Abstract
Background: Coronary access after transcatheter aortic valve replacement (TAVR) can be challenging and complicate percutaneous coronary intervention (PCI).
Aims: We aimed to investigate the incidence, characteristics, and predictors of unplanned PCI after TAVR.
Methods: In a single-centre registry, TAVR candidates were systematically screened for concomitant coronary artery disease (CAD) through the use of coronary angiography prior to TAVR. Rates of unplanned PCI were prospectively collected and independently adjudicated.
Results: Among 3,015 patients undergoing TAVR between August 2007 and December 2020, 67 patients (2.2%) underwent unplanned PCI after TAVR. The indication for unplanned PCI was acute coronary syndrome in more than half of the cases. Patients with unplanned PCI were younger (80.2±6.5 years vs 81.9±6.4 years; p=0.028) and more likely to be male (75% vs 50%; p<0.001) than those without unplanned PCI. In a multivariable analysis, the number of diseased vessels, male sex, and younger age were independently associated with an increased risk of unplanned PCI. The cumulative incidence rates of unplanned PCI at 1, 5, and 10 years were 0.1%, 0.4%, and 0.6% in patients with no CAD at the time of TAVR, 0.7%, 2.5%, and 3.4% in patients with single-vessel disease, and 1.5%, 5.4%, and 7.4% in patients with multivessel disease, respectively.
Conclusions: The lifetime risk of unplanned PCI after TAVR is low in patients with no CAD at the time of TAVR but accumulates over time in patients with known CAD, particularly multivessel disease. ClinicalTrials.gov: NCT01368250.
Introduction
A majority of patients with severe aortic stenosis (AS) can be safely and effectively treated with both transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) based on periprocedural risk and anatomic criteria12. However, the lifetime management of patients with AS introduces an additional level of complexity to the Heart Team’s decision. The selection of the optimal treatment strategy for younger patients with a longer life expectancy requires the integration of downstream cardiac risk due to concomitant coronary artery disease (CAD), combined valvulopathies, and repeat and future cardiac interventions3.
Coronary access after TAVR is a concern with important repercussions on lifetime management of patients with severe AS. Previous studies have detailed the challenges of selective coronary access for percutaneous coronary intervention (PCI) after TAVR, particularly in the presence of supra-annular devices featuring tall stent frames12. The need for repeat TAVR in younger patients is anticipated to further exacerbate the complexity of coronary access, particularly in the setting of low coronary offtake in relation to the neoskirt and misalignment of the two stent frames345. The complexity of coronary access directly translates into adverse clinical outcomes in patients presenting with acute coronary syndrome (ACS)6 and needs to be anticipated before the implantation of the first transcatheter heart valve (THV). Thus, in order to tailor lifetime management to an individual context, it is crucial to estimate during treatment for AS the probability that PCI will be required in the future.
In the present study, we aimed to evaluate the incidence, characteristics, and predictors of unplanned PCI after TAVR in a prospective TAVR registry.
Methods
Study population
The Bern TAVR registry is a prospective registry enrolling consecutive patients undergoing TAVR at Bern University Hospital, which forms part of the nationwide SwissTAVI Registry (ClinicalTrials.gov: NCT01368250). The registry was approved by the ethics committee, and all patients provided written informed consent for participation. The study was conducted in compliance with the Declaration of Helsinki.
Patients undergoing TAVR were systematically screened for concomitant CAD by means of coronary angiography prior to TAVR. Concomitant CAD was treated with PCI before, during, or after TAVR based on the Heart Team's decision, taking into account myocardium at risk, lesion complexity, and symptom status7. Functional ischaemia testing was not routinely performed during the study period.
Data collection and outcome measures
Baseline clinical characteristics, procedural, and follow-up data were prospectively recorded in a web-based database. Computed tomographic imaging data were independently re-evaluated by dedicated imaging specialists, as previously described, and integrated into the database8. The presence of CAD was defined by a history of surgical and/or percutaneous coronary revascularisation, previous myocardial infarction (MI), and/or at least 1 significant lesion (diameter stenosis ≥50%) in a major native coronary artery by visual assessment with coronary angiography prior to TAVR9.
