Abstract
Background: The staging classification of aortic stenosis (AS) which characterises the extent of cardiac damage has been validated in patients undergoing transcatheter aortic valve implantation (TAVI). Short-term changes in cardiac damage after TAVI and their association with long-term prognosis remain unknown.
Aims: This study aims to investigate the early evolution of cardiac damage after TAVI and the association of residual cardiac damage with clinical outcomes in TAVI recipients.
Methods: AS patients undergoing TAVI were consecutively enrolled and classified into five stages of cardiac damage (0-4). Early change in cardiac damage was defined as any change of stage at 30 days (Δcardiac damage between baseline pre-TAVI and 30 days post-TAVI).
Results: Within 30 days post-TAVI, the baseline cardiac damage stage had changed in 22.2% of 644 TAVI recipients, accompanied by improvements in the degree of dyspnoea and left ventricular ejection fraction (LVEF). Two-year mortality was associated with residual cardiac damage within 30 days post-TAVI (hazard ratio [HR] 2.97, 95% confidence interval [CI]: 2.07-4.25; p<0.001). Compared to unchanged cardiac damage post-TAVI, further cardiac damage within 30 days was associated with a higher crude risk of 2-year mortality (HR 22.04, 95% CI: 9.87-49.20; p<0.001). Cardiac deterioration within 30 days post-TAVI was an independent risk factor for 2-year mortality (HR 19.564, 95% CI: 8.047-47.565; p<0.001).
Conclusions: This investigation provided insight into the early evolution of cardiac damage in TAVI recipients and confirmed the predictive value of both residual and early changes in cardiac damage post-TAVI. Cardiac deterioration within 30 days is associated with poor clinical prognosis.
Introduction
Transcatheter aortic valve implantation (TAVI) has brought in a new era in the treatment of aortic stenosis (AS). It is now preferred over surgical aortic valve replacement (SAVR) in most intermediate to high surgical risk patients1. Current guidelines recommend risk stratification of AS patients undergoing TAVI depending on aortic valve-related parameters and symptoms23. Despite the valvular factors in the pathophysiological process, extra-valvular involvement results in structural and functional cardiac damage (CD). The extent of cardiac damage, representing the "total burden" of AS, involves the left ventricle (LV), the left atrium (LA), the mitral and tricuspid valves, the pulmonary vasculature, and the right ventricle (RV)4.
It is well accepted that the culprit for symptoms and adverse events after aortic valve replacement (AVR) is not merely the effects of the procedure, but also the cardiac function, general status and comorbidity of the recipients. Recently, attempts have been made to develop an AVR-specific risk stratification system. Prognostic tools such as the traditional Society of Thoracic Surgeons (STS) risk score mostly depend on general status and comorbidity5. Still, current indications for risk stratification following TAVI have not incorporated a systematic assessment of the extent of cardiac damage. Despite valve replacement therapy, irreversible myocardial damage has been associated with poor outcomes in patients with severe AS undergoing AVR. Généreux et al proposed a staging system to quantify the extent of cardiac damage in AS patients based on data from the PARTNER (Placement of AoRTic TraNscathetER Valves) Trials6. The prognostic value of this staging classification has been validated in multiple cohorts of Western AS patients7. These studies were all based on the extent of cardiac damage before TAVI58910. However, the influence of TAVI on the stage of cardiac damage cannot be ignored. Up to now, too little attention has been paid to the evolution of cardiac damage after TAVI, not to mention its association with long-term prognosis in TAVI recipients.
Therefore, this study sought to investigate the short-term evolution of cardiac damage after TAVI and the association of residual cardiac damage with long-term outcomes. We aimed to test the hypothesis that post-TAVI residual cardiac damage is predictive of outcomes in TAVI recipients.
Methods
Study population
For this study, we consecutively enrolled all patients >18 years of age treated with TAVI at the Second Affiliated Hospital of Zhejiang University. Patients with severe AS who underwent TAVI at the same hospital were prospectively enrolled. Patients with bicuspid aortic stenosis underwent TAVI using the Hangzhou Solution, which has been described in detail elsewhere1112. The study was approved by the medical ethics committee of the Second Affiliated Hospital of Zhejiang University and conducted according to the principles of the Declaration of Helsinki. All patients provided their written informed consent for TAVI and the use of their anonymised data for research. Specifically, we evaluated consecutive patients presenting at the enrolled medical centre between 1 March 2013 and 1 March 2020. Study exclusion criteria were as follows: (1) implantation of transcatheter or surgical prostheses in any position in the native cardiac valves before the TAVI procedure (valve-in-valve procedures); (2) patients diagnosed with pure aortic regurgitation; (3) incomplete or unavailable baseline and post-TAVI data.
Cardiac damage staging classification
Comprehensive transthoracic echocardiograms were obtained using a uniform image acquisition protocol. All individual echocardiographic data were independently reviewed by two cardiologists that were blinded to the clinical information and outcome data. Using the staging classification system proposed by Généreux et al6, we classified patients into the five stages depending on the presence or absence of extra-valvular cardiac damage as detected by transthoracic echocardiography: stage 0, no other cardiac damage seen; stage 1, LV damage (left ventricular ejection fraction [LVEF] <60%, LV mass index >95 g/m2 for women, >115 g/m2 for men, or LV diastolic dysfunction ≥grade II); stage 2, left atrium or mitral valve damage (presence of enlarged left atrium or atrial fibrillation, or mitral regurgitation [MR] ≥moderate); stage 3, pulmonary artery vasculature or tricuspid valve damage or dysfunction (pulmonary artery systolic pressure [PASP] ≥60 mmHg, or tricuspid regurgitation [TR] ≥moderate); and stage 4, RV damage, as defined by the presence of moderate to severe RV dysfunction (tricuspid annular plane systolic excursion [TAPSE] <16 mm). If patients met the criteria for multiple stages, they were assigned to the highest (worst) stage. Early change in cardiac damage was defined as the evolution of cardiac damage stage at 30 days (Δcardiac damage between baseline and 30 days). Accordingly, patients were further divided into three groups: improvement (improved by at least 1 stage), no change, and deterioration (worsened by at least 1 stage).
