Abstract
Aims: Myocardial injury occurs frequently following transcatheter aortic valve implantation (TAVI). The aim of this study was to assess timing, predictors, and prognostic value of periprocedural myocardial injury and chronic troponin elevation after TAVI.
Methods and results: Two hundred and seventy-six patients (logistic EuroSCORE 26.6±17.1%) underwent transvascular TAVI. Troponin, CK-MB, and NT-proBNP levels were measured before and after TAVI (1 hr, 4 hrs, 24 hrs, 48 hrs, 72 hrs, seven days, three, and six months). Myocardial injury (according to VARC-2 recommendation defined as ΔTroponin ≥15x URL) occurred in 143/276 patients (51.8%) during the first 72 hours following TAVI. Use of a self-expanding prosthesis (p=0.02), coronary artery disease (p=0.04), higher left ventricular ejection fraction (LVEF) (p<0.001), and procedure time (p<0.001) were independent predictors for the development of myocardial injury after TAVI. Thirty-day (4.2% vs. 6.1%; p=0.48) and one-year mortality (19.4% vs. 26.5%; p=0.15) were not related to the incidence of periprocedural myocardial injury. However, patients with chronic troponin elevation after TAVI had an increased one-year mortality risk (HR 4.5, 95% CI: 2.0-10.0; p<0.001).
Conclusions: Myocardial injury defined as ΔTroponin ≥15x URL after TAVI seems to be a procedure-related issue without impact on 30-day and one-year survival. However, monitoring of post-procedural troponin might be useful for prognostication after TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) has emerged as an alternative treatment strategy to surgical aortic valve replacement (SAVR) in high-risk patients1. In the currently treated patient population, which is characterised by severe comorbidities, the overall prognosis is significantly influenced by periprocedural complications2,3. Periprocedural myocardial infarction is a very rare complication (1-2%) after TAVI, which can occur through coronary ostia occlusion following valve deployment2,3. However, periprocedural myocardial injury indicated by a significant rise in cardiac biomarkers such as troponin during the first 72 hours after TAVI occurs in ≥95% of the patients – depending on the definition4-7.
In patients undergoing SAVR, myocardial injury is a common operative complication and its extent is related to both repeat myocardial infarction and death beyond the immediate postoperative period8. In TAVI patients, definition, timing, predictors, and prognostic value of myocardial injury have not yet been fully elucidated4-9. Cardiac biomarker elevation is significantly higher in transapical TAVI patients through direct trauma of the apex compared to patients undergoing transfemoral TAVI. However, a debate is still ongoing as to whether myocardial injury without evidence of coronary obstruction is associated with an increased mortality rate4-6.
The aims of this study were to: 1) investigate the incidence and timing of myocardial injury defined as troponin increase ΔTroponin ≥15x URL according to the updated Valve Academic Research Consortium-2 (VARC-2) criteria; 2) identify and compare predictors of myocardial injury in a study cohort of patients undergoing transvascular TAVI with the use of self-expanding and balloon-expandable transcatheter heart valves (THV); 3) evaluate the relationship between the extent of myocardial injury and outcome in patients undergoing uncomplicated transvascular TAVI; and 4) assess the prognostic value of troponin elevation after TAVI.
Methods
PATIENT POPULATION
From January 2010 to April 2013, 282 consecutive patients underwent TAVI with the use of the third-generation CoreValve (Medtronic, Minneapolis, MN, USA) or the Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) prosthesis and were included into this prospective study after written informed consent. Procedural success was achieved in 276 of 282 (98%) patients with two periprocedural deaths (0.7%) due to annular rupture and cardiogenic shock after balloon aortic valvuloplasty (BAV) and four conversions to open heart surgery (1.4%) due to device embolisation (n=2) or pericardial tamponade (n=2). In this analysis, patients with conversion to open heart surgery (n=4) were excluded as the surgical trauma itself and extracorporeal circulation led to myocardial injury. Details about patient screening, valve implantation techniques, and adjunctive medication have been described previously in detail10,11.
Invasive coronary angiography was performed in all patients before TAVI and was assessed in the Heart Team discussion. In case of significant CAD (i.e., >50% diameter stenosis on quantitative coronary angiography [QCA]), patients in this study were fully revascularised before the TAVI procedure (staged PCI) except for lesions in small vessels (<2.5 mm), distal lesions, and chronic total occlusions with negative stress test.
The primary endpoint of this study was all-cause mortality at one year. Clinically significant myocardial injury was defined according to the biomarker cut-off value of the VARC-2 definition of periprocedural myocardial infarction as cardiac biomarker elevation within 72 hrs after the index procedure, consisting of at least one sample post procedure with a peak value exceeding 15x the URL for troponin I (>1.5 ng/mL) or 5x for CK-MB (>18 ng/mL). If cardiac biomarkers were increased at baseline (>99th percentile), a further increase of at least 50% post procedure was required, and the peak value had to exceed the previously stated limit9.
Information about the cause of death was obtained from the treating hospital, referring cardiologist, or general practitioner. The study was approved by the local ethics committee of the University of Bonn. The investigators initiated the study, had full access to and analysed the data, and wrote the manuscript. All authors vouch for the data and analysis.
