Abstract
Background: The optimal timing to perform percutaneous coronary interventions (PCI) in transcatheter aortic valve implantation (TAVI) patients remains unknown.
Aims: We sought to compare different PCI timing strategies in TAVI patients.
Methods: The REVASC-TAVI registry is an international registry including patients undergoing TAVI with significant, stable coronary artery disease (CAD) at preprocedural workup. In this analysis, patients scheduled to undergo PCI before, after or concomitantly with TAVI were included. The main endpoints were all-cause death and a composite of all-cause death, stroke, myocardial infarction (MI) or rehospitalisation for congestive heart failure (CHF) at 2 years. Outcomes were adjusted using the inverse probability treatment weighting (IPTW) method.
Results: A total of 1,603 patients were included. PCI was performed before, after or concomitantly with TAVI in 65.6% (n=1,052), 9.8% (n=157) or 24.6% (n=394), respectively. At 2 years, all-cause death was significantly lower in patients undergoing PCI after TAVI as compared with PCI before or concomitantly with TAVI (6.8% vs 20.1% vs 20.6%; p<0.001). Likewise, the composite endpoint was significantly lower in patients undergoing PCI after TAVI as compared with PCI before or concomitantly with TAVI (17.4% vs 30.4% vs 30.0%; p=0.003). Results were confirmed at landmark analyses considering events from 0 to 30 days and from 31 to 720 days.
Conclusions: In patients with severe aortic stenosis and stable coronary artery disease scheduled for TAVI, performance of PCI after TAVI seems to be associated with improved 2-year clinical outcomes compared with other revascularisation timing strategies. These results need to be confirmed in randomised clinical trials.
Introduction
Coronary artery disease (CAD) frequently coexists with severe aortic stenosis (AS) due to overlapping risk factors1. As both disease conditions may cause similar symptoms, assessment and management of these coexisting pathologies can be challenging. Current international guidelines recommend percutaneous coronary intervention (PCI) of coronary artery lesions with >70% stenosis in proximal segments (or >50% in case of left main disease) in patients scheduled for transcatheter aortic valve implantation (TAVI) based on angiographic assessment (class IIa recommendation, Level of Evidence C)2.
As available evidence mainly comes from non-randomised, observational studies with inherent limitations, the optimal diagnosis and treatment of CAD in patients scheduled for TAVI are yet to be defined3. Assessment of CAD severity in the setting of AS, the extent of revascularisation and the optimal timing of both procedures remain a matter of debate456. Historically, percutaneous revascularisation prior to TAVI represented standard clinical practice due to concerns regarding ischaemic and haemodynamic complications during rapid ventricular pacing. Currently, the chronology of interventions is subject to individualised decision-making based on clinical and anatomical variables with potential (dis)advantages for each timing strategy2.
Despite the high clinical relevance, available data are scarce, and their general applicability is limited by small sample sizes, the exclusive use of balloon-expandable valves (BEV) and limitation of PCI procedures to selected subgroups789. Randomised trials investigating the role of physiological assessment of CAD and revascularisation timing strategies in patients with severe AS undergoing TAVI are ongoing.
Against the background of the mentioned limitations of the previous single-centre studies, we sought to compare different PCI timing strategies in patients scheduled for TAVI in this large, international, multicentre Management of myocardial REVASCularization in patients undergoing Transcatheter Aortic Valve Implantation with coronary artery disease (REVASC-TAVI) registry with regard to adverse clinical outcomes, including all-cause death, myocardial infarction (MI), stroke and rehospitalisation for congestive heart failure (CHF) at 2 years after TAVI.
Methods
Patient population and procedures
Among 2,402 patients enrolled in the REVASC-TAVI registry between January 2015 and September 2021 from 30 centres in Europe, North and South America and Japan, a total of 1,603 TAVI patients were scheduled to undergo either staged (before or after) or concomitant PCI. The REVASC-TAVI registry is an investigator-initiated registry designed to collect data of patients with severe AS scheduled for TAVI and significant coronary artery lesions, diagnosed during pre-TAVI angiography, as described previously10. All patients were discussed by a multidisciplinary Heart Team and found eligible for TAVI. TAVI and PCI procedures were performed according to local standards. The chronology of both procedures as well as valve type selection was at the operator’s discretion. Data collection and analysis was approved by local ethics committees of the participating centres and complied with the Declaration of Helsinki. All patients provided written informed consent.
Definitions
Significant CAD was defined according to international guidelines on myocardial revascularisation1112. In detail, revascularisation was indicated in the presence of an angiographic stenosis ≥70% (or ≥50% in case of protected left main [LM] or bypass graft) as determined by visual estimation, or functionally significant stenosis (instantaneous wave-free ratio [iFR] value ≤0.89 or fractional flow reserve [FFR] value ≤ 0.80) in at least 1 major coronary artery with a diameter of at least 2.5 mm, detected in a coronary angiography performed during pre-TAVI workup, or LM minimal lumen area (MLA) <6 mm2 at intravascular ultrasound (IVUS) assessment. PCI before TAVI was defined as an elective PCI procedure performed in a different session prior to TAVI. Of note, PCI for acute coronary syndromes was excluded by definition. PCI after TAVI was defined as an elective PCI procedure performed after TAVI in a staged procedure. Concomitant PCI was defined as an elective PCI procedure performed within a single session, either before or after transcatheter heart valve (THV) implantation. The management of CAD, including indication for PCI, use of functional invasive or non-invasive tests to assess myocardial ischaemia, use of intravascular imaging, PCI strategy and duration of antithrombotic therapy, was at the operator’s discretion at each centre and according to current international guidelines.
Definition of endpoints and follow-up
All centres contributed anonymised individual patient-level data using a dedicated electronic case report form. Baseline characteristics, angiographic characteristics and procedural details of PCI and TAVI procedures as well as follow-up data were collected by local co-investigators at each institution. Data were then collected in a joint database for statistical analysis. All inconsistencies were resolved directly by communicating with the responsible local investigators.
All clinical endpoints, procedural data for TAVI and in-hospital complications were site-reported and categorised according to Valve Academic Research Consortium (VARC)-2 criteria, which were applicable when the registry data were collected13. Major endpoints of interest were all-cause death as well as a composite of all-cause death, stroke, MI or rehospitalisation for CHF at 2 years.