Data collection
Data collection included baseline characteristics, procedural data, and predischarge outcomes. Baseline characteristics consisted of clinical, laboratory, and echocardiographic data. Predischarge outcomes were obtained from the local hospital database and were rigorously assessed for quality. The primary clinical outcome was all-cause mortality, defined according to the Valve Academic Research Consortium-3 criteria13. After patients were discharged, clinical follow-up data were prospectively collected during scheduled outpatient clinic visits or direct telephone interviews. All data were stored in the TORCH database using standard data management, privacy, and security procedures.
Statistical analysis
Continuous variables with normal and non-normal distributions were presented as means±standard deviations or medians and interquartile ranges (IQR), respectively. Variable distributions between the two groups were compared via the Student's t-test and the Mann-Whitney U test, as appropriate. Categorical data were presented as frequency (percentages) and were compared using the chi-square or Fisher’s exact tests. A p-value <0.05 was considered statistically significant. The p-values were from a 2-sided test. We used multivariable logistic regression models to identify independent predictors of early cardiac damage within 30 days after TAVI. The candidate variables were selected a priori for inclusion in the univariable models and then variates with p<0.05 were entered in a multivariate logistic regression model to identify independent factors of cardiac damage within 30 days. Two-year survival rates after TAVI within each group are displayed using Kaplan-Meier curves and the log-rank test was used for comparisons. For the time-to-event analysis, we used the date of the TAVI procedure as the starting point date. The unadjusted and adjusted mortality risks at 2-year follow-up were estimated using Cox proportional hazards regression models. Covariates for this analysis were selected a priori based on their known association with the outcomes of interest or clinical judgment; these were then selected for the multivariate analyses based on their statistical significance. In consideration of the collinearity of pre- and post-TAVI cardiac damage, we selected the post-TAVI residual cardiac damage in the multivariate Cox models.
All statistical analyses were performed using SPSS software (version 22.0, IBM) and R statistical software (version 4.0.3, The R Foundation for Statistical Computing). Figures were created using GraphPad Prism software (version 6.0).
Results
Baseline patient characteristics
During the study period (1 March 2013 to 1 March 2020), 785 consecutive patients underwent TAVI at the Second Affiliated Hospital of the Zhejiang University School of Medicine. The patient selection flowchart is shown in Figure 1. Patients were excluded due to previously implanted transcatheter or surgical prostheses, pure aortic regurgitation, and incomplete data. Therefore, a total of 644 patients were enrolled in the current study.
Baseline demographic and medical characteristics according to different cardiac damage stages are illustrated in Table 1. The included patients had a mean age of 75.8 years at baseline, and 58.1% were male. Participants were classified into five groups (from 0 to 4) according to their baseline stage of cardiac damage. The specific components of cardiac damage in each stage align with previous studies' schemes68. In general, older patients with more advanced stages had higher STS scores. They were more likely to have higher levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP), a higher prevalence of atrial fibrillation, and severe cardiac symptoms (i.e., New York Heart Association [NYHA] Class ≥3). There were also no between-group differences in other concomitant diseases, including a history of dyslipidaemia, diabetes, chronic obstructive pulmonary disease (COPD), percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), and stroke.