Regular clinical follow-up was scheduled at 30 days, 1 year, 5 years, and 10 years after TAVR, and the data were obtained by standardised interviews, documentation from referring physicians, and hospital discharge summaries. All adverse events, including unplanned PCI, were systematically collected and adjudicated by a dedicated clinical event committee. The Clinical Trials Unit Bern was responsible for central data monitoring to verify the completeness and accuracy of data, and to perform independent statistical analysis. In the registry, unplanned PCI included all PCI following TAVR, excluding staged PCI planned at the time of TAVR. PCI was performed in accordance with guidelines at the corresponding time of intervention7. For the purpose of the present study, coronary revascularisation resulting from mechanical coronary obstruction complicating TAVR was excluded.
Statistical analysis
Categorical variables are reported as frequencies and percentages. Continuous variables are presented as mean values±standard deviation (SD) or median values with interquartile ranges (IQR). Univariable Cox proportional hazard models were used to calculate hazard ratios (HR), 95% confidence intervals (CI), and p-values. The cumulative rate of PCI over time was represented through a cumulative incidence curve. Multivariable Fine and Gray regressions (with all-cause death as a competing risk) were used to build a prediction model for the incidence of unplanned PCI. All variables potentially related to unplanned PCI were tested in a univariable model, and variables with a p-value <0.2 were subsequently entered into a multivariable model. All p-values were 2-sided, and a p-value <0.05 was considered significant for all tests. All statistical analyses were performed with Stata 17.0 (StataCorp).
Results
Incidence of unplanned PCI
Among 3,015 patients undergoing TAVR between August 2007 and December 2020, 109 patients (3.6%) underwent staged PCI after TAVR and 67 patients (2.2%) underwent unplanned PCI during follow-up. The median follow-up time available for living patients was 1,095 (IQR 366-1,824) days, and the median time to death was 799 (IQR 268-1,485) days. The cumulative incidence rates of unplanned PCI were 0.4%, 1.6%, and 2.3% at 1, 5, and 10 years, respectively (Figure 1). The median interval from TAVR to unplanned PCI was 605 (IQR 292-1,340) days.