LABORATORY METHODS
Troponin I (Trop I), N-terminal pro-brain natriuretic peptide (NT-proBNP), and creatine kinase MB (CK-MB) levels were measured before and 1 hr, 4 hrs, 24 hrs, 48 hrs, 72 hrs, seven days, three months, and six months after TAVI (CTNI Flex, PBNP Flex, and MMB Flex reagent cartridge, Dimension Vista; Siemens Healthcare Diagnostics GmbH, Munich, Germany). Based on the 99th percentile in a healthy population and the requirement of a ≤10% coefficient variation, the upper reference limits (URL) for Trop I and CK-MB levels in our institution were 0.10 ng/mL and 3.6 ng/mL, respectively.
STATISTICAL ANALYSIS
Statistical analysis is available in the Online Appendix.
Results
MYOCARDIAL INJURY FOLLOWING TAVI
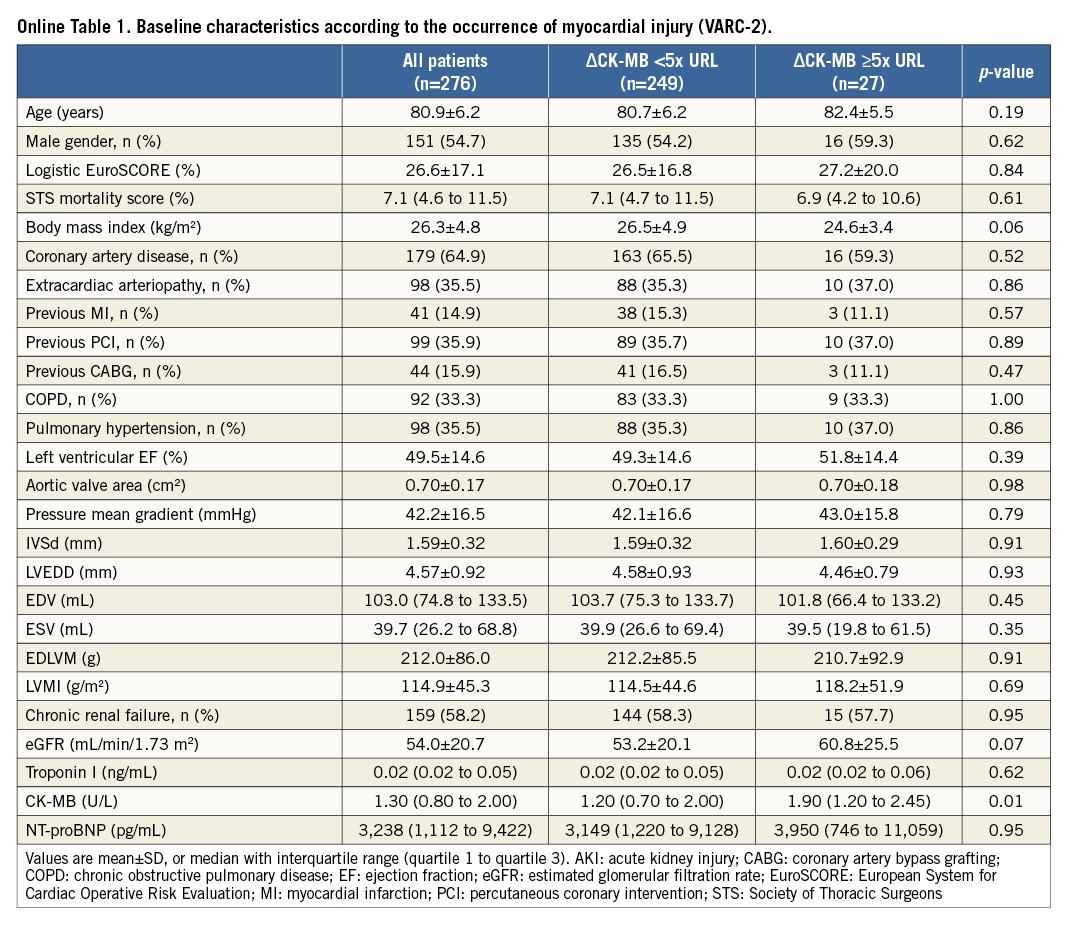
Myocardial injury according to the troponin cut-off value of the VARC-2 definition of myocardial infarction based on serial measurements (ΔTroponin ≥15x URL) occurred in 143 of 276 patients (51.8%) (Figure 1A). Using the CK-MB based definition of myocardial injury, the incidence was 9.1% (25 of 276 patients) (Figure 1B). Baseline troponin (Figure 2) and CK-MB levels (Online Figure 1) did not differ between patients with the occurrence of myocardial injury after TAVI and those without. At baseline, median NT-proBNP levels were significantly lower in patients with myocardial injury after TAVI (p<0.001).
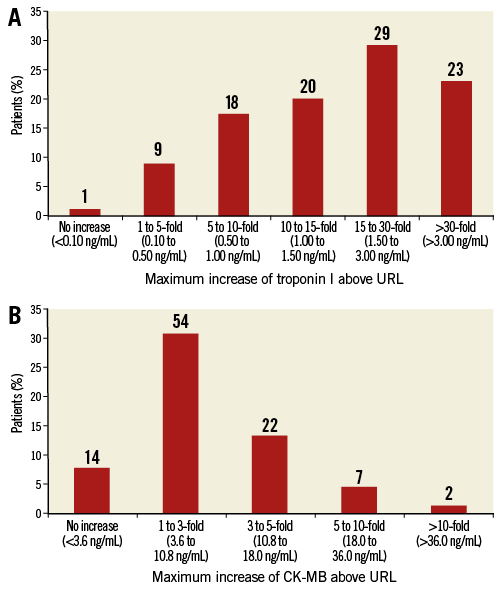
Figure 1. Maximum cardiac biomarker increase following TAVI. Maximum increase in troponin I (A) and CK-MB (B) level above upper reference limit (URL) during the first 72 hrs after transcatheter aortic valve replacement (TAVI).