Baseline echocardiographic data in individual groups of extra-aortic involvement are presented in Supplementary Table 1. As expected, patients in the groups with advanced stages of cardiac damage had a lower left ventricular ejection fraction, higher pulmonary artery systolic pressure and tricuspid annular plane systolic excursion indices, as well as a higher prevalence of significant mitral and tricuspid regurgitation.
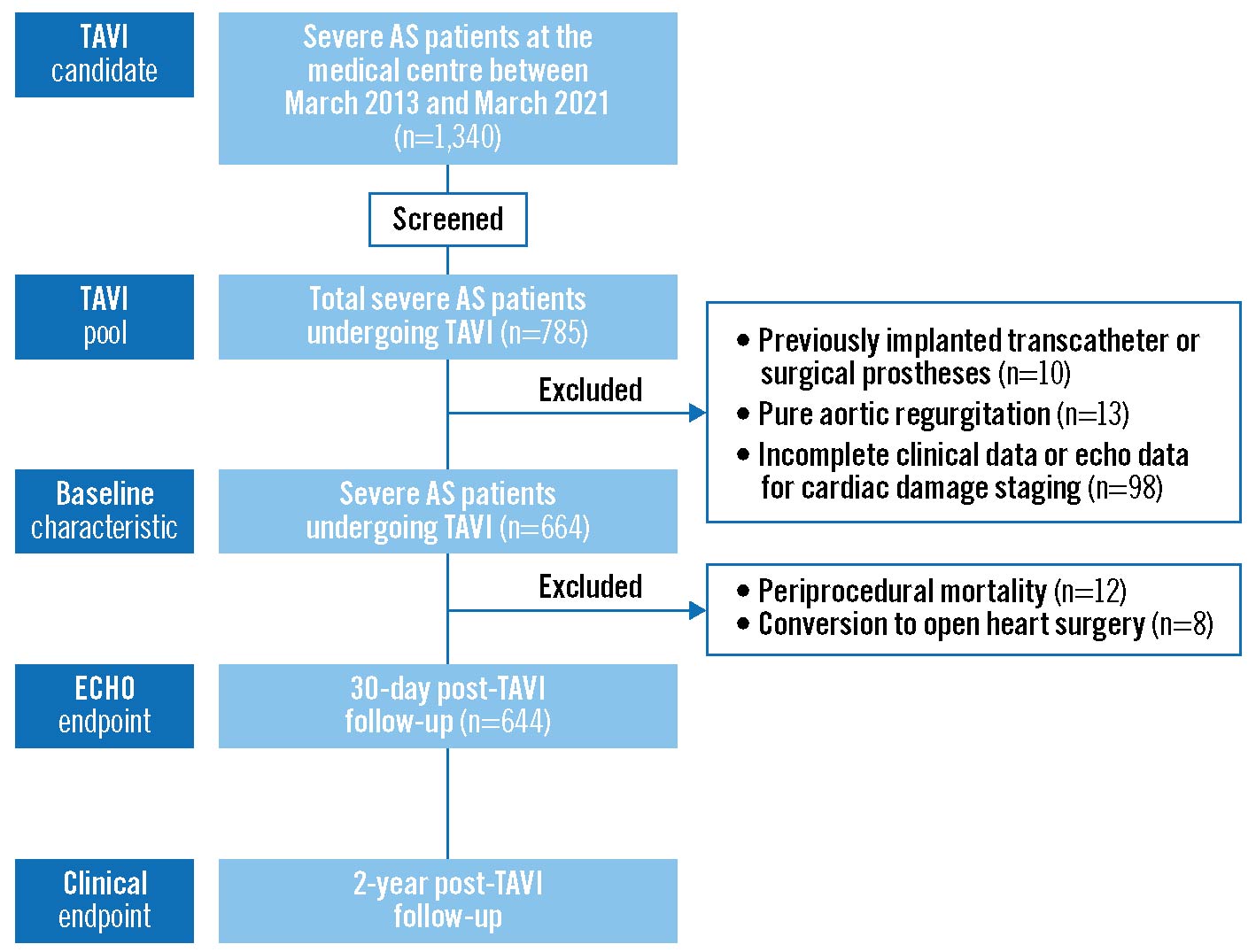
Figure 1. Flowchart of patient enrolment, including study inclusion and exclusion criteria. AS: aortic stenosis; ECHO: echocardiographic; TAVI: transcatheter aortic valve implantation
Table 1.
| Stage 0 | Stage 1 | Stage 2 | Stage 3 | Stage 4 | p-value | ||
|---|---|---|---|---|---|---|---|
| N | 18 (2.8%) | 74 (11.5%) | 427 (66.3%) | 72 (11.2%) | 53 (8.2%) | ||
| Age (y) | 69.0 (66.2-71.5) | 75.5 (70.2-80.0) | 76.0 (70.0-80.0) | 76.0 (72.0-81.0) | 77.0 (71.0-82.0) | 0.039 | |
| Male | 6 (33.3%) | 39 (52.7%) | 252 (59.0%) | 42 (58.3%) | 35 (66.0%) | 0.139 | |
| BMI (kg/m2) | 22.3 (20.4-23.7) | 21.5 (19.5-23.2) | 23.3 (20.9-25.8) | 21.7 (19.6-24.2) | 22.0 (19.2-24.6) | <0.001 | |
| STS | 2.8 (1.9-5.1) | 5.1 (3.4-8.4) | 4.4 (2.8-8.0) | 5.9 (4.1-9.3) | 8.3 (4.6-11.7) | <0.001 | |
| Smoker | 2 (11.1%) | 8 (10.8%) | 83 (19.5%) | 8 (11.1%) | 10 (18.9%) | 0.188 | |
| Dyslipidaemia | 6 (33.3%) | 9 (12.2%) | 73 (17.1%) | 16 (22.5%) | 10 (18.9%) | 0.211 | |
| Diabetes | 2 (11.1%) | 21 (28.8%) | 91 (21.3%) | 14 (19.4%) | 8 (15.1%) | 0.295 | |
| Hypertension | 9 (50.0%) | 35 (47.3%) | 251 (59.1%) | 37 (51.4%) | 19 (35.8%) | 0.011 | |
| Atrial fibrillation | 0 (0.0%) | 0 (0%) | 51 (11.9%) | 30 (41.7%) | 23 (43.4%) | <0.001 | |
| COPD | 1 (5.6%) | 18 (24.3%) | 100 (23.4%) | 15 (20.8%) | 13 (24.5%) | 0.481 | |
| Previous PCI | 1 (5.6%) | 10 (13.5%) | 50 (11.7%) | 7 (9.7%) | 7 (13.2%) | 0.864 | |
| Prior CABG | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) | 0 (0.0%) | 1 (1.9%) | 0.304 | |
| Prior stroke | 0 (0.0%) | 3 (4.1%) | 20 (4.7%) | 3 (4.2%) | 6 (11.3%) | 0.217 | |
| NYHA Class | 1 | 0 (0.0%) | 0 (0.0%) | 3 (0.7%) | 0 (0.0%) | 0 (0.0%) | <0.001 |
| 2 | 5 (27.8%) | 12 (16.2%) | 77 (18.0%) | 3 (4.2%) | 4 (7.5%) | ||
| 3 | 11 (61.1%) | 39 (52.7%) | 217 (50.8%) | 39 (54.9%) | 17 (32.1%) | ||
| 4 | 2 (11.1%) | 23 (31.1%) | 130 (30.4%) | 29 (40.8%) | 32 (60.4%) | ||
| NT-proBNP (pg/mL) | 113.0(83.2-232.5) | 1,359.5(306.0-2,753.2) | 1,941.0(669.0-5,368.0) | 4,688.0(1,853.0-12,051.0) | 9,733.0(3,231.0-23,435.0) | <0.001 | |
| cTnI (ng/ml) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.1) | 0.0 (0.0-0.1) | 0.728 | |
| CK-MB (U/L) | 12.0 (9.2-15.0) | 13.0 (9.0-17.0) | 12.0 (9.0-15.0) | 12.0 (10.0-17.0) | 13.0 (10.5-17.0) | 0.051 | |
| eGFR (ml/min) | 66.8 (58.9-74.8) | 57.91 (54.0-61.8) | 59.19 (56.8-61.6) | 50.83 (43.0-58.6) | 45.59 (37.9-53.3) | 0.003 | |
| Albumin (g/L) | 37.7 (36.9-40.0) | 37.3 (35.2-40.1) | 37.3 (34.9-39.4) | 36.0 (34.2-39.0) | 35.3 (33.5-36.6) | 0.573 | |
| Values are median (interquartile range), n (%), or mean SD. BMI: body mass index; CABG: coronary artery bypass grafting; CK-MB: creatine kinase MB isoenzyme; COPD: chronic obstructive pulmonary disease; cTnI: cardiac troponin I; eGFR: estimated glomerular filtration rate; N: number; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SD: standard deviation; STS: Society of Thoracic Surgeons | |||||||
Short-term clinical outcomes following TAVI
Regarding the unbalanced distribution of the population across the five groups, patients were conventionally regrouped into groups of stage 0-1, stage 2, or stage 3-4. Figure 2 exhibits the short-term clinical changes in degree of dyspnoea and LVEF after TAVI.