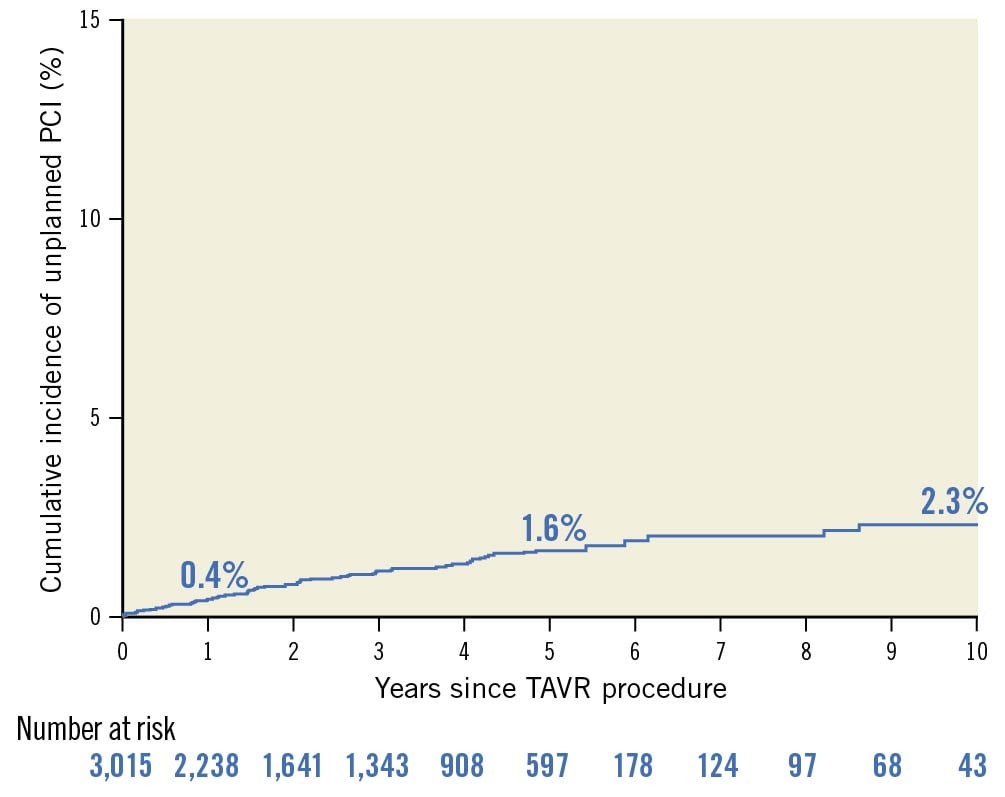
Figure 1. Lifetime risk of unplanned PCI after TAVR. Cumulative incidence of unplanned PCI after TAVR considering competing risk with death is shown. PCI: percutaneous coronary intervention; TAVR: transcatheter aortic valve replacement
Baseline and procedural characteristics
Baseline characteristics of patients with and without unplanned PCI after TAVR are shown in Table 1. Patients who underwent unplanned PCI after TAVR were younger (80.2±6.5 years vs 81.9±6.4 years; p=0.028), more likely to be male (75% vs 50%; p<0.001), and more frequently had peripheral artery disease (21% vs 13%; p=0.048) than those without unplanned PCI. There were no significant differences in traditional risk factors (hypertension, diabetes mellitus, and dyslipidaemia), chronic kidney disease, or previous cerebrovascular events between the 2 groups. Pre-TAVR coronary angiographies revealed that 94% of patients who underwent unplanned PCI had CAD at baseline, compared with 59% of patients who did not undergo unplanned PCI (p<0.001). CAD was more likely to be multivessel disease in patients with unplanned PCI than in those without unplanned PCI (p=0.025). Patients with unplanned PCI were more likely to have had previous myocardial infarction (30% vs 14%; p<0.001) and a history of PCI (52% vs 26%; p<0.001).
Table 1. Baseline characteristics.
|
Cohort |
Control |
Unplanned PCI |
p-value* |
|
|---|---|---|---|---|
|
N=3,015 |
N=2,948 |
N=67 |
||
|
Age, years |
81.9±6.4 |
81.9±6.4 |
80.2±6.5 |
0.028 |
|
Gender, male |
1,521 (50%) |
1,471 (50%) |
50 (75%) |
<0.001 |
|
Body mass index, kg/cm2 |
26.6±5.3 |
26.6±5.3 |
27.6±5.0 |
0.154 |
|
STS calculated risk of mortality |
5.2±4.1 |
5.2±4.1 |
4.6±2.8 |
0.176 |
|
Risk factors |
||||
|
Hypertension |
2,628 (87%) |
2,566 (87%) |
62 (93%) |
0.128 |
|
Diabetes mellitus |
805 (27%) |
785 (27%) |
20 (30%) |
0.240 |
|
Dyslipedaemia |
2,009 (67%) |
1,957 (66%) |
52 (78%) |
0.060 |
|
Renal failure (GFR<60) |
2,027 (67%) |
1,983 (67%) |
44 (66%) |
0.941 |
|
History of cerebrovascular accident |
352 (12%) |
342 (12%) |
10 (15%) |
0.288 |
|
Peripheral artery disease |
408 (14%) |
394 (13%) |
14 (21%) |
0.048 |
|
Coronary artery disease |
||||
|
Coronary artery disease |
1,797 (60%) |
1,734 (59%) |
63 (94%) |
<0.001 |
|
Number of vessels involved |
n=1,797 |
n=1,734 |
n=63 |
0.025 |
|
1VD |
656 (37%) |
64 (37%) |
13 (21%) |
|
|
2VD |
510 (28%) |
490 (28%) |
20 (32%) |
|
|
3VD |
631 (35%) |
601 (35%) |
30 (48%) |
|
|
History of MI |
429 (14%) |
409 (14%) |
20 (30%) |
<0.001 |
|
Previous CABG |
347 (12%) |
335 (11%) |
12 (18%) |
0.170 |
|
Previous PCI |
806 (27%) |
771 (26%) |
35 (52%) |
<0.001 |
|
Computed tomography |
||||
|
Left coronary height, mm |
14.8±3.6 |
14.8±3.6 |
15.2±3.8 |
0.230 |
|
Right coronary height, mm |
17.7±3.4 |
17.7±3.4 |
18.3±2.7 |
0.024 |
|
Aortic valve calcium volume, mm3 |
307.9±329.5 |
309.0±331.5 |
259.3±228.5 |
0.138 |
|
Sinus of Valsalva diameter, mm |
32.1±4.7 |
32.0±4.7 |
32.9±2.7 |
0.298 |
|
Procedural data |
||||
|
Femoral main access |
2,728 (90%) |
2,669 (91%) |
59 (88%) |
0.816 |
|
Transcatheter heart valve type |
n=3,011 |
n=2,944 |
n=67 |
0.936 |
|
Balloon-expandable |
1,530 (51%) |
1,497 (51%) |
33 (49%) |
|
|
Self-expanding |
1,334 (44%) |
1,304 (44%) |
30 (45%) |
|
|
Mechanically expandable |
147 (5%) |
143 (5%) |
4 (6%) |
|
|
Concomitant revascularisation |
243 (8%) |
238 (8%) |
5 (7%) |
0.479 |
|
*p-values from univariable Cox regressions with time-to-unplanned PCI as the outcome. Aortic valve calcium volume was quantified as previously described8. CABG: coronary artery bypass surgery; GFR: glomerular filtration rate; MI: myocardial infarction; PCI: percutaneous coronary intervention; STS: Society of Thoracic Surgeons; VD: vessel disease |
||||
Details of TAVR procedures are shown in Table 1. TAVR was performed via transfemoral access in 90% of patients, without a significant difference between groups. There was no significant difference in the type of THV used between groups.
Predictors of unplanned PCI
In a multivariable model, the number of diseased vessels, male sex, and younger age were independently associated with an increased risk of unplanned PCI after TAVR, while traditional risk factors, previous peripheral artery disease, previous myocardial infarction and a history of PCI were not (Table 2, Supplementary Figure 1, Supplementary Figure 2). The cumulative incidence curves stratified by the presence of CAD and number of diseased vessels prior to TAVR are shown in