data from ongoing randomised trials evaluating the optimal pharmacotherapy after TAVI. For the time being, pharmacotherapy after TAVI should be applied according to current guidelines1.
MECHANISMS OF STRUCTURAL VALVE DETERIORATION
Currently, mechanisms leading to (haemodynamic) SVD are incompletely understood. Various reasons might account for elevated transvalvular gradients following procedural success. In the present analysis, patients with device failure according to VARC-2 criteria were excluded from further analyses to focus on actual valve deterioration during follow-up.
Recently, bioprosthesis thrombosis after TAVI has become a major concern18. The incidence of valve thrombosis after TAVI ranges from 0.6-2.8% in the literature19,20,21, whereas subclinical leaflet thrombosis has been observed in 6-40%18,22. In our current analysis, valve thrombosis was present in 0.87% of the entire cohort and 8.5% of patients with haemodynamic SVD. Interestingly, it was observed in patients with moderate (mean transvalvular gradients ≥20 mmHg and <40 mmHg) and those with severe (mean transvalvular gradients ≥40 mmHg) haemodynamic SVD.
So far, the clinical relevance of bioprosthesis thrombosis has not been fully understood18. Data regarding subclinical leaflet thrombosis are mostly derived from routine CT scans after TAVI. Midterm follow-up suggested no impact on mortality or stroke rates in these patients22. However, thorough validation of elevated transvalvular gradients to predict (subclinical) leaflet thrombosis remains an important unmet need.
In contrast to the uncertainty regarding optimal pharmacotherapy after TAVI until ongoing randomised trials have bridged the gap of evidence, OAC seems effective in treating actual bioprosthesis thrombosis after TAVI15,20,21. In our cohort, all cases of valve thrombosis were on antiplatelet therapy at the time point of detection, and thrombosis resolved after initiation of OAC with either phenprocoumon or apixaban, with a reduction of mean transvalvular gradients to <20 mmHg. The duration of required OAC has yet to be established.
Trials comparing different valve designs will also provide further information on the potential role of valve design on transvalvular gradients. Presumably there will be predominance of elevated gradients in balloon-expandable valves, which is most likely due to their deployment in an intra-annular position introducing bias towards higher transvalvular gradients, when compared to self-expanding valves placed in a supra-annular position.
Future research should focus on dissecting the mechanisms of haemodynamic SVD, to enable differentiation of isolated elevated gradients, subclinical leaflet thrombosis and symptomatic valve thrombosis and their clinical impact.
Limitations
This is a single-centre observational study. Only patients with device success and available echocardiographic examinations during follow-up were included. Consequently, the major findings of the current analysis cannot be extrapolated to patients surviving in the absence of echocardiographic follow-up.
Conclusions
During the first 12 months after TAVI, 10.3% of the entire cohort had moderate or greater haemodynamic SVD and valve thrombosis was observed in 0.87% of all patients. Pharmacotherapy and procedural factors seem to play a key role during manifestation. Future histopathological and imaging studies should focus on dissecting the mechanisms of haemodynamic SVD, subclinical leaflet thrombosis and manifest transcatheter heart valve thrombosis, while prospective randomised trials are required to investigate the optimal pharmacotherapy after TAVI.
|
Impact on daily practice So far, data regarding the prevalence and predictors of haemodynamic SVD remain scarce. This study provides evidence that 10.3% of all patients treated with latest-generation balloon-expandable valves developed moderate or greater haemodynamic SVD during the first 12 months after TAVI. Valve thrombosis was present in 0.87% of the entire cohort. Procedural factors and pharmacotherapy seem to play a key role during manifestation. After accounting for death as a competing event, oral anticoagulation and valve-in-valve procedures were significantly associated with haemodynamic SVD. |
Conflict of interest statement
M. Joner received lecture fees and a research grant from Edwards Lifesciences and Boston Scientific. The other authors have no conflicts of interest relevant to this work to disclose.
Supplementary data
To read the full content of this article, please download the PDF.
Supplemental Video 1 - GD
Supplemental Video 2 - GD
Moving image 1. Case of valve thrombosis after valve-in-valve TAVI, assessed using 4D-CT.
Moving image 2. Case of valve thrombosis after valve-in-valve TAVI, assessed using 4D-CT.