Statistical analysis
For the purpose of the analysis, the entire patient population was divided into 3 groups, based on the timing of the PCI procedure relative to the initial diagnostic coronary angiogram (PCI before TAVI, PCI after TAVI or concomitant PCI with TAVI). Continuous variables are presented as means with standard deviation or medians with interquartile range (IQR) and were compared using the Student’s t-test or Mann-Whitney U test for paired samples, as appropriate. Categorical variables are summarised as frequencies and proportions and were compared using the chi-square, Fisher’s exact or McNemar tests for paired samples, as appropriate. To account for the non-randomised study design and to reduce the imbalance in baseline characteristics and the effect of a potential selection bias, an inverse probability treatment weighting (IPTW) analysis was performed, adjusted for variables selected based on their p-value in univariate analysis and on their potential influence on outcome. The selected variables were age, sex, Society of Thoracic Surgeons (STS) score, Canadian Cardiovascular Society (CCS) class, chronic obstructive pulmonary disease (COPD), diabetes mellitus, hypertension, New York Heart Association (NYHA) Class, atrial fibrillation, estimated glomerular filtration rate, prior pacemaker, prior stroke, prior coronary artery bypass graft, prior MI, left ventricular ejection fraction, mean transvalvular gradient, multivessel CAD, LM or proximal left anterior descending (LAD) artery CAD (Supplementary Figure 1).
Time-to-event curves for the main outcome variables were estimated using the Kaplan-Meier method. Hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated using an IPTW-adjusted Cox proportional hazards model. Additionally, a sensitivity analysis was performed after excluding cases with in-hospital mortality related to TAVI. Likewise, time-to-event curves were estimated using the Kaplan-Meier method with IPTW adjustment. A landmark analysis was performed for all-cause death and the composite endpoint from 0 to 30 days and from 31 to 720 days, respectively. All tests were 2-sided, and a p-value<0.05 was considered the threshold for statistical significance. All statistical analyses were performed using R software, version 3.6.3 (R Foundation for Statistical Computing).
Results
Baseline characteristics
A total of 1,617 TAVI patients from the REVASC-TAVI registry underwent PCI. After excluding 14 patients with no available data about PCI timing (n=7) or those who had undergone unplanned PCI due to acute coronary ostia occlusion during TAVI (n=7), a total of 1,603 patients were included in the present analysis. PCI was planned and performed either before TAVI (65.6%; n=1,052), after TAVI (9.8%, n=157) or concomitantly with TAVI (24.6%, n=394), respectively (Central illustration). PCI was performed within a median time interval of 35 days (13-63 days) before TAVI and 40 days (20-57 days) after TAVI. Baseline characteristics after IPTW analysis of the overall cohort and the 3 PCI timing groups are depicted in Table 1. The standardised mean difference of included variables (Supplementary Figure 1) indicates excellent adjustment for baseline variables.
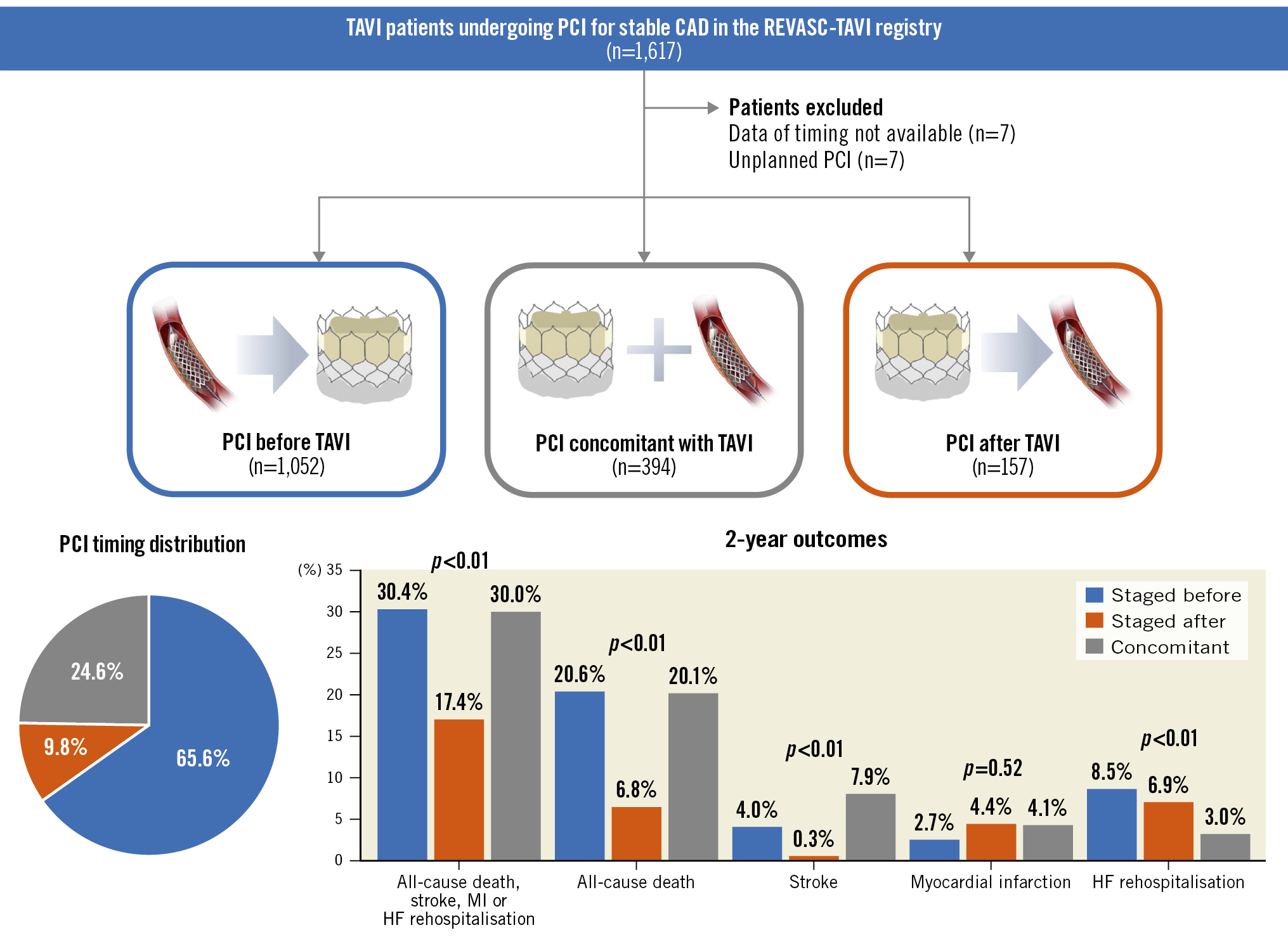
Central illustration. Outcomes of patients undergoing transcatheter aortic valve implantation and percutaneous coronary intervention for stable coronary artery disease from the international, multicentre REVASC-TAVI registry. CAD: coronary artery disease; HF: heart failure; MI: myocardial infarction; PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation
Table 1. Baseline characteristics after inverse probability treatment weighting analysis.