At baseline, a higher proportion of AS patients in the advanced stage group (stage 3-4) suffered from NYHA Class IV symptoms compared to patients with cardiac damage stage ≤2. Within 30 days post-TAVI, all groups obtained symptomatic relief regarding their NYHA Functional Class. As for left ventricular systolic function, it was worst in the advanced stage group at baseline (p<0.001). After the TAVI procedure, baseline left ventricular ejection fraction improved significantly in the stage 2 and stage 3-4 groups.
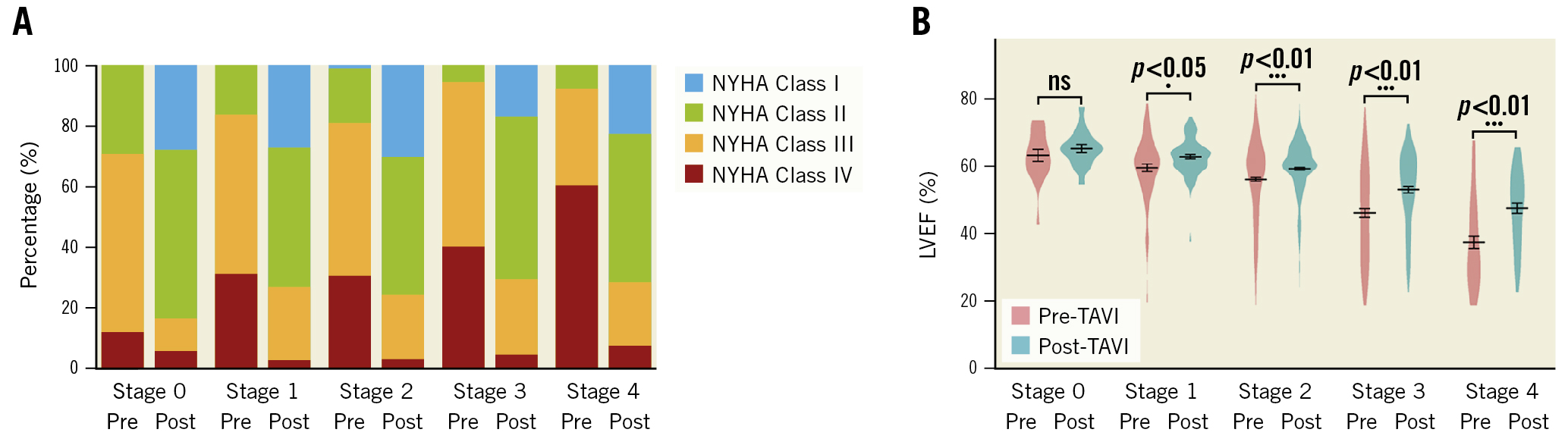
Figure 2. Changes in NYHA Functional Class and LVEF from baseline to 30 days after TAVI. A) NYHA Functional Class (with various colours representing different degrees of dyspnoea) and B) LVEF. LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; TAVI: transcatheter aortic valve implantation
Evolution of cardiac damage following TAVI
Short-term changes in the cardiac damage stage for each group using paired data are shown in Figure 3. The influence of the TAVI procedure on the cardiac function of AS patients cannot be underestimated, even in the short term. The cardiac damage stage changed in 22.2% of TAVI recipients within 30 days post-TAVI. As many as 19.9% of TAVI recipients obtained benefits in a reversal of cardiac damage. Specifically, the number of patients in the stage 0-1 group increased from 92 to 157, while the proportion of the stage 3-4 group declined from 19.4% at baseline to 10.5% within 30 days post-TAVI.
To understand the long-term evolution of cardiac function, we investigated the longitudinal changes in the stages of cardiac damage from baseline to 30 days and at 1 year after TAVI (Figure 3). To guarantee the completeness of the investigation for an entire year, we restricted the analytic cohort to living patients with echocardiographic assessment at 1 year (n=612). The extent of cardiac damage generally improved over time post-TAVI. In general, most of the reversal of cardiac damage occurred within the first month post-TAVI. In TAVI recipients with a baseline stage 3-4 (n=117), 59% of them saw early improvements in cardiac damage within 30 days after TAVI. In contrast, there were eighteen patients suffering from early cardiac damage of cardiac damage post-TAVI in the entire cohort.
The predictors of early cardiac deterioration post-TAVI are displayed in Supplementary Table 2. In univariate analysis, factors including body mass index (BMI), COPD, estimated glomerular filtration rate (eGFR), left ventricular end-diastolic diameter (LVEDD), post-TAVI acute kidney injury, and myocardial infarction were associated with early cardiac deterioration. After using multivariate logistic regression analysis, a history of COPD (odds ratio [OR] 4.33, 95% confidence interval [CI]: 1.55-12.14; p=0.005) and perioperative myocardial infarction (OR 46.94, 95% CI: 2.55-865.49; p=0.01) were independently associated with further cardiac damage within 30 days post-TAVI.
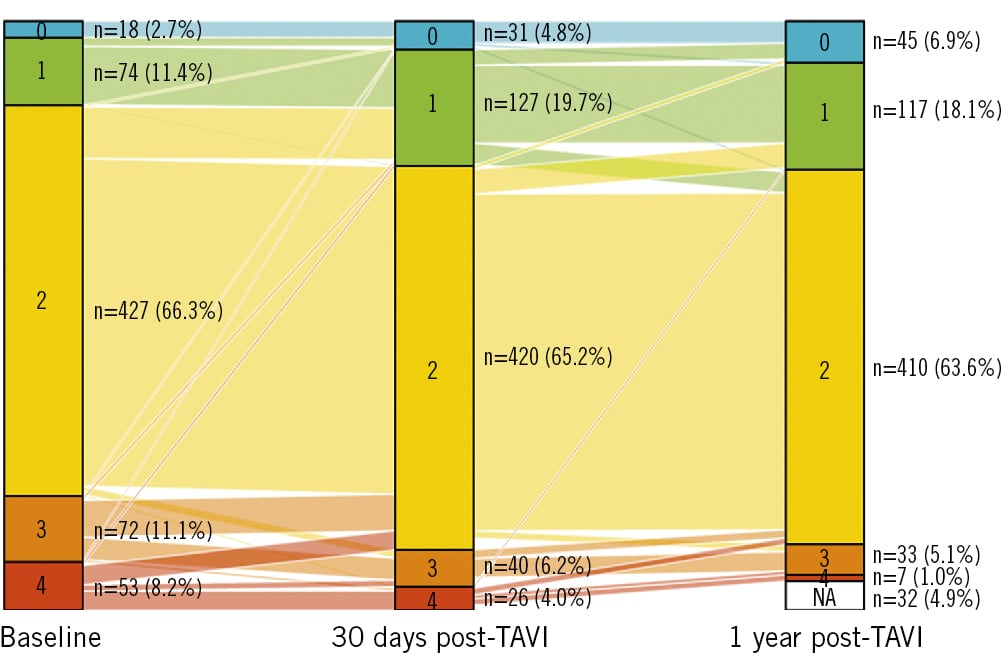
Figure 3. Evolution of cardiac damage stage from baseline to 30 days and at 1 year after TAVI. The different colours denote the different stages of cardiac damage. TAVI: transcatheter aortic valve implantation
Prognostic value of the cardiac damage staging system
During a median follow-up period of 2 years after TAVI, a total of 43 (6.7%) deaths occurred in 644 TAVI recipients. As Figure 4A illustrates, 2-year all-cause mortality gradually increased with advancing stages of baseline cardiac damage (HR 1.59, 95% CI: 1.10-2.30; p=0.014, for linear trend). In addition, the cardiac damage stage 30 days post-TAVI showed a more significant association with 2-year outcomes (HR 2.97, 95% CI: 2.07-4.25; p<0.001) (Figure 4B). Figure 4C shows the relationship between the early evolution of cardiac damage within 30 days post-TAVI and 2-year all-cause mortality. As compared to patients with unchanged cardiac damage, patients with cardiac deterioration were associated with a higher crude risk of 2-year mortality (HR 22.04, 95% CI: 9.87-49.20; p<0.001). On the other hand, 2-year outcomes were similar in those whose cardiac damage improved or remained stable in the entire cohort (HR 1.13, 95% CI: 0.45-2.85; p=0.80).
Univariate and multivariate survival analyses for the total study population are presented in Table 2. Age, STS score, a history of COPD, atrial fibrillation at 30 days post-TAVI, cardiac damage (per stage increase), and cardiac deterioration within 30 days were associated with higher 2-year mortality rates in univariate analyses. Following the multivariate adjustment, cardiac deterioration within 30 days post-TAVI was an independent risk factor for 2-year mortality (HR 19.564, 95% CI: 8.047-47.565; p<0.001). In addition, the STS score was also related to prognosis (HR 1.059, 95% CI: 1.022-1.098; p=0.002).
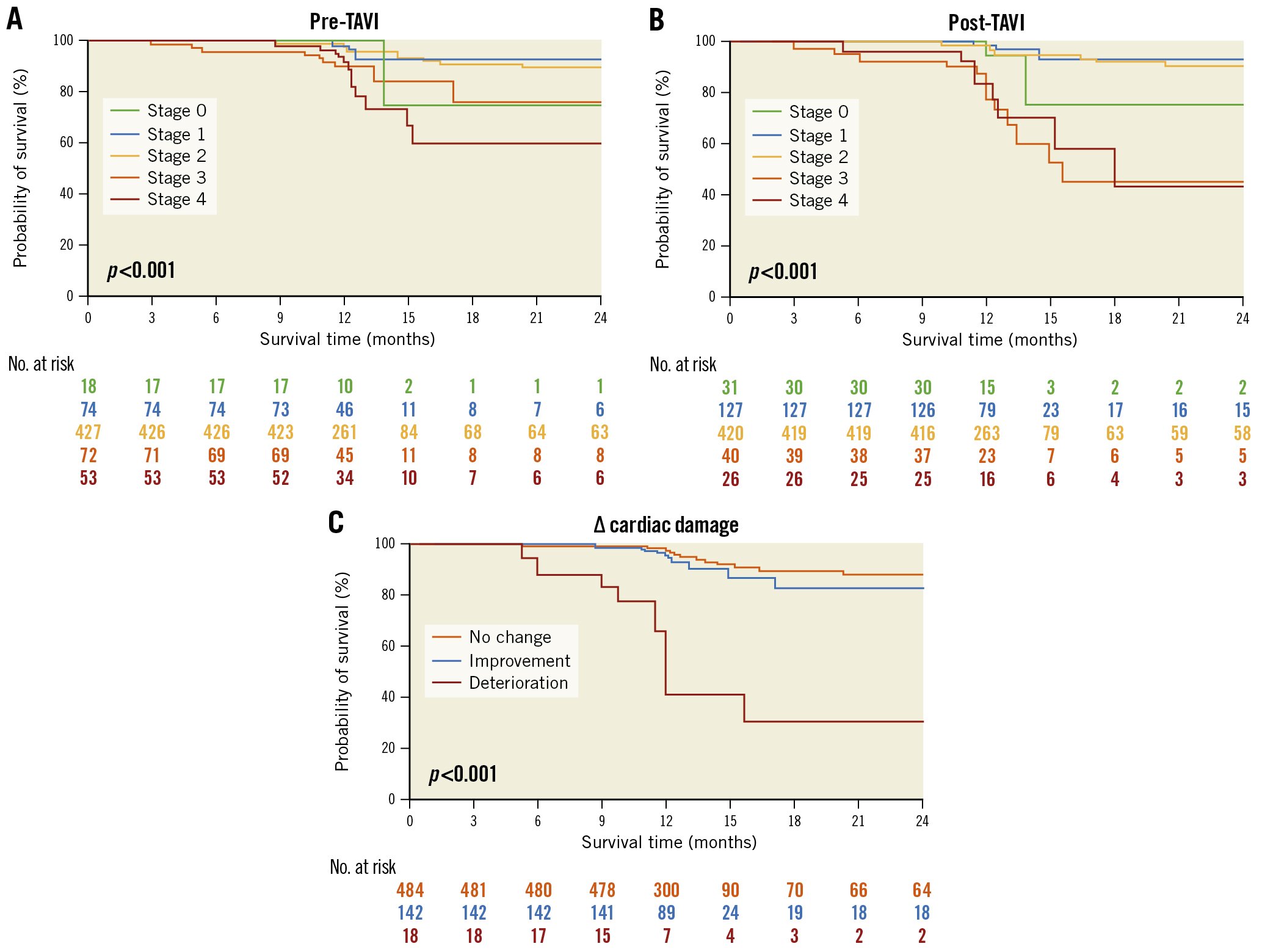
Figure 4. Kaplan-Meier analyses for 2-year survival for the entire patient population. Analyses according to: A) baseline cardiac damage stage; B) 30 days post-TAVI cardiac damage stage, with different colours representing different stages and C) change in cardiac damage stage within 30 days post-TAVI, with red representing deterioration, blue representing improvement, and yellow representing no change. TAVI: transcatheter aortic valve implantation
Table 2. Univariable and multivariable Cox proportional hazards analysis on 2-year survival for TAVI recipients.
| Univariable 95% CI | Multivariable-adjusted 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | Lower limit | Upper limit | p-value | HR | Lower limit | Upper limit | p-value |
| Age | 1.089 | 1.031 | 1.15 | 0.02 | 1.059 | 0.996 | 1.125 | 0.066 |
| Male | 1.956 | 0.674 | 1.356 | 0.8 | ||||
| BMI | 0.92 | 0.83 | 1.019 | 0.105 | ||||
| STS | 1.069 | 1.04 | 1.099 | <0.001 | 1.059 | 1.022 | 1.098 | 0.002 |
| NYHA ≥Class III | 0.403 | 0.149 | 1.09 | 0.073 | ||||
| NT-proBNP (per 1000 pg/ml increase) | 1.013 | 1.001 | 1.025 | 0.029 | 1.002 | 0.987 | 1.018 | 0.78 |
| Smoker | 1.286 | 0.427 | 3.874 | 0.655 | ||||
| Hypertension | 1.823 | 0.693 | 4.796 | 0.224 | ||||
| Diabetes | 1.1 | 0.476 | 2.539 | 0.824 | ||||
| Atrial fibrillation/flutter | 2.974 | 1.463 | 6.044 | 0.003 | ||||
| COPD | 2.234 | 1.11 | 4.494 | 0.024 | 1.631 | 0.752 | 3.536 | 0.215 |
| LVEF | 0.989 | 0.966 | 1.012 | 0.346 | ||||
| V max (m/sec) | 0.75 | 0.5 | 1.125 | 0.165 | ||||
| MPG (mmHg) | 0.986 | 0.966 | 1.005 | 0.152 | ||||
| AVA (m2) | 1.67 | 0.474 | 5.886 | 0.425 | ||||
| CD stage at 30 days (per stage increase) | 2.967 | 2.073 | 4.247 | <0.001 | ||||
| Early deterioration of CD at 30 days | 21.4 | 9.915 | 46.188 | <0.001 | 19.564 | 8.047 | 47.565 | <0.001 |
| AVA: aortic valve area; BMI: body mass index; CD: cardiac damage; CI: confidence interval; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; LVEF: left ventricular ejection fraction; MPG: mean pressure gradient; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortice valve implantation; V max: peak aortic velocity | ||||||||
Discussion
The study is the first to confirm the prognostic impact of both residual and early changes in cardiac damage within 30 days post-TAVI in a large, unselected, real-world cohort of TAVI recipients. This preliminary investigation demonstrates the following findings: (1) the early evolution of cardiac damage and resolution of symptoms shortly after TAVI; (2) the potential reversal of cardiac damage after the TAVI procedure, especially for patients with baseline stage 3-4; (3) a stepwise increase in all-cause mortality for each increment in residual cardiac damage stage; (4) early cardiac deterioration is associated with poor clinical prognosis. On account of early changes in cardiac function post-TAVI, our results highlight the significance of the reassessment of residual cardiac damage to enhance risk stratification for TAVI recipients.
Prevalence and pathogenesis of extra-valvular cardiac damage in AS
Prior studies have demonstrated a high prevalence of extra-valvular cardiac damage in severe AS patients, with reported rates ranging from 51% to 60% for left ventricular hypertrophy, 8% to 51% for atrial fibrillation, 13% to 20% for MR, 11% to 27% for TR, 10% to 36% for severe pulmonary hypertension, and 24% to 29% for RV dysfunction1415. The prevalence is generally in line with our analytical cohort.
To some extent, structural and functional cardiac damage are pathophysiological consequences of severe AS4. Due to the progressive chronic pressure overload, the LV myocardium makes a compensatory concentric hypertrophic response to maintain cardiac output and reduce wall stress. This initial adaptive change leads to a deterioration of both LV systolic and diastolic function1617. Gradually, elevated LV filling pressures may facilitate the development of LA dilation, atrial fibrillation, and mitral regurgitation. Further, the long-standing LA pressure overload will lead to high pulmonary artery pressure, right atrial remodelling, tricuspid regurgitation, and, ultimately, RV dysfunction1819. Notably, individual differences exist in myocardial susceptibility to pressure or volume overload. These may lead to more advanced cardiac damage despite a lower grade of valve disease severity20. Our preliminary study found that patients with a history of COPD and perioperative myocardial infarction were more likely to experience further cardiac damage within 30 days post-TAVI, suggesting the significance of comprehensive management of concomitant diseases in TAVI recipients.
Prognostic value of cardiac damage in AS
Généreux et al proposed the first cardiac damage staging scheme, demonstrating its prognostic value with ~45% increased mortality risk at 1 year for each stage increment in AS patients undergoing AVR6. Our findings confirmed the prognostic value of this classification for TAVI recipients in the Chinese population. Recently, Généreux et al further investigated the value of the change in cardiac damage stage at 1 year, which was associated with 2-year mortality (HR for improvement=0.49; no change=1.0; worsening=1.95; p=0.023). Although this study included 1,974 patients at baseline, 854 patients were subsequently excluded due to death and missing echocardiographic data. Additionally, the finding was restricted to living patients with echocardiographic assessment at one year, with the prognostic time frame from 1 year to 2 years21. From a development point of view, we seemed to end up at the same point, in that the extent and evolution of cardiac damage post-AVR were more critical for AVR recipients compared to the cardiac damage at baseline. By contrast, the majority of patients in our study were consecutive TAVI recipients. We reported the prognostic implications of the stage of 30-day post-TAVI cardiac damage for the first time. Irrespective of the 1-year outcome following TAVI, 30-day post-TAVI cardiac damage can be leveraged to provide a 2-year prognostic value for TAVI recipients. Our findings focus on the post-AVR cardiac damage stage at an earlier point in time and its association with a prognosis with a longer time frame (over the next two years). Early detection of irreversible cardiac damage is beneficial for earlier intervention, adjunctive pharmacotherapy and better prognosis.
Recently, this novel approach has been validated in multiple cohorts of AS patients with and without symptoms1415. These studies have noted that the prognostic value of the staging scheme is independent of whether cardiac damage is caused by AS per se or by concomitant comorbidities. This analysis is consistent with previous work, suggesting the importance of global cardiac health in AS patients before the TAVI procedure. Multiple studies have emphasised a significant adverse prognostic impact of pulmonary artery vasculature or tricuspid valve damage and RV dysfunction in TAVI recipients222324. Notably, the prognostic implication of a more advanced stage is independent of AS patients suffering from other concomitant comorbidities.
Evolution of cardiac damage after TAVI
Studies considering the evolution of extra-aortic cardiac injury after a TAVI procedure are still limited. Previous studies have demonstrated the concept of reversible myocardial remodelling after the removal of a pathological insult following AVR101621. It was suggested that left ventricular hypertrophy regressed by 20% to 30% 1-year post-AVR25. Nevertheless, the timeline is different when it comes to the reversal of cardiac damage with various forms of myocardial responses to afterload reduction.
The most interesting finding in our study is that most reversals occurred in the short period of time following TAVI. Therefore, 30 days post-TAVI turns out to be a reasonable timepoint for echocardiographic reassessment in TAVI recipients. In general, 22.2% of TAVI recipients experienced cardiac damage stage changes (mostly regressions) within 30 days post-TAVI.
Even more to the point, approximately one-half of the patients with baseline stage 3-4 recovered significantly after TAVI. Overall, the proportion of the stage 3-4 group declined from 19.4% (pre-TAVI) to 10.5% (post-TAVI). Our observations were consistent with a recent study suggesting that acute relief in obstruction to LV ejection with TAVI was associated with improvements in RV function and RV-pulmonary artery coupling26. This finding has important implications because previous studies have long debated whether an AVR intervention is favourable for patients with cardiac damage stage 3-4. Based on our results, it is plausible that patients with an advanced baseline stage may benefit immediately from the TAVI intervention (Central illustration).
Until now, very little was found in the literature on the prognostic value of the residual and early changes in cardiac damage for TAVI recipients. Of importance, our study is the first to demonstrate an association between short-term residual cardiac damage and long-term prognosis in TAVI recipients. According to our results, two-year mortality was associated with residual cardiac damage within 30 days post-TAVI (HR 2.97, 95% CI: 2.07-4.25; p<0.001, for linear trend). Moreover, early cardiac deterioration within 30 days post-TAVI was an independent risk factor for 2-year mortality (HR 8.747, 95% CI: 2.801-27.32; p<0.001).
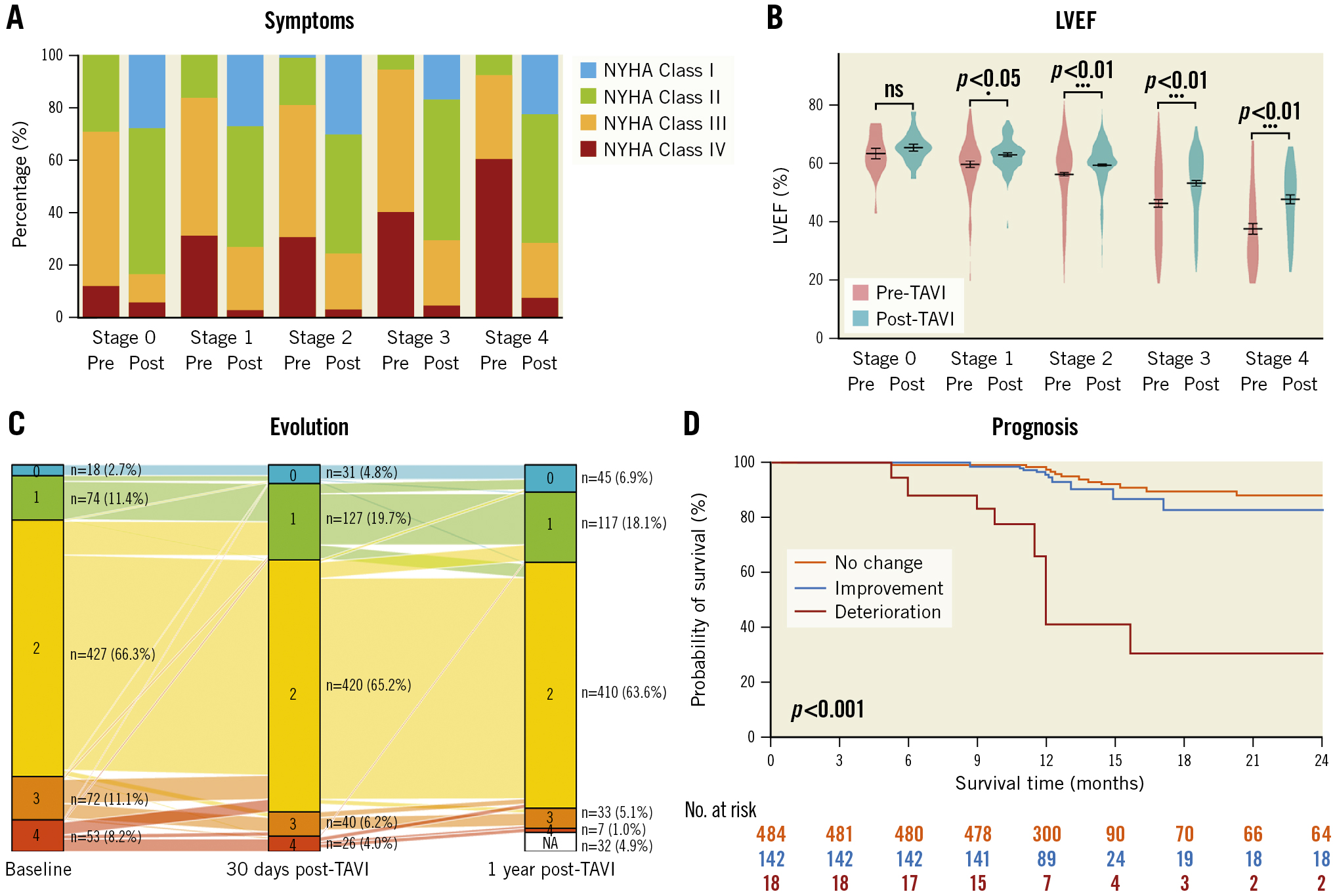
Central illustration. Evolution of NYHA Functional Class, LVEF, cardiac damage stage and 2-year survival. A) NYHA Functional Class (with various colours representing the different degrees of dyspnoea); B) LVEF from baseline to 30 days after TAVI; C) Evolution of cardiac damage stage from baseline to 30 days and at 1 year after TAVI; D) Kaplan-Meier estimates of all-cause mortality. LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; TAVI: transcatheter aortic valve implantation
Clinical implications
First, the stage classification is easy to obtain and based on a multiparameter matrix approach. Integrating more sensitive indices of cardiac dysfunction (speckle tracking imaging) and myocardial structural changes (cardiac magnetic resonance) will further refine the utility of the schemes2728. Furthermore, the comprehensive characterisation of AS implies the emphasis should be on the disease, not just the valve. Our findings suggest the necessity of reassessing cardiac function following TAVI. For TAVI recipients whose cardiac damage remains unpromising, closer surveillance and earlier intervention for comorbidities may be beneficial. Lastly, the concept of cardiac damage staging could extend to other valvular heart diseases, including aortic regurgitation, mitral regurgitation, and tricuspid regurgitation29.
Limitations
First, we acknowledge that our data comes from a single-centre, prospective, observational study. As a preliminary study, we utilised a staging scheme based on a mainly Caucasian population. It may not be sufficient to fully validate the accuracy of the staging system because of inevitable bias and racial differences. Secondly, concerns about the causality also deserve mention due to the small sample size. Given the small number of events, results from the multivariable adjustment should be interpreted with caution. At the same time, other unconsidered factors or concomitant conditions might serve as confounders. Thirdly, the specificity and sensitivity of the proposed staging classification remain to be improved, because its criteria are mostly based on conventional echocardiographic parameters. More evaluations are needed to determine whether other imaging parameters and blood biomarkers could further improve the prognostic value. Finally, the underlying cause of worsening cardiac damage in a small portion of TAVI recipients remains to be unravelled. The most likely culprits are other conditions apart from AS (e.g., coronary artery disease, hypertension, amyloidosis, new-onset atrial fibrillation). Further prospective studies are warranted to determine predictors of early deterioration of cardiac damage after TAVI.
Conclusions
Our preliminary study attempts to shed light on the early changes in cardiac damage and confirm their prognostic value in TAVI recipients. The extent of cardiac damage might regress after the TAVI procedure, especially for AS patients with baseline stage 3-4. By contrast, cardiac deterioration within 30 days is associated with poor clinical prognosis in an AS population following TAVI.
Impact on daily practice
The prognostic value of the cardiac damage staging system has been validated in multiple cohorts of AS patients. However, no evidence exists on short-term changes in cardiac damage post-TAVI, let alone their association with long-term prognosis. This investigation confirms the predictive value of both residual and early change in cardiac damage post-TAVI. Notably, early deterioration of cardiac damage is associated with poor clinical prognosis in an AS population following TAVI. Our study has important implications: it is necessary to reassess residual cardiac damage within short periods after TAVI. It can be leveraged to gain long-term benefits for better risk stratification and prognostication in AS patients undergoing TAVI.
Funding
This work was supported by Zhejiang Province Science and Technology Department Key R&D Program (2021C03097, 2022C03063) and Binjiang Institute of Zhejiang University (ZY202205SMKY001).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