the Central illustration. The cumulative incidence rates of unplanned PCI at 1, 5, and 10 years were 0.1%, 0.4%, and 0.6%, respectively, in patients with no CAD. In patients with single-vessel disease, the unplanned PCI rates were 0.7%, 2.5%, and 3.4%, respectively. In patients with multivessel disease, unplanned PCI was performed in 1.5%, 5.4%, and 7.4% at 1, 5 and 10 years, respectively. The effect of CAD was largely consistent after excluding patients with a history of coronary artery bypass grafting (Supplementary Figure 1).
Table 2. Predictors of unplanned PCI under competing risk with death.
|
Variables |
Subhazard ratio (95% CI) |
p-value |
|---|---|---|
|
Univariable |
||
|
Coronary artery disease |
||
|
None |
[Ref.] |
|
|
Single-vessel disease |
5.59 (1.82-17.14) |
0.003 |
|
Multivessel disease |
12.26 (4.43-33.90) |
<0.001 |
|
Age, years |
0.96 (0.93-0.99) |
0.006 |
|
Gender, male |
3.03 (1.75-5.26) |
<0.001 |
|
Body mass index, kg/m² |
1.04 (1.00-1.07) |
0.058 |
|
Hypertension |
2.02 (0.81-5.01) |
0.130 |
|
Diabetes mellitus |
1.22 (0.72-2.05) |
0.462 |
|
Dyslipidaemia |
1.83 (1.03-3.25) |
0.040 |
|
Peripheral artery disease |
1.60 (0.89-2.88) |
0.117 |
|
History of MI |
2.46 (1.47-4.14) |
0.001 |
|
Previous CABG |
1.54 (0.84-2.83) |
0.163 |
|
Previous PCI |
2.95 (1.83-4.76) |
<0.001 |
|
Concomitant PCI |
0.72 (0.29-1.81) |
0.489 |
|
Aortic valve calcification, mm³* |
1.00 (1.00-1.00) |
0.099 |
|
Multivariable |
||
|
Coronary artery disease |
||
|
None |
[Ref.] |
|
|
Single-vessel disease |
4.10 (1.29-13.10) |
0.017 |
|
Multivessel disease |
8.63 (2.85-26.09) |
<0.001 |
|
Age, years |
0.96 (0.92-1.00) |
0.039 |
|
Gender, female |
0.37 (0.20-0.67) |
0.001 |
|
Body mass index, kg/m² |
1.04 (0.99-1.09) |
0.141 |
|
Hypertension |
1.84 (0.64-5.22) |
0.255 |
|
Dyslipidaemia |
0.85 (0.44-1.64) |
0.631 |
|
Peripheral artery disease |
1.16 (0.61-2.22) |
0.655 |
|
History of MI |
1.36 (0.74-2.48) |
0.320 |
|
History of PCI |
1.14 (0.63-2.06) |
0.669 |
|
Previous CABG |
0.50 (0.24-1.03) |
0.061 |
|
Aortic valve calcification, cm³* |
0.46 (0.19-1.11) |
0.085 |
|
*Aortic valve calcification was quantified in contrast-enhanced images using a predefined Hounsfield unit threshold of 850, as previously described8. Multivariable model selecting variables with a p-value <0.2 in the univariable analysis. Multivariable analysis was based on 2,460 patients with CT imaging data. Single imputation of the mode (or mean for BMI) for missing data: n=3 CABG, n=3 PCI and n=10 BMI. A multivariable model without aortic valve calcification in the entire cohort (N=3,015) is shown in Supplementary Table 1. A multivariable model with a limited number of variables with a p-value <0.1 in the univariable analysis is shown in Supplementary Table 2. BMI: body mass index; CABG: coronary bypass grafting; CT: computed tomography; MI: myocardial infarction; PCI: percutaneous coronary intervention |
||
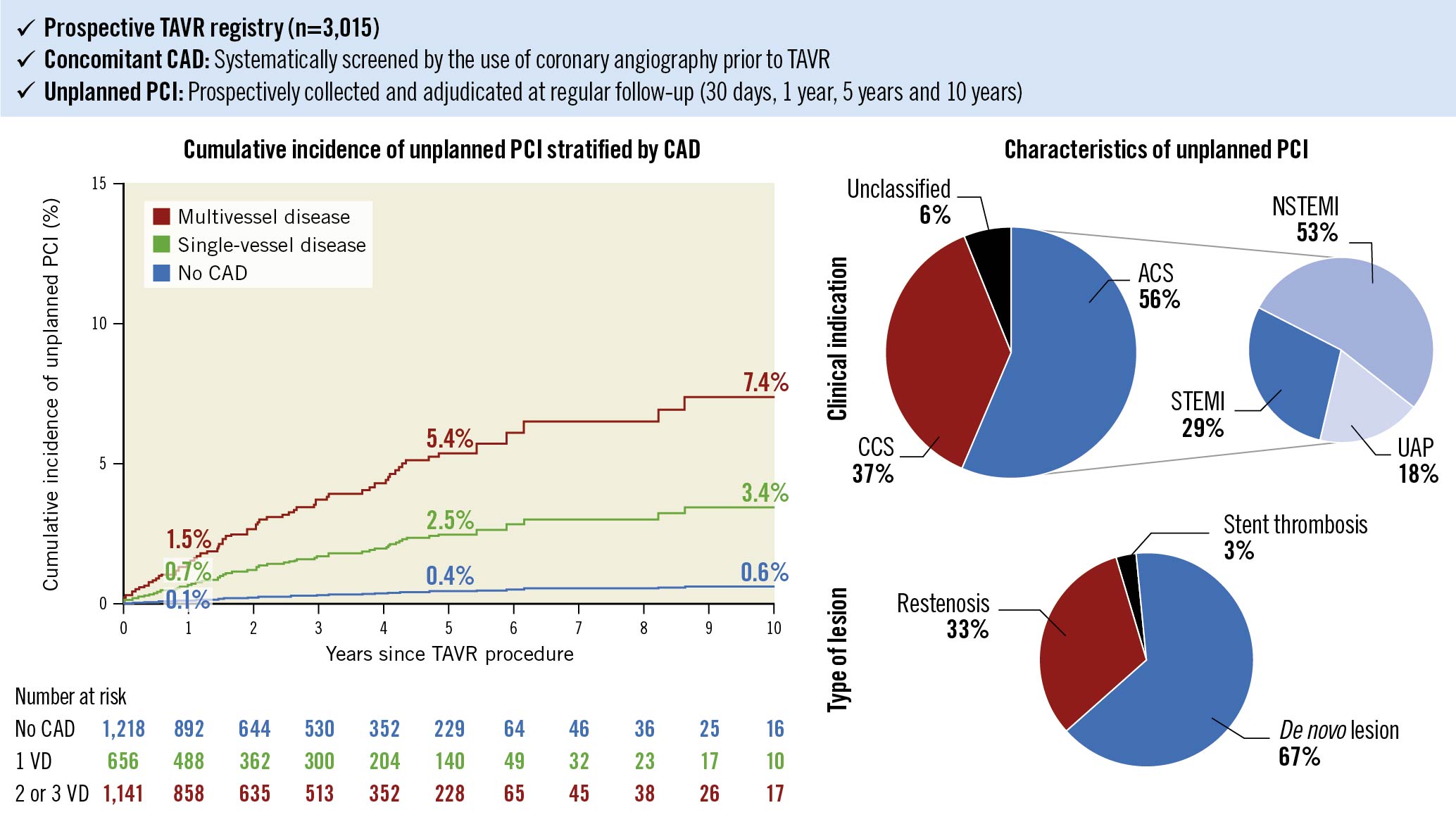
Central Illustration. Long-term risk of unplanned PCI after TAVR.
Cumulative incidence curve of unplanned PCI after TAVR stratified by baseline CAD. The red line denotes multivessel disease, the green line denotes single-vessel disease, and the blue line denotes no CAD. Clinical indications and types of lesions of unplanned PCI after TAVR. ACS: acute coronary syndrome; CAD: coronary artery disease; CCS: chronic coronary syndrome; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction; TAVR: transcatheter aortic valve replacement; UAP: unstable angina pectoris; VD: vessel disease
Clinical indications and procedural characteristics of unplanned PCI
The clinical indications and procedural characteristics of unplanned PCI are shown in Table 3. More than half of the patients underwent unplanned PCI for treatment of ACS (56%). Non-ST-elevation myocardial infarction (NSTEMI) was the most frequent indication (30%), followed by ST-elevation myocardial infarction (STEMI; 16%), and unstable angina pectoris (UAP; 10%) (Central illustration). The most frequent target vessels were, in descending order, the left anterior descending artery (LAD; 51%), followed by the right coronary artery (RCA; 30%), the left circumflex artery (LCx; 18%), the left main (LM; 16%), and coronary artery bypass grafts (9%). Sixty-seven percent of lesions were de novo lesions, 33% were restenotic lesions, and 3% of lesions occurred in the setting of stent thromboses (Central illustration). Femoral access was preferentially used over radial access, and PCI of the target vessel was successful in 99% of cases.
Table 3. Clinical indication for unplanned PCI.
|
Unplanned PCI |
|
|---|---|
|
N=67 |
|
|
Days between TAVR and unplanned PCI |
817±711 |
|
median (25%; 75% IQR) |
605 (292; 1,340) |
|
Reason for unplanned PCI |
|
|
Chronic coronary syndrome |
25 (37%) |
|
Unstable angina pectoris |
7 (10%) |
|
NSTEMI |
20 (30%) |
|
STEMI |
11 (16%) |
|
Others |
4 (6%) |
|
Successful PCI |
66 (99%) |
|
Type of lesion |
|
|
De novo stenosis |
45 (67%) |
|
Restenosis |
22 (33%) |
|
Stent thrombosis |
2 (3%) |
|
Target vessel |
|
|
Left main trunk |
11 (16%) |
|
Left anterior descending artery |
34 (51%) |
|
Left circumflex artery |
12 (18%) |
|
Right coronary artery |
20 (30%) |
|
Graft |
6 (9%) |
|
Vascular access |
|
|
Femoral |
37 (61%) |
|
Radial |
24 (39%) |
|
IQR: interquartile range; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction; TAVR: transcatheter aortic valve replacement |
|
Discussion
In this prospective TAVR registry, the incidence of unplanned PCI after TAVR was 0.4%, 1.6%, and 2.3% at 1, 5, and 10 years, respectively. The number of diseased vessels diagnosed by pre-TAVR coronary angiography, male sex, and younger age were independently associated with an increased risk of unplanned PCI after TAVR. Fewer than 1% of patients without CAD before TAVR underwent unplanned PCI within 10 years after TAVR, while 3% and 7% of patients with single-vessel disease and multivessel disease, respectively, underwent unplanned PCI within 10 years. The primary indication for unplanned PCI was ACS in more than half of the cases; the LAD was the most frequent target vessel and most lesions were de novo. Unplanned PCI was successful in 99% of patients.
The high PCI success rate in the present study is in line with the 96-100% success rate in previous studies10111213141516. However, the relatively high rate of femoral access for PCI (61%) may indicate the operators’ anticipation of challenging coronary cannulation. In a recent, dedicated, prospective study, Reobtain Coronary Ostia Cannulation Beyond Transcatheter Aortic Valve Stent (RE-ACCESS), unsuccessful coronary cannulation was observed in 7.7% of patients with a previously implanted THV, and semi-selective cannulation was reported in 12.0% for the left coronary artery and in 31.7% for the RCA. Difficulties in achieving selective cannulation after TAVR were also reflected by the longer times and the larger amounts of contrast dye used to engage each coronary ostium. Challenging cannulation was more commonly observed in patients treated with the Evolut R/PRO (Medtronic) THV2. The TAVR with Commissural Alignment Followed by Coronary Access (ALIGN-ACCESS) study demonstrated that commissural alignment improves the rate of selective coronary access after TAVR with supra-annular THV; however, aligned supra-annular THV still carry a higher risk of unfeasible/non-selective coronary access than the SAPIEN 3 (Edwards Lifesciences) THV17. Along the same lines, in a multicentre study including 118 patients presenting with STEMI after TAVR, the PCI failure rate, median door-to-balloon time, procedural time, fluoroscopy time, dose-area product, and contrast volume were all higher in TAVR patients presenting with STEMI compared with all-comer STEMI patients6. Furthermore, it can be expected that coronary access will be even more challenging after repeat TAVR345. Thus, the risk of unplanned PCI after TAVR should always be taken into account during the Heart Team decision-making process, particularly in younger patients at risk of requiring repeat TAVR in the future.
Evidence on the incidence and characteristics of unplanned PCI following TAVR is scarce. The Revascularization After Transcatheter Aortic Valve Implantation (REVIVAL) study summarised unplanned PCI cases after TAVR from several centres. Estimates of PCI after TAVR may have been biased by selective reporting and retrospective data collection. In addition, the study did not have a control group and did not differentiate PCI for mechanical obstruction complicating TAVR from PCI due to CAD18. Thus, the present study is the first to systematically report the incidence, characteristics, and predictors of unplanned PCI after TAVR from a prospective registry. In line with the previous multicentre study, unplanned PCI after TAVR was infrequent, and the most common indication for PCI was ACS. In contrast to the study by Stefanini and colleagues, we documented no acute decrease in the incidence of unplanned PCI over time, and the median interval from TAVR to unplanned PCI was longer. Both of these observations may be explained by the exclusion of unplanned PCI for acute coronary obstruction due to TAVR and the high completeness of follow-up in the present study.
To the best of our knowledge, our study is the first to identify baseline clinical factors associated with an increased risk of unplanned PCI after TAVR. Patients with single-vessel disease and multivessel disease had a 4-fold and 9-fold increased risk of unplanned PCI after TAVR, respectively, compared to those without CAD. Male sex and younger age were also independently associated with an increased risk of unplanned PCI. This finding has important clinical implications for the lifetime management of patients with AS. If no relevant CAD is documented at the time of intervention for AS, the long-term risk of unplanned PCI later in life is exceedingly low. This observation seems to expand the range of transcatheter treatment options for low-risk patients with isolated AS to all THV, irrespective of the height of the stent frame. Conversely, if a TAVR candidate does have relevant CAD at the time AS requires intervention, is male, and has a long life expectancy, it may be preferable to consider surgery or preserve future coronary access by using an intra-annular THV with a short stent frame.
Study limitations
The findings of our cohort study should be interpreted in light of several limitations. First, although the current study was based on a large prospective TAVR registry including over 3,000 patients, the incidence of unplanned PCI was relatively rare, and the number of patients with unplanned PCI after TAVR was modest. Furthermore, more detailed data reflecting the complexity of selective coronary cannulation, such as procedural duration, fluoroscopic time, the amount of contrast dye used, and the number of catheters used, were not systematically collected. On the other hand, the robustness of our findings is enhanced by the prospective data collection and independent event adjudication as well as the systematic assessment of CAD through the use of coronary angiography prior to TAVR. Second, although the data on unplanned PCI were systematically collected at regular follow-ups, the number of patients reaching 10-year follow-up was limited. Finally, the present cohort predominantly included octogenarians at increased surgical risk. As shown in this study, the risk of unplanned PCI is higher in younger patients due to their longer life expectancy. The overall incidence of unplanned PCI after TAVR is expected to further increase as the indication for TAVR is expanded to younger and low-risk patient populations.
Conclusions
Unplanned PCI after TAVR is rare in patients with no CAD prior to TAVR, while it is more common in those with CAD, particularly in the setting of multivessel disease. The assessment of CAD prior to TAVR is essential in the lifetime management of patients with AS.
Impact on daily practice
PCI after TAVR can be challenging due to impaired coronary access. In a prospective TAVR registry, the number of diseased vessels diagnosed by pre-TAVR coronary angiography, male sex, and younger age were independently associated with an increased risk of unplanned PCI after TAVR. Unplanned PCI after TAVR is rare in patients with no CAD prior to TAVR, while it is more common in those with CAD, particularly in the setting of multivessel disease. The assessment of concomitant CAD prior to TAVR is crucial for optimal lifetime management of patients with severe AS.
Conflict of interest statement
S. Windecker reports research and educational grants to the institution from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardinal Health, CardioValve, Corflow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Janssen-Cilag, Johnson & Johnson, Medicure, Medtronic, Merck Sharp & Dohm, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Suisse, Pfizer, Polares, Regeneron, Sanofi-Aventis, Servier, Sinomed, Terumo, Vifor, and V-Wave. S. Windecker serves as unpaid advisory board member for and/or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol-Myers Squibb, Edwards Lifesciences, Janssen, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, V-Wave, and Xeltis, but has not received personal payments from pharmaceutical companies or device manufacturers. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding from industry without impact on his personal remuneration. S. Windecker is an unpaid member of the Pfizer Research Award selection committee in Switzerland and of the Women as One Awards Committee. He is member of the Clinical Study Group of the Deutsches Zentrum für Herz Kreislauf-Forschung and of the Advisory Board of the Australian Victorian Heart Institute. He is chairperson of the ESC Congress Program Committee and Deputy Editor of JACC CV Interventions. T. Pilgrim received research grants to the institution from Boston Scientific, Edwards Lifesciences, and Biotronik; speaker fees/consultancy from Boston Scientific, Biotronik, Medtronic, and Abbott; and consultancy (clinical event adjudication committee) for HighLife SAS. D. Heg has no personal conflicts; his employer, CTU Bern, University of Bern, has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in the design, conduct, or analysis of clinical studies funded by not-for-profit and for-profit organisations. In particular, pharmaceutical and medical device companies provide direct funding to some of these studies. For an up-to-date list of CTU Bern’s conflicts of interest see http://www.ctu.unibe.ch/research/declaration_of_interest/index_eng.html. The other authors have no conflicts of interest to declare with regards to the contents of this article.
Supplementary data
To read the full content of this article, please download the PDF.