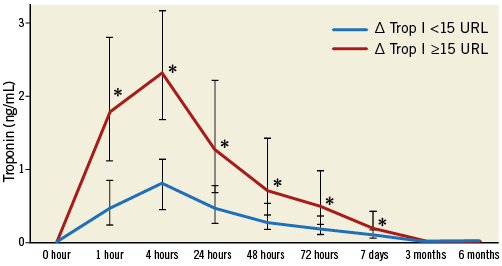
Figure 2. Cardiac biomarker increase according to occurrence of myocardial injury. Median troponin I levels with interquartile range according to the occurrence of myocardial injury after TAVI and at follow-up (ΔTrop ≥15x URL) after TAVI.
After the TAVI procedure, troponin and CK-MB levels (p<0.001) had already increased at 1 hr after deployment of the valve, with significantly higher levels in patients with development of myocardial injury. The periprocedural troponin elevation was highly correlated with CK-MB increase after TAVI at 4 hrs (r=0.82, p<0.001) and at 24 hrs (r=0.87, p<0.001). In patients with myocardial injury, this troponin elevation persisted until day seven (p<0.001).
PREDICTORS OF MYOCARDIAL INJURY
Patients with the occurrence of myocardial injury after TAVI had a higher left ventricular ejection fraction at baseline (53.8±13.2% vs. 44.8±14.5%; p<0.001) and had a lower logistic EuroSCORE (p=0.02) (Table 1). Furthermore, patients with periprocedural myocardial injury had a significantly lower left ventricular mass index (LVMI) (p=0.03), lower end-diastolic volumes (EDV) (p=0.007), and lower end-systolic volumes (ESV) (p<0.001) than patients without the development of myocardial injury. The peak troponin level at 4 hrs after TAVI showed a moderate correlation with pre-procedural LVEF (r=0.31; p<0.001), LVMI (r=–0.24; p<0.001), EDV (r=–0.19; p=0.002) and ESV (r=–0.26; p<0.001).
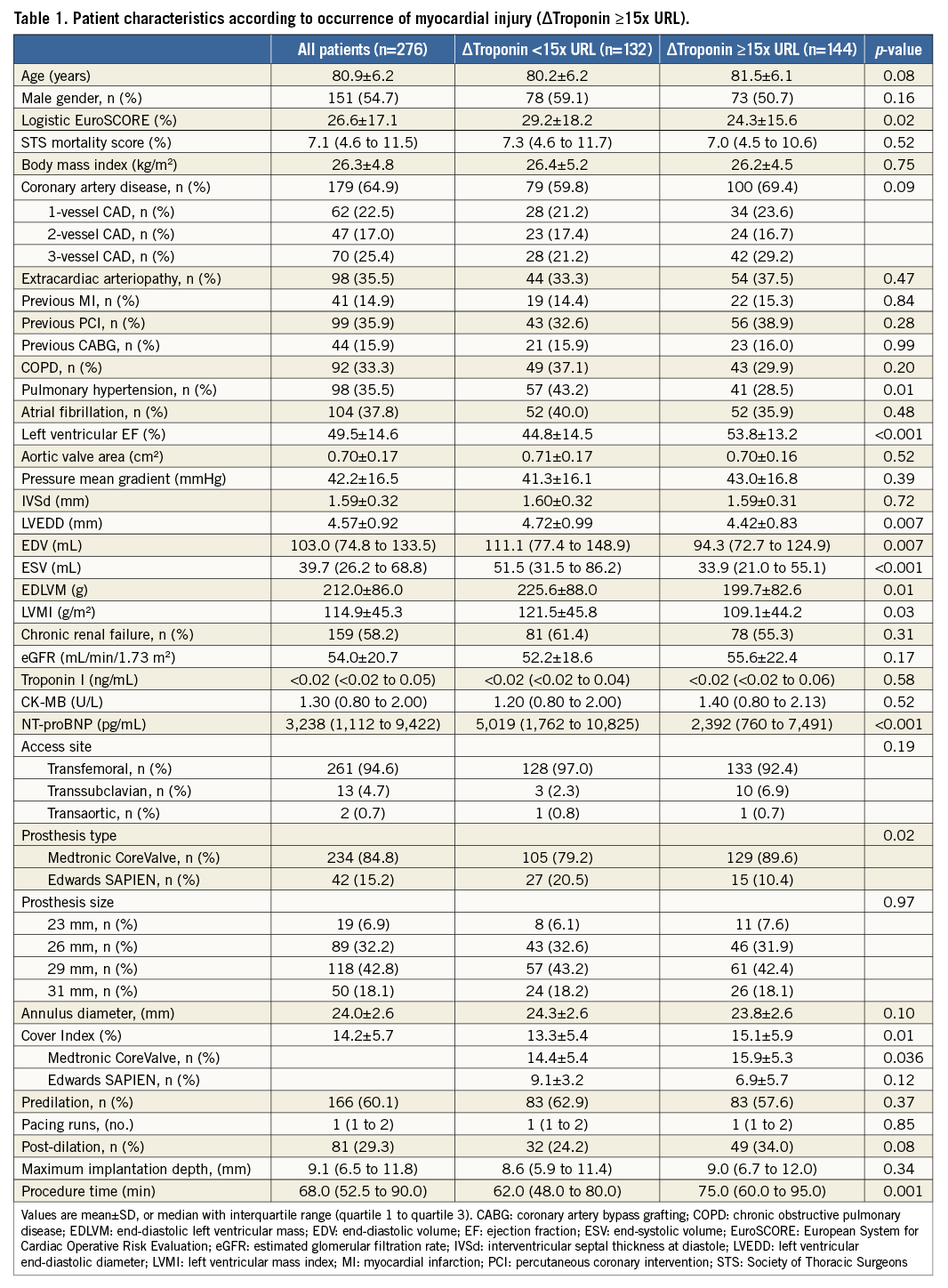
Patients undergoing TAVI with the CoreValve prosthesis developed myocardial injury significantly more often than patients with the Edwards SAPIEN valve (p=0.02). With regard to other procedural characteristics, longer intervention time (75.0 [IQR: 60.0 to 95.0] min vs. 62.0 [IQR: 48.0 to 80.0] min; p=0.001) and the degree of oversizing reflected by a higher cover index (15.1±5.9% vs. 13.3±5.4%; p=0.01) were related to the development of myocardial injury.