| Overall cohort | PCI before TAVI (n=1,052) |
PCI after TAVI (n=157) |
Concomitant PCI ( n=394) |
SMD | |
|---|---|---|---|---|---|
| Age, years | 82.0 [78.3, 85.1] | 82.2 [78.5, 85.3] | 82.0 [79.0, 85.2] | 82.0 [78.0, 85.0] | 0.083 |
| Female | 41.1 | 40.9 | 42.3 | 39.9 | 0.024 |
| STS score | 5.0 [3.2, 5.1] | 5.0 [3.2, 5.0] | 5.0 [3.3, 5.1] | 5.0 [3.0, 5.1] | 0.070 |
| NYHA Class III/IV | 62.8 | 62.2 | 61.9 | 64.7 | 0.028 |
| CCS class >1 | 31.1 | 31.1 | 28.9 | 33.5 | 0.045 |
| Hypertension | 85.6 | 84.8 | 85.9 | 86.6 | 0.017 |
| Diabetes mellitus | 32.2 | 31.5 | 33.6 | 31.9 | 0.021 |
| Previous surgical aortic valve replacement | 2.2 | 1.8 | 2.3 | 2.8 | 0.064 |
| Previous coronary artery bypass grafting | 7.7 | 8.5 | 5.8 | 8.8 | 0.029 |
| Previous myocardial infarction | 18.3 | 19.8 | 17.0 | 17.5 | 0.029 |
| Previous stroke | 8.5 | 8.8 | 10.6 | 5.6 | 0.050 |
| Chronic obstructive pulmonary disease | 15.1 | 14.6 | 15.3 | 15.6 | 0.010 |
| eGFR, ml/min | 55.1 [44.0, 63.0] | 55.1 [45.0, 64.1] | 55.1 [42.8, 62.2] | 55.1 [44.6, 63.1] | 0.078 |
| Previous pacemaker | 7.4 | 8.1 | 4.7 | 9.2 | 0.045 |
| Atrial fibrillation | 25.8 | 27.5 | 21.7 | 28.0 | 0.063 |
| Mean transaortic gradient, mmHg | 44.0 [36.0, 50.0] | 44.0 [36.0, 50.0] | 43.2 [35.0, 49.0] | 43.0 [37.0, 51.0] | 0.069 |
| Aortic valve area, cm2 | 0.7 [0.6, 0.8] | 0.7 [0.6, 0.8] | 0.7 [0.6, 0.8] | 0.7 [0.6, 0.8] | 0.048 |
| Left ventricular ejection fraction, % | 58.0 [48.0, 62.0] | 58.0 [48.0, 63.0] | 59.5 [48.0, 60.0] | 56.0 [50.0, 62.0] | 0.022 |
| Bicuspid valve | 4.0 | 4.6 | 4.7 | 2.4 | 0.099 |
| Aspirin | 74.7 | 81.5 | 71.4 | 69.4 | 0.190 |
| Clopidogrel | 48.9 | 73.5 | 34.7 | 32.7 | 0.595 |
| Ticagrelor | 0.7 | 1.3 | 0.3 | 0.4 | 0.080 |
| Prasugrel | 1.0 | 1.9 | 0.7 | 0.1 | 0.124 |
| Vitamin K antagonist | 7.4 | 6.9 | 4.5 | 11.6 | 0.175 |
| NOAC | 14.2 | 15.4 | 14.0 | 12.8 | 0.049 |
| DAPT | 39.8 | 56.4 | 29.4 | 26.9 | 0.674 |
| DAT | 7.6 | 8.2 | 7.0 | 7.4 | 0.357 |
| TAT | 5.4 | 8.1 | 3.9 | 3.3 | 0.394 |
| Data are median [interquartile range] or %. CCS: Canadian Cardiovascular Society; DAPT: dual antiplatelet therapy; DAT: dual antithrombotic therapy; eGFR: estimated glomerular filtration rate; NOAC: novel oral anticoagulant; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SMD: standardised mean difference; STS: Society of Thoracic Surgeons; TAT: triple antithrombotic therapy. TAVI: transcatheter aortic valve implantation | |||||
Coronary artery disease characteristics
Angiographic characteristics of CAD found at pre-TAVI coronary angiography are depicted in Table 2. There were no differences with regard to the number of diseased vessels (p=0.136), multivessel CAD (p=0.484), CAD involving proximal segments (p=0.392), or LM or proximal segments (0.868) between the 3 groups. In contrast, bifurcation lesions were more frequent in patients undergoing PCI after TAVI compared to those treated before or concomitantly with TAVI (44.0% vs 32.0% vs 21.8%; p<0.001).
Table 2. Angiographic characteristics after inverse probability treatment weighting analysis.
| PCI before TAVI (n=1,052) |
PCI after TAVI (n=157) |
Concomitant PCI (n=394) |
p-value | |
|---|---|---|---|---|
| Diseased vessels | ||||
| 1 vessel | 55.1 | 56.3 | 60.3 | 0.136 |
| 2 vessels | 26.9 | 22.4 | 27.7 | |
| 3 vessels | 18.0 | 21.3 | 11.9 | |
| Coronary segment involved | ||||
| LM | 13.1 | 15.5 | 14.7 | 0.688 |
| LAD | 64.4 | 71.8 | 59.7 | 0.042 |
| Proximal LAD | 32.0 | 31.8 | 29.9 | 0.830 |
| Mid-LAD | 39.5 | 48.4 | 34.7 | 0.021 |
| Distal LAD | 7.9 | 4.9 | 5.1 | 0.286 |
| Diagonal | 13.4 | 24.0 | 9.1 | <0.001 |
| LCx | 37.5 | 40.0 | 29.7 | 0.094 |
| Proximal LCx | 17.5 | 16.8 | 13.3 | 0.431 |
| Mid-LCx | 10.9 | 21.0 | 8.9 | <0.001 |
| Distal LCx/PDA | 4.9 | 5.6 | 1.5 | 0.063 |
| Obtuse marginal | 13.2 | 7.9 | 13.0 | 0.182 |
| RCA | 47.7 | 40.4 | 46.5 | 0.246 |
| Proximal RCA | 28.5 | 25.0 | 30.2 | 0.470 |
| Mid-RCA | 20.9 | 20.6 | 19.9 | 0.941 |
| Distal RCA/PL/PDA | 12.2 | 11.3 | 8.0 | 0.310 |
| Bypass graft | 3.9 | 0.4 | 3.8 | 0.006 |
| Calcific disease | 25.4 | 17.5 | 21.0 | 0.142 |
| Bifurcation lesions | 32.0 | 44.0 | 21.8 | <0.001 |
| Multivessel CAD | 44.9 | 43.7 | 39.7 | 0.484 |
| Proximal CAD | 64.6 | 59.8 | 65.8 | 0.392 |
| LM/proximal LAD CAD | 38.0 | 36.4 | 38.3 | 0.868 |
| Data are presented as %. CAD: coronary artery disease; LAD: left anterior descending; LCx: left circumflex artery; LM: left main; PCI: percutaneous coronary intervention; PDA: posterior descending artery; PL: posterolateral; RCA: right coronary artery; TAVI: transcatheter aortic valve implantation | ||||
Procedural PCI characteristics
A total of 2,014 lesions were included and were treated either before TAVI (n=1,357), after TAVI (n=225) or concomitantly with TAVI (n=432) (Table 3). Among the concomitant TAVI and PCI group, PCI was performed before and after THV deployment in 296/432 (68.5%) and 136/432 (31.5%) lesions, respectively. Assessment of coronary lesion severity using iFR/FFR or IVUS/optical coherence tomography was infrequent, in 9.5% and 7% of the overall cohort, respectively, with no relevant differences across the groups. Target vessel stenosis >90% or chronic total occlusions were more frequent in lesions treated before TAVI compared to those treated after or concomitantly with TAVI (p=0.021). LM or proximal LAD stenoses were more frequently treated before TAVI or concomitantly with TAVI as compared to after TAVI (11.3% vs 13.7% vs 7.1%; p=0.043 and 23.7% vs 25.2% vs 15.6%; p=0.014). In contrast, left circumflex stenoses were more frequently treated after TAVI as compared to before or concomitantly with TAVI (21.8% vs 17.5% vs 12.7%; p=0.009). Access routes differed significantly, with higher rates of the radial approach in PCI procedures performed before TAVI as compared to those performed after or concomitantly with TAVI (p<0.001). Overall, lesion preparation using rotational or orbital atherectomy was low, 4.5% and 0.4% of the overall cohort, respectively, without differences across the groups. However, haemodynamic support differed significantly, with significantly higher rates in the concomitant PCI group (p<0.001).
Table 3. Procedural PCI characteristics.
| Overall cohort (n=2,014) |
PCI before TAVI (n=1,357) |
PCI after TAVI (n=225) |
Concomitant PCI (n=432) |
p-value | |
|---|---|---|---|---|---|
| Target vessel stenosis | |||||
| >70% | 1,587 (81.3) | 1,035 (79.6) | 193 (87.7) | 359 (83.5) | 0.021 |
| >90% | 306 (15.7) | 222 (17.1) | 23 (10.5) | 61 (14.2) | |
| CTO | 49 (2.5) | 39 (3.0) | 4 (1.8) | 6 (1.4) | |
| Target vessel | |||||
| LM | 229 (11.4) | 154 (11.3) | 16 (7.1) | 59 (13.7) | 0.043 |
| LAD | 912 (45.3) | 610 (45.0) | 99 (44.0) | 203 (47.0) | 0.698 |
| Prox LAD | 465 (23.1) | 321 (23.7) | 35 (15.6) | 109 (25.2) | 0.014 |
| Mid-LAD | 560 (27.8) | 366 (27.0) | 74 (32.9) | 120 (27.8) | 0.186 |
| Distal LAD | 77 (3.8) | 58 (4.3) | 9 (4.0) | 10 (2.3) | 0.179 |
| Diagonal | 107 (5.3) | 79 (5.8) | 14 (6.2) | 14 (3.2) | 0.093 |
| LCx | 341 (17.0) | 238 (17.5) | 49 (21.8) | 55 (12.7) | 0.009 |
| Prox LCx | 198 (9.8) | 131 (9.7) | 32 (14.2) | 35 (8.1) | 0.041 |
| Mid-LCx | 133 (6.6) | 93 (6.9) | 16 (7.1) | 24 (5.6) | 0.606 |
| Distal LCx | 48 (2.4) | 38 (2.8) | 7 (3.1) | 3 (0.7) | 0.033 |
| Obtuse marginal | 127 (6.3) | 90 (6.6) | 10 (4.4) | 27 (6.2) | 0.457 |
| RCA | 585 (29.0) | 405 (29.8) | 56 (24.9) | 124 (28.7) | 0.312 |
| Prox RCA | 358 (17.8) | 241 (17.8) | 35 (15.6) | 82 (19.0) | 0.796 |
| Mid-RCA | 246 (12.2) | 172 (12.7) | 17 (7.6) | 57 (13.2) | 0.074 |
| Distal RCA | 123 (6.1) | 99 (7.3) | 13 (5.8) | 11 (2.5) | 0.009 |
| Bypass graft | 41 (2.0) | 33 (2.4) | 1 (0.4) | 7 (1.6) | 0.117 |
| Access route | |||||
| Right radial | 938 (48.8) | 827 (64.3) | 74 (34.4) | 37 (8.7) | <0.001 |
| Left radial | 75 (3.9) | 55 (4.3) | 6 (2.8) | 14 (3.3) | |
| Femoral | 910 (47.3) | 404 (31.4) | 135 (62.8) | 371 (87.7) | |
| Use of iFR/FFR | 159 (9.5) | 116 (9.7) | 6 (6.2) | 37 (9.5) | 0.536 |
| Use of IVUS/OCT | 117 (7.0) | 82 (6.9) | 8 (7.8) | 27 (6.9) | 0.937 |
| Use of atherectomy | |||||
| Rotational atherectomy | 87 (4.5) | 54 (4.1) | 14 (6.5) | 19 (4.4) | 0.445 |
| Orbital atherectomy | 7 (0.4) | 6 (0.5) | 0 (0) | 1 (0.2) | |
| Haemodynamic support | 22 (1.1) | 8 (0.6) | 0 (0) | 14 (3.3) | <0.001 |
| Device type | |||||
| DES | 1,529 (88.5) | 998 (87.0) | 192 (93.2) | 339 (90.6) | 0.011 |
| BMS | 7 (0.4) | 3 (0.3) | 0 (0) | 4 (1.1) | |
| POBA | 23 (1.3) | 15 (1.3) | 4 (1.9) | 4 (1.1) | |
| BVS | 33 (1.9) | 23 (2.0) | 2 (1.0) | 8 (2.1) | |
| Number of stents | 1.0 [1.0, 1.0] | 1.0 [1.0, 1.0] | 1.0 [1.0, 1.0] | 1.0 [1.0, 1.0] | 0.483 |