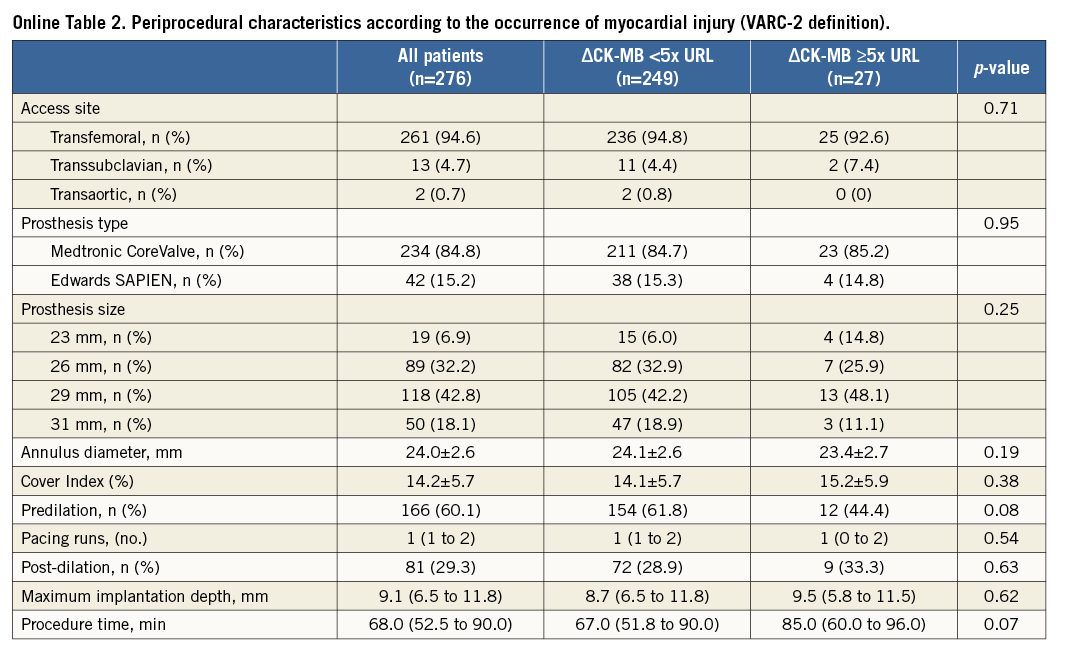
Concerning the CK-MB based definition of myocardial injury (ΔCK-MB ≥5x URL), baseline and procedural characteristics of patients with the occurrence of myocardial injury did not differ significantly from those without (Online Table 1, Online Table 2).
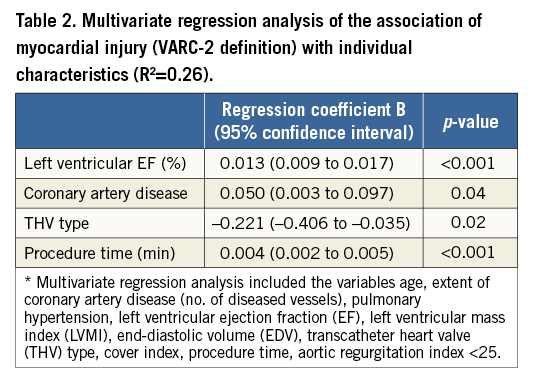
In a multivariate regression analysis, the use of a self-expanding prosthesis (p=0.02), higher LVEF (p<0.001), the extent of coronary artery disease (CAD) (p=0.04), and procedure time (p<0.001) were independent predictors of myocardial injury after TAVI (Table 2).
CLINICAL OUTCOMES AND THE OCCURRENCE OF MYOCARDIAL INJURY AFTER TAVI
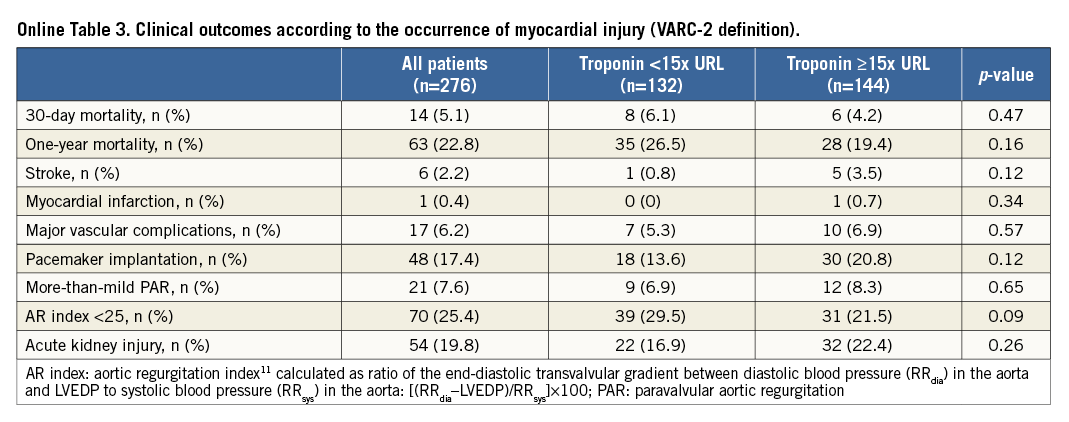
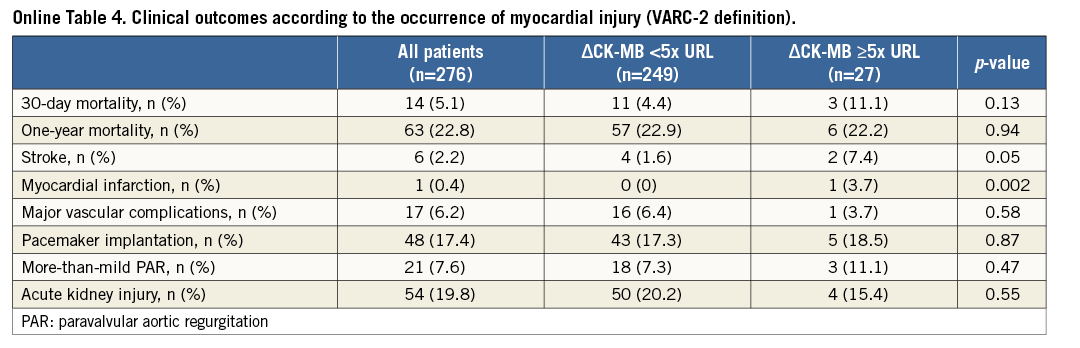
The development of myocardial injury was not related to 30-day (4.2% vs. 6.1%; p=0.48) and one-year mortality (19.4% vs. 26.5%; p=0.15) (Figure 3) or to procedural complications (Online Table 3). The CK-MB based definition of myocardial injury was also not related to outcome (Online Table 4).
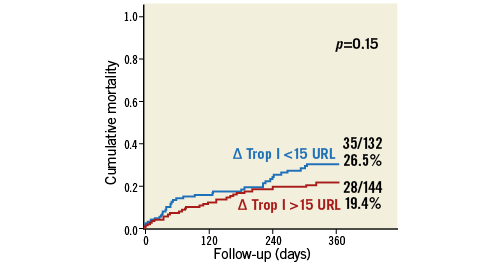
Figure 3. All-cause mortality according to the occurrence of myocardial injury. Cumulative one-year mortality according to the occurrence of myocardial injury defined as ΔTrop ≥15x URL.
CLINICAL OUTCOMES ACCORDING TO CHRONIC TROPONIN ELEVATION BEFORE AND AFTER TAVI
In 13.5% of the patients, baseline troponin was elevated above the URL (>0.10 ng/mL). From day three on, non-survivors after TAVI had significantly higher troponin levels compared to survivors (p<0.001). Non-survivors also showed elevated troponin levels at follow-up after three (p<0.001) and six months (p<0.001) (Figure 4).
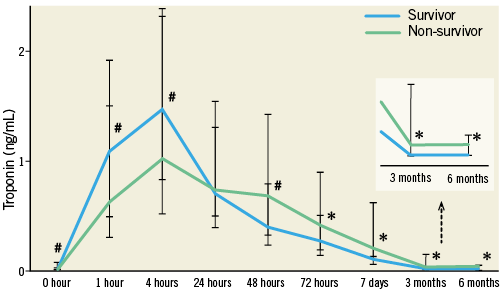
Figure 4. Cardiac biomarker increase according to one-year all-cause mortality. Median troponin I levels with interquartile range after TAVI and at follow-up according to one-year all-cause mortality after TAVI.
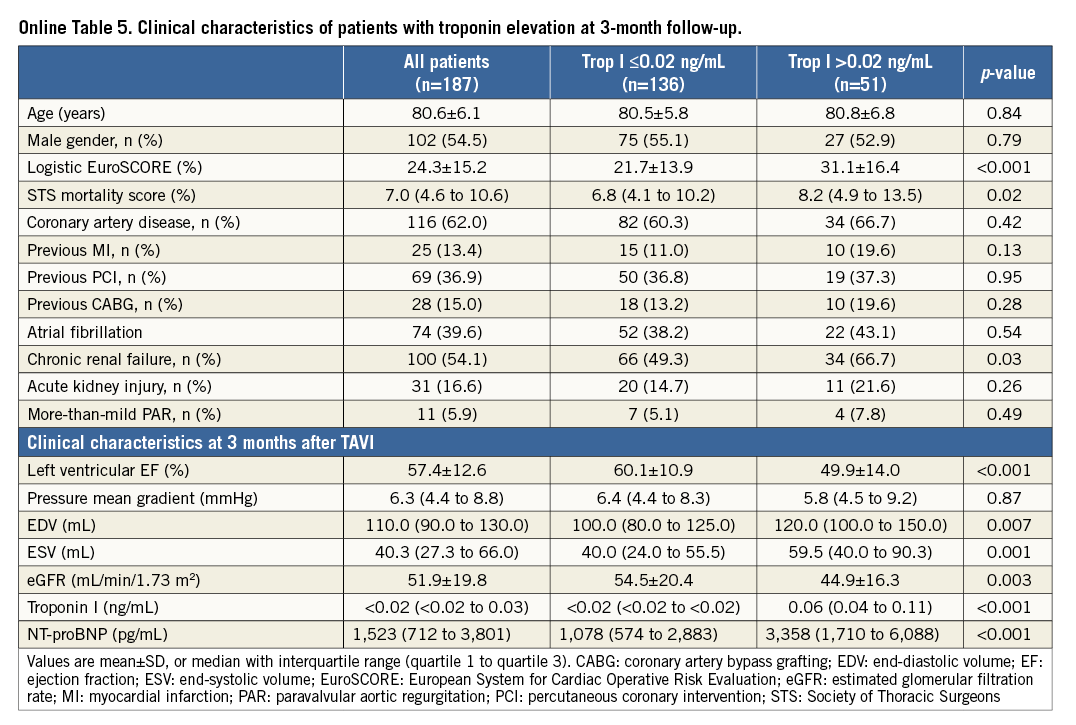
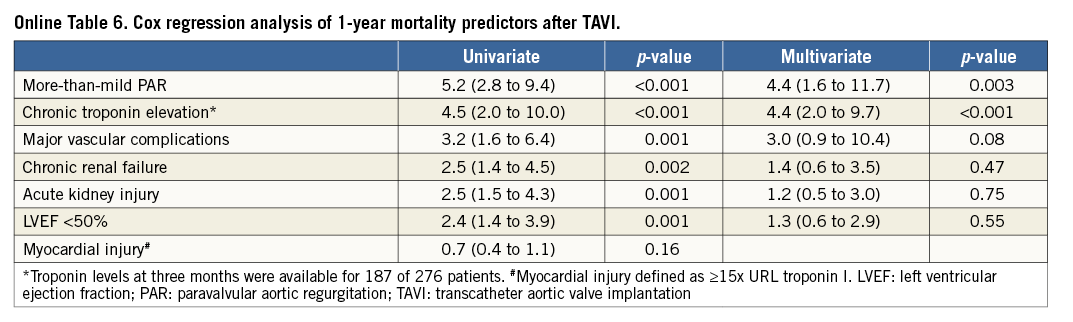
Troponin levels were available for 187 of 243 survivors (77.0%) at three months after TAVI: 27.3% (51 of 187) patients had a troponin elevation >0.02 ng/mL (optimum cut-off in ROC curve analysis; Youden Index 0.38; p<0.001). Patients with chronic troponin elevation had significantly lower LVEF (49.9±14.0 mL vs. 60.1±10.9 mL; p<0.001) and higher NT-proBNP levels (p<0.001) than troponin-negative individuals (Online Table 5). In patients with chronic troponin elevation, one-year mortality risk was increased (29.4% vs. 7.4%; p<0.001) (Figure 5). In multivariate Cox regression analysis with adjustment for independent univariate predictors of outcome, the occurrence of more-than-mild paravalvular AR (Hazard ratio [HR] 4.4, 95% confidence interval [CI]: 1.6 to 11.7, p=0.003) and an elevated troponin after TAVI at follow-up (HR 4.5, 95% CI: 2.0-10.0; p<0.001) were independently associated with outcome after one year (Online Table 6).
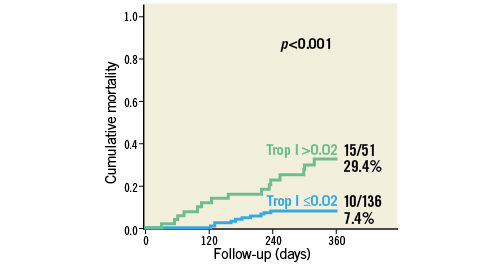
Figure 5. All-cause mortality according to chronic troponin elevation. Cumulative one-year mortality according to chronic troponin elevation (>0.02 ng/mL) at three months after TAVI.
Discussion
This prospective study of 276 percutaneous TAVI patients represents the largest study cohort so far for the assessment of myocardial injury with serial cardiac biomarker measurements following a standardised protocol in non-transapical TAVI patients. We were able to demonstrate that myocardial injury defined according to a troponin elevation ≥15x URL (in accordance with the troponin cut-off value for the VARC-2 definition of TAVI-related myocardial infarction) occurs in more than half of the patients during the first 72 hours after TAVI. The periprocedural troponin elevation was associated with the use of a self-expanding valve, the extent of CAD, and procedure time, but was not related to complications or outcome. Our results might influence the VARC definition of myocardial infarction and injury, when validated in other series, as higher troponin cut-off values for the definition of both myocardial injury and infarction should be used in TAVI patients. Finally, persistently increased troponin levels after TAVI may be considered for the purpose of prognostication in TAVI patients.
PREDICTIVE FACTORS OF PERIPROCEDURAL MYOCARDIAL INJURY AFTER TAVI
Periprocedural myocardial infarction, which is predominantly caused by coronary obstruction through leaflet parts of the native aortic valve after BAV or deployment of the valve, occurs in fewer than 1% of patients undergoing TAVI2,3. The updated VARC-2 criteria recommend the use of a relatively conservative definition of myocardial infarction based on a biomarker increase AND clinical signs of infarction. This may explain –at least in part– the low rate of myocardial infarction reported after TAVI9. However, TAVI –independent from the access route– seems to be systematically associated with some degree of myocardial injury as determined by troponin or CK-MB release following the procedure4-6. In contrast to patients with acute coronary syndrome (ACS) or undergoing cardiac surgery8,12-14, the source and release pattern of troponin in TAVI patients is less clear.
Rodes-Cabau et al were the first to report on the incidence of troponin increase after TAVI in their study, in which 97% of transfemoral patients (N=38) and 100% of the transapical patients (N=63) showed a troponin increase. In contrast to our study, the prevalence of CAD –independent of whether fully revascularised or not before the procedure– was not associated with myocardial injury4,6. A debate is still ongoing as to whether the extent of CAD and especially the revascularisation status reflected by residual SYNTAX score is associated with impaired clinical outcome and a higher cardiovascular event rate after TAVI15-17. In our study, which focused on transvascular TAVI patients, the extent of baseline CAD, which was quantified by QCA, was associated with the incidence of myocardial injury, although all patients were revascularised if indicated before TAVI. However, myocardial injury also occurred in patients without evidence of CAD (45.8% vs. 56.4%; p=0.09). Therefore, the early rise of troponin after the procedure might be caused by direct trauma of the myocardium through the valve implantation itself rather than ischaemia with subsequent apoptosis of myocardial cells12.
Recent studies hypothesise that factors such as global myocardial ischaemia as a result of a myocardial oxygen demand-supply mismatch during several steps of the procedure including predilation and pacing, which lead to transient hypotension, microembolism induced by device delivery, and myocardial tissue compression by the expansion of the device, should be considered as potential sources of myocardial injury3,4,6. Our study is the first to demonstrate that the THV type and the deployment technique of the prosthesis itself seem to be major sources for cardiac biomarker release (Figure 6). Therefore, we hypothesise that self-expanding valves with a higher degree of oversizing, which was also reflected by the cover index in our study, might lead to a greater myocardial tissue compression and trauma than balloon-expandable valves.
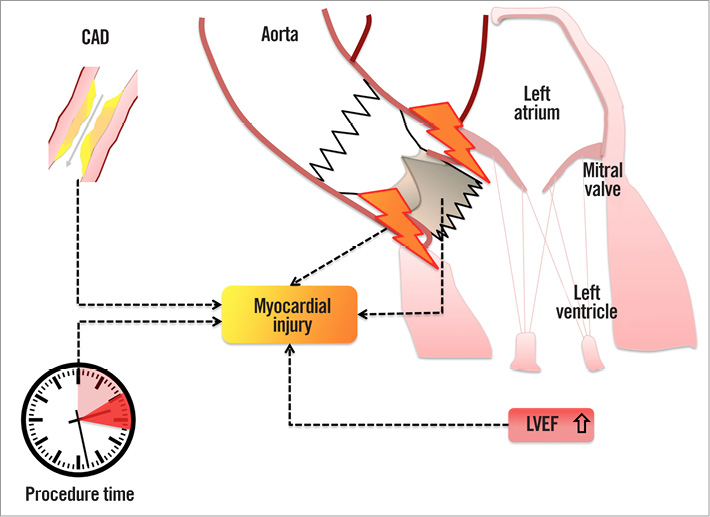
Figure 6. Variables contributing to myocardial injury in patients undergoing TAVI. Myocardial injury following transvascular TAVI is associated with the use of a self-expanding transcatheter heart valve, prolonged procedure time, the prevalence of coronary artery disease (CAD), and a higher left ventricular ejection fraction (LVEF) at baseline.
Surprisingly, a higher LVEF as well as lower NT-proBNP levels at baseline, respectively, were found to be related to the development of myocardial injury in our study cohort. This might be explained by the fact that patients with preserved LVEF have more myocardium at risk, whereas patients with low ejection fraction (and high NT-proBNP levels) might have a greater amount of infarcted, scarred, or inactive myocardium, which tends to release lower levels of troponin or CK-MB through TAVI-related myocardial tissue compression, etc6,18. Another mechanism, which might contribute to relative ischaemia, could be the decreased cardiac output through acute afterload reduction after TAVI and consecutive hypercontractility, especially in patients with higher LVEF at baseline and smaller LV dimensions. This hypothesis would be in line with the unexpectedly lower LVMI in patients developing myocardial injury after TAVI in our study. Hypercontractility would lead to a vicious circle with elevated LVEDP, increased oxygen consumption, and further decrease of coronary blood flow in systole19. In this context, the prevalence of CAD could even aggravate the underlying supply-demand mismatch and additionally lead to subendocardial ischaemia (Type 2 myocardial infarction)12.
TROPONIN RELEASE PATTERN AFTER TAVI AND OUTCOME
In contrast to previous reports4-6, our analysis, comprising only non-transapical patients without conversion to open heart surgery (excluding access-related myocardial injury), did not find any prognostic value of either the maximum troponin or CK-MB elevation to predict post-procedural mortality.
However, our study demonstrated that elevated troponin levels after the TAVI procedure during follow-up at three and six months –regardless of the occurrence of myocardial injury– are related to mortality. In heart failure patients, it has been demonstrated that patients with an elevated troponin level typically present with a more decompensated profile, including higher filling pressures, lower ejection fraction, and worse cardiac performance15,20. In line with this hypothesis, patients with a chronic troponin elevation after TAVI, which was associated with lower LVEF during follow-up in our study, had a more than fourfold increased one-year mortality risk. Thus, a persistent minor troponin elevation might be helpful to identify patients with an increased risk of mortality after TAVI as described for heart failure and ACS patients21,22.
STUDY LIMITATIONS
The exact pathomechanism of myocardial injury in TAVI patients remains speculative and cannot be answered by a clinical study. The extent of CAD has not been quantified by an objective tool such as the SYNTAX score, as all patients were fully revascularised before TAVI as part of a staged procedure, if indicated. Only experimental studies can further verify the involvement of the identified clinical predictors in myocardial injury. Since our study is hypothesis-generating, larger studies are needed for the validation of our study results, since it may be underpowered for mortality due to its small sample size and the low periprocedural rate of “true” myocardial infarction. However, it represents the largest study cohort so far with a focus on transvascular patients. In addition, future studies should evaluate next-generation valves and the associated procedure-related myocardial trauma with focus on deployment technique and new features such as repositionability.
Conclusions
Myocardial injury after TAVI, defined as an increase in troponin I ≥15x URL, seems to be a procedure-related issue without impact on survival. Chronic troponin elevation during follow-up may be considered for the purpose of prognostication in TAVI patients.
| Impact on daily practice Cardiac biomarker elevation is significantly higher in transapical transcatheter aortic valve implantation (TAVI) patients through direct trauma of the apex compared to patients undergoing transfemoral TAVI. Myocardial injury, defined according to a troponin elevation ≥15x URL (VARC-2), occurs in more than half of the patients with uncomplicated course during the first 72 hours after transvascular TAVI. Periprocedural troponin elevation is associated with periprocedural factors such as the use of a self-expanding valve, the extent of coronary artery disease, and procedure time but did not have any impact on outcome. According to our study, higher troponin cut-off values are needed for the definition of myocardial injury and infarction in TAVI patients. Furthermore, a persistent (“chronic”) troponin elevation seems to be helpful to identify patients with an increased risk of mortality after TAVI. |
Funding
J.M. Sinning is supported by a research grant from the University Hospital Bonn (BONFOR). No extramural funding was used to support this work.
Conflict of interest statement
J.M. Sinning, E. Grube, G. Nickenig, and N. Werner receive research grants and speaker honoraria from Medtronic and Edwards Lifesciences. E. Grube works as proctor for Medtronic. The other authors have no conflicts of interest to declare.
SUPPLEMENTARY DATA
Online Appendix. Statistical analysis
Data are presented as mean±standard deviation if normally distributed or as median and interquartile range (IQR: quartile 1 to quartile 3) if not normally distributed. Continuous variables were tested for normal distribution with the use of the Kolmogorov-Smirnov test. Categorical variables are given as frequencies and percentages. For continuous variables, a Student’s t-test was used for comparison between two groups. When comparing more than two groups, ANOVA or the Kruskal-Wallis test was used. Pearson’s and Spearman’s correlation coefficients were used to establish associations. For categorical variables, the χ2 or Fisher’s exact test were used for further analysis. Multiple linear regression analysis was applied to identify factors which were independently associated with myocardial injury (p<0.1 in univariate analysis), and included age, coronary artery disease (CAD), pulmonary hypertension, LVEF, LVMI, EDV, post-dilation, cover index, THV type, procedure time, aortic regurgitation (AR) index <25.
Survival after TAVI was determined with the use of the Kaplan-Meier method. The log-rank test was used to determine statistical differences in terms of survival. We performed univariate and multivariate Cox regression analysis to assess the predictive value of myocardial injury and chronic troponin elevation (Troponin >0.02 ng/mL) in comparison with other one-year mortality predictors.
Based on the results of previous studies4-7 with an approximately two times increased mortality risk for patients with myocardial injury after TAVR, we estimated a one-year all-cause mortality of 20% for patients without myocardial injury and planned a study of independent cases and controls with one control per case. If the true mortality rate for experimental subjects was 40% as suggested by these studies4-7, we would need to study 118 individuals with and 118 control individuals without myocardial injury to be able to reject the null hypothesis that the mortality rates after TAVR are independent from the occurrence myocardial injury with a probability (power) 0.9. The Type I error probability associated with this test of the null hypothesis is 0.05. We decided to use the Fisher’s exact test to evaluate this null hypothesis.
All tests were two-sided, and a p-value of <0.05 was considered statistically significant. Statistical analyses were conducted with PASW Statistics version 21.0.0.0.0 64-bit (IBM Corporation, Somers, NY, USA) and MedCalc version 11.1.1.0 (MedCalc Software, Mariakerke, Belgium).
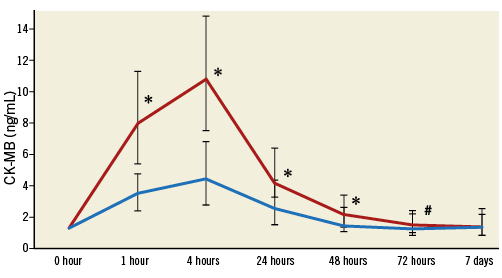
Online Figure 1. Median CK-MB levels with interquartile range according to the occurrence of myocardial injury after TAVR (ΔCK-MB ≥5x URL) after TAVR.