| Total stent length, mm | 23.0 [16.0, 34.0] | 23.0 [16.0, 35.0] | 24.5 [18.0, 37.3] | 20.0 [15.0, 30.0] | 0.005 |
| Stent diameter, mm | 3.00 [2.75, 3.50] | 3.00 [2.75, 3.50] | 3.00 [2.50, 3.50] | 3.00 [3.00, 3.50] | <0.001 |
| Use of guiding extension | 74 (4.6) | 64 (5.6) | 5 (5.2) | 5 (1.3) | 0.002 |
| Procedural success | 1,924 (97.4) | 1,291 (97.1) | 215 (98.2) | 418 (97.9) | 0.526 |
| Crossing difficulty | 49 (2.6) | 39 (3.1) | 1 (0.5) | 9 (2.1) | 0.066 |
| Data are presented as n (%) or median [interquartile range]. BMS: bare metal stent; BVS: bioresorbable vascular scaffold; CTO: chronic total occlusion; DES: drug-eluting stent; FFR: fractional flow reserve; iFR: instantaneous wave-free ratio; IVUS: intravascular ultrasound; LAD: left anterior descending; LCx: left circumflex artery; LM: left main; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; POBA: plain old balloon angioplasty; RCA: right coronary artery; TAVI: transcatheter aortic valve implantation | |||||
Procedural TAVI characteristics and in-hospital outcomes
Procedural characteristics and in-hospital outcomes are detailed in Table 4. The majority of TAVI procedures were performed with local anaesthesia and via transfemoral access, without significant differences across treatment groups (p=0.247 and p=0.325, respectively). The balloon-expandable SAPIEN 3/Ultra (Edwards Lifesciences) and the self-expanding Evolut R/PRO (Medtronic) platforms were most frequently implanted, with higher utilisation of BEV in patients undergoing PCI after TAVI or concomitantly with TAVI (p<0.001). Contrast volume differed significantly and was highest with concomitant PCI as compared to PCI before or after TAVI (230 ml vs 110 ml vs 140 ml; p<0.001), resulting from two interventions in one procedure in this group.
In-hospital all-cause mortality differed significantly and was higher with concomitant PCI or PCI before TAVI as compared to PCI after TAVI (3.7% vs 2.2% vs 0.0%; p=0.005). Likewise, disabling strokes were higher with PCI before TAVI or concomitant PCI with TAVI as compared to PCI after TAVI (1.3% vs 1.0% vs 0.0%; p=0.082). Major vascular complications and major bleeding differed significantly and were lower with PCI before TAVI as compared to PCI after TAVI or concomitant PCI with TAVI (3.5% vs 6.5% vs 8.3%; p=0.077 and 3.4% vs 6.8% vs 9.9%; p=0.025). The rates of acute kidney injury also differed significantly, with the highest rates in patients treated concomitantly with TAVI as compared to those treated before or after TAVI (p=0.011).
Table 4. Procedural TAVI characteristics and in-hospital outcomes after inverse probability treatment weighting (IPTW) analysis.
| PCI before TAVI (n=1,052) |
PCI after TAVI (n=157) |
Concomitant PCI (n=394) |
p-value | ||
|---|---|---|---|---|---|
| Procedural characteristics | |||||
| Anaesthesia | N/A | 2.0 | 2.1 | 4.9 | 0.247 |
| Local | 87.5 | 85.4 | 84.2 | ||
| General | 10.6 | 12.5 | 10.9 | ||
| Access route | Transfemoral | 94.6 | 92.8 | 94.8 | 0.325 |
| Transapical | 1.4 | 4.1 | 1.3 | ||
| Transsubclavian | 2.8 | 3.1 | 2.9 | ||
| Direct aortic | 1.0 | 0 | 0.7 | ||
| Transcarotid | 0.1 | 0 | 0.3 | ||
| Transcaval | 0.1 | 0 | 0 | ||
| THV type | S3/S3 Ultra | 34.3 | 59.4 | 46.1 | <0.001 |
| SXT | 0.9 | 0 | 0.2 | ||
| Evolut R/PRO | 33.8 | 19.7 | 30.7 | ||
| CoreValve | 3.3 | 0.9 | 4.8 | ||
| Portico | 8.2 | 3.9 | 5.6 | ||
| Lotus | 1.3 | 0.7 | 1.5 | ||
| ACURATE neo/neo2 | 15.0 | 14.9 | 9.7 | ||
| Allegra | 0.4 | 0 | 1.0 | ||
| Other | 2.6 | 0.6 | 0.3 | ||
| Need for second valve | 1.4 | 0.5 | 0.7 | 0.249 | |
| Post-dilatation | 24.9 | 10.8 | 17.0 | 0.006 | |
| Contrast volume, ml | 110 [80, 155] | 140 [100, 187] | 230 [150, 300] | <0.001 | |
| In-hospital outcomes | |||||
| All-cause death | 2.2 | 0.0 | 3.7 | 0.005 | |
| Disabling stroke | 1.3 | 0.0 | 1.0 | 0.082 | |
| Non-disabling stroke | 1.2 | 2.0 | 1.6 | 0.810 | |
| Myocardial infarction | 0.9 | 0.0 | 0.3 | 0.055 | |
| Permanent pacemaker implantation | 12.4 | 10.8 | 10.6 | 0.738 | |
| Life-threatening bleeding | 2.2 | 0.6 | 2.0 | 0.233 | |
| Major bleeding | 3.4 | 6.8 | 9.9 | 0.025 | |
| Minor bleeding | 6.7 | 10.1 | 10.5 | 0.262 | |
| Major vascular complications | 3.5 | 6.5 | 8.3 | 0.077 | |
| Minor vascular complications | 8.5 | 13.4 | 11.1 | 0.226 | |
| Acute kidney injury | RIFLE 1 | 5.4 | 4.1 | 8.6 | 0.011 |
| RIFLE 2/3 | 2.6 | 0 | 5.1 | ||
| Length of stay, days | 5.0 [2.0, 7.0] | 5.0 [3.0, 7.0] | 4.0 [2.0, 7.0] | 0.722 | |
| Data are presented as % or median [IQR]. N/A: not applicable; PCI: percutaneous coronary intervention; RIFLE: Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve | |||||
Clinical follow-up
The median follow-up after TAVI was 393 days. No deaths were reported among patients scheduled to undergo PCI after TAVI within the median time frame of 40 days. The incidence of all-cause death at 2 years differed significantly and was lower in patients undergoing PCI after TAVI as compared to patients treated before or concomitantly with TAVI (6.8% vs 20.6% vs 20.1%; p<0.001; PCI before vs after TAVI: hazard ratio [HR] 3.21 [1.47-7.00]; p=0.003; concomitant PCI vs PCI after TAVI: HR 3.23 [1.42-7.39]; p=0.005) (Figure 1, Central illustration). Likewise, occurrence of the composite endpoint differed significantly and was lower in patients undergoing PCI after TAVI as compared to patients treated before or concomitantly with TAVI (17.4% vs 30.4% vs 30.0%; p=0.003; PCI before vs after TAVI: HR 2.0 [1.16-3.45]; p=0.013; concomitant PCI vs PCI after TAVI: HR 2.03 [1.09-3.79]; p=0.026) (Figure 2, Central illustration).
A sensitivity analysis confirmed these results after excluding cases with in-hospital death related to TAVI. The incidence of all-cause death at 2 years differed significantly and was lower in patients undergoing PCI after TAVI as compared to patients treated before or concomitantly with TAVI (7.6% vs 18.6% vs 17.1%; p<0.01) (Supplementary Figure 2). Likewise, occurrence of the composite endpoint differed significantly and was lower in patients undergoing PCI after TAVI as compared to patients treated before or concomitantly with TAVI (18.7% vs 28.6% vs 27.5%; p=0.05) (Supplementary Figure 3).
A landmark analysis demonstrated that from 0 to 30 days and from 31 to 720 days, the rate of all-cause death differed significantly and was lower in patients undergoing PCI after TAVI as compared to patients treated before or concomitantly with TAVI (0-30 days: 0% vs 2.5% vs 3.6%; p<0.001; 31-720 days: 6.8% vs 18.6% vs 17.1%; p=0.002) (Figure 1). Likewise, the composite endpoint rate differed significantly and was lower in patients undergoing PCI after TAVI as compared to patients treated before or concomitantly with TAVI (0-30 days: 0.8% vs 4.4% vs 6.0%; p=0.002; 31-720 days: 16.8% vs 27.2% vs 25.5%; p=0.045) (Figure 2).
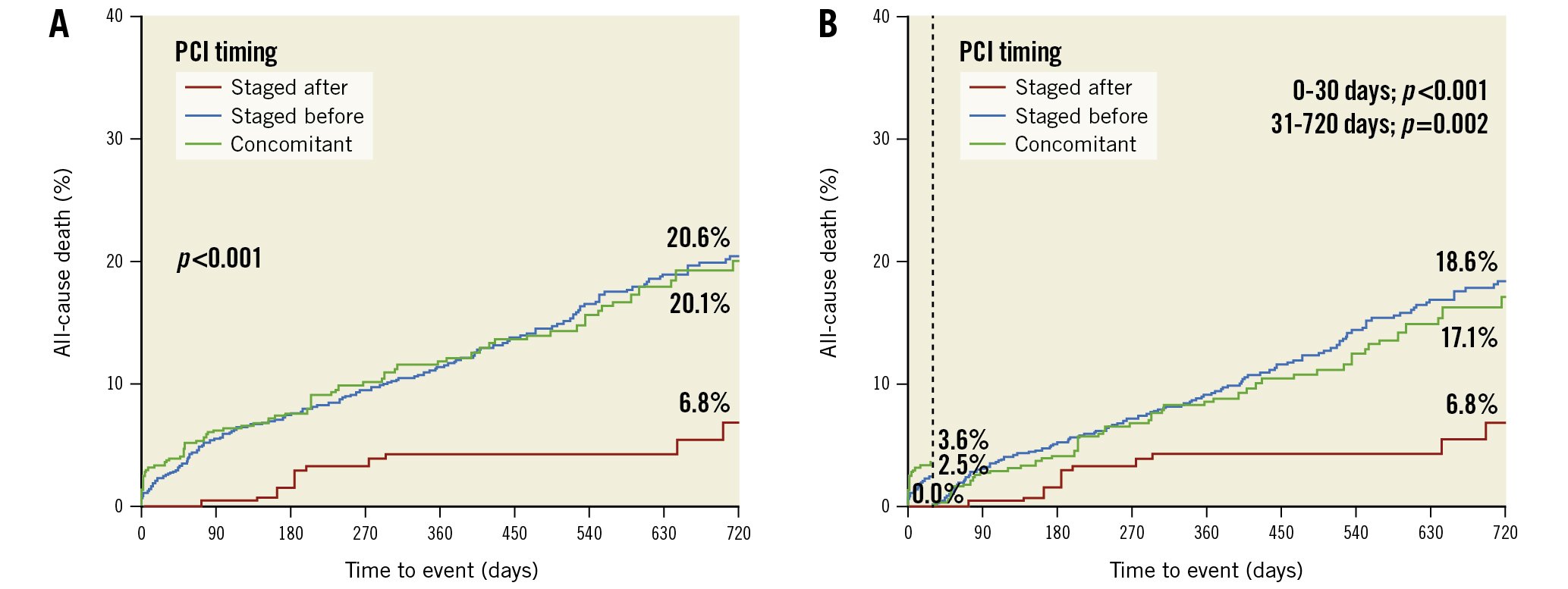
Figure 1. All-cause death according to PCI timing strategy in patients undergoing TAVI. A) Time-to-event curves for all-cause death using the Kaplan-Meier method with inverse probability treatment weighting (IPTW) adjustment and B) landmark analysis for all-cause death from 0-30 days (vertical dotted line) and 31-720 days. PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation
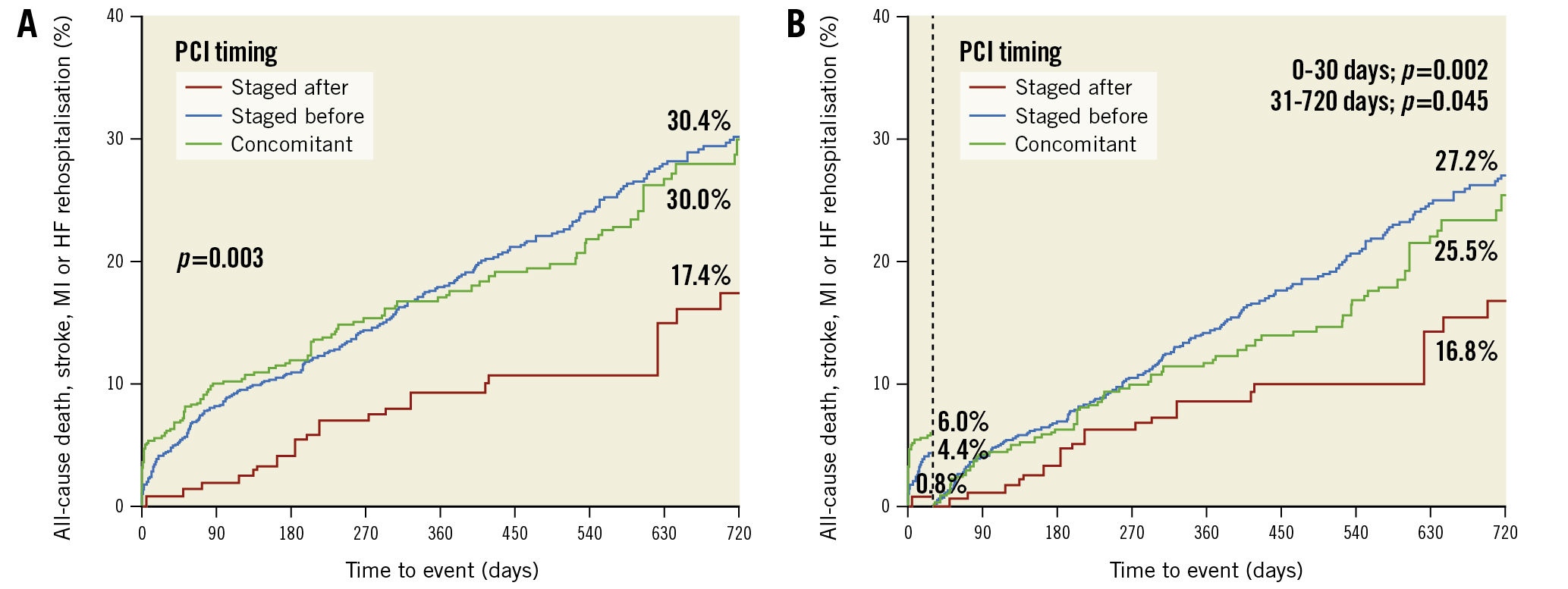
Figure 2. Composite endpoint according to PCI timing strategy in patients undergoing TAVI. A) Time-to-event curves for the combined endpoint using the Kaplan-Meier method with inverse probability treatment weighting (IPTW) adjustment and B) landmark analysis for the combined endpoint from 0-30 days (vertical dotted line) and 31-720 days. HF: heart failure; MI: myocardial infarction; PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation
Discussion
The current study aimed to investigate the clinical outcomes of different PCI timing strategies in patients undergoing TAVI from a large international multicentre registry. With regard to this objective, the most salient findings can be described as follows:
(i) In this international, multicentre study using balloon-expandable and self-expanding THV platforms, two-thirds of all patients scheduled for PCI underwent revascularisation before TAVI.
(ii) Performance of concomitant PCI and TAVI was associated with the highest rates of acute kidney injury, likely due to a significantly higher use of contrast medium. Moreover, in-hospital mortality was highest in this group.
(iii) Major vascular complications and major bleeds differed across treatment groups, with the lowest rates in patients undergoing PCI before TAVI.
(iv) Although the 3 study groups had similar CAD complexity and extension, PCI after TAVI was associated with significantly lower rates of all-cause death as well as the composite of all-cause death, stroke, MI or unplanned rehospitalisation for CHF at 2 years as compared with any other PCI timing strategy.
Although CAD frequently coexists with severe AS, there remain many unanswered questions regarding the optimal management of these 2 pathologies, including assessment of CAD severity, extent of myocardial revascularisation and timing of PCI and TAVI procedures314. Current guidelines give a class IIa recommendation for PCI in lesions with >70% diameter stenosis in proximal segments (Level of Evidence C) and suggest performing both procedures combined or staged according to the clinical situation and pattern of CAD2. Overall, data from randomised clinical trials are scarce. The ACTIVATION trial investigated the role of PCI in patients undergoing TAVI and demonstrated similar rates of death and rehospitalisation at 1 year for PCI and no PCI prior to TAVI in patients with severe aortic stenosis and minimal angina6. In addition, a previous analysis from the REVASC-TAVI registry demonstrated that complete myocardial revascularisation was similar to a strategy of incomplete revascularisation in reducing the risk of all-cause death, as well as the risk of a combination of death, stroke, MI, and rehospitalisation for heart failure at 2 years, regardless of the clinical and anatomical situations in TAVI patients with significant stable CAD10. With regard to the timing of procedures, TAVI candidates have historically undergone staged PCI prior to TAVI in a separate session, which is also reflected by this international all-comers study, with almost two-thirds of patients undergoing staged PCI before TAVI. This approach is justified by the concern that untreated significant coronary lesions might cause ischaemic and haemodynamic complications during valve implantation, although this concern remains theoretical and does not reflect clinical reality. Moreover, concerns regarding the feasibility of coronary access after TAVI, especially with long stent-frame prostheses were raised1516. Of note, the THV type differed significantly in this analysis, with a more frequent use of balloon-expandable THV platforms in patients undergoing staged PCI after TAVI, which are most likely to maintain direct access to coronary ostia. In this regard, dedicated implantation techniques for self-expanding THVs have been proposed in recent years to facilitate optimal commissural alignment and simplify coronary access after TAVI171819.
The performance of PCI before TAVI with the subsequent need for dual antiplatelet therapy was shown to increase bleeding risk in the randomised POPular-TAVI and ACTIVATION trials620. In our cohort, we observed contrary results, with lower rates of major vascular complications and major bleedings in patients undergoing staged PCI before TAVI, which indicates that bleeding complications are multifactorial in this elderly patient population21. This might, to some extent, be attributed to a significantly higher radial approach rate in patients undergoing PCI prior to TAVI in this analysis and indicates that guideline recommendations favouring transradial access should generally be considered in this patient population.
There are certain clinical and anatomical conditions, including filiform subtotal coronary artery lesions, that require timely treatment and exclude the possibility of performing PCI after TAVI. However, in most cases, performance of PCI staged after TAVI seems beneficial in various matters. First, successful treatment of AS eliminates left ventricular pressure overload and microvascular dysfunction and permits adequate physiological assessment of coronary lesion severity and identification of patients deriving benefit from revascularisation62223. Second, (intermittent) haemodynamic compromise during PCI procedures due to AS may cause renal or cerebral ischaemia, and indeed, previous observational studies demonstrated a higher stroke risk when performing PCI before TAVI7. We also observed a higher disabling stroke rate in patients undergoing PCI before TAVI or concomitantly with TAVI at 2 years (Central illustration). Finally, we observed significantly lower all-cause death rates as well as a combination of all-cause death, myocardial infarction, stroke and rehospitalisation for HF in patients undergoing PCI after TAVI, which was confirmed in the landmark analysis considering events before and after 30 days. This favourable clinical course in patients treated in a separate session after TAVI underlines the prognostic importance of an individualised revascularisation timing strategy, as well as accurate THV type selection to preserve coronary access after TAVI. So far, available evidence from previous studies has been inconsistent, with similar event rates among different timing strategies, including bleeding events, vascular complications and acute kidney injury as well as all-cause mortality at 2 years in 1 study9 and favourable (adverse event-free) survival at 2 years in another study7. As the generalisability of these studies is limited by a rather small patient population, this analysis of the REVASC-TAVI registry supports performing PCI after TAVI in most cases after a thorough clinical evaluation, as suggested by current guidelines2. In this regard, results from the ongoing TAVI-PCI Trial currently randomising patients to either PCI before or after TAVI using a balloon-expandable THV (ClinicalTrials.gov: NCT04310046) are eagerly awaited. Performance of PCI in the same session, also suggested by current guidelines, seems unfavourable, despite several potential logistic advantages, as rates of acute kidney injury and in-hospital mortality are significantly higher with this approach compared to any other revascularisation timing approach.
Limitations
This observational, multicentre study exhibits the inherent limitations of a retrospective, non-randomised study design. In particular, the timing of both procedures was at the discretion of the treating physician without consistent selection criteria and may have been influenced by anatomical factors, comorbidities or clinical conditions not captured in this analysis. Although angiographic characteristics were well balanced across treatment groups after IPTW adjustment, further information provided by the SYNTAX score is not available. Moreover, the number of patients differed significantly across treatment groups; the PCI after TAVI group had the fewest patients. Therefore, a selection bias cannot be excluded, limiting the generalisability of the results. As TAVI represented the target intervention in this registry, a greater level of granularity in complications during PCI procedures, beyond the reported key endpoints, is not available in this registry. Moreover, patients treated with staged PCI before TAVI who died before undergoing TAVI, or vice versa, were not captured in this registry. In addition, THV types differed significantly across the treatment groups, with a more frequent use of balloon-expandable THV platforms as compared with self-expanding valves in patients undergoing PCI after TAVI, also limiting the generalisability of the results. Furthermore, although clinical events were categorised according to standardised definitions, events were not adjudicated by an independent event adjudication committee. The results of this analysis are limited to patients with chronic coronary syndrome and cannot be extrapolated to those with acute coronary syndromes.
Conclusions
In patients with severe AS and stable CAD scheduled for TAVI, performance of PCI after TAVI seems to be associated with improved 2-year clinical outcomes compared with other revascularisation timing strategies. Further randomised trials with different available THV platforms are warranted to confirm these results.
Impact on daily practice
Significant coronary artery disease is common in patients undergoing TAVI. The optimal timing to perform PCI in TAVI patients with chronic coronary syndrome remains unknown. These registry data demonstrate that in patients with severe aortic stenosis and stable CAD scheduled for TAVI, performance of PCI after TAVI seems to be associated with improved 2-year clinical outcomes compared with other revascularisation timing strategies. Results from randomised clinical trials with different THV platforms are warranted to confirm these results.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; UFR Medecine, Université de Paris, Paris, France.
Conflict of interest statement
T. Rheude received speaker fees from AstraZeneca and SIS Medical. F.L. Ribichini is aproctor for Edwards Lifesciences and Medtronic. T. Pilgrim received research grants to the institution from Biotronik, Boston Scientific, and Edwards Lifesciences; andspeakers/consultancy fees from Medtronic, Boston Scientific, Biotronik, Abbott, and HighLife SAS. O. De Backer received institutional research grants and/or consulting fees from Abbott and Boston Scientific. W-K. Kim received personal fees from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, Meril Life Sciences, and Shockwave Medical. I.J. Amat-Santos is aproctor for Medtronic, Boston Scientific, and Meril Life Sciences. P. Garot received speaker/consultant fees from Abbott, Biosensors, Boston Scientific, Edwards Lifesciences, GE HealthCare, and Terumo. He serves as amedical director and is ashareholder of CERC (Cardiovascular European Research Center), aCRO based in Massy, France. M. Savontaus is aconsultant for Medtronic and Boston Scientific. C. Gandolfo is aproctor for Edwards Lifesciences. L. Sondergaard is Chief Medical Officer and Divisional VP for Medical Affairs at Abbott Structural Heart; and has received consultant fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic, and SMT. M. Taramasso reports consultant or consultancy fees from Abbott Vascular, Edwards Lifesciences, Medtronic, Boston Scientific, Shenqi Medical, MEDIRA, ReCross, Hi-D Imaging, VentraMed, CoreMedic, Simulands, and CardioValve. M. Adam reports personal fees/speaker honoraria from Abbott, Boston Scientific, Edwards Lifesciences, JenaValve, and Medtronic. S. Baldus reports lecture fees from JenaValve and lecture and speaker fees from Edwards Lifesciences. M. Andreas is a proctor/consultant/speaker for Edwards Lifesciences, Abbott, Medtronic, Boston Scientific, Zoll, and AbbVie; and received institutional research grants from Edwards Lifesciences, Abbott, Medtronic, and LSI. C. Tamburino is aconsultant for Medtronic. M. Joner reports institutional grant support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, and Infraredx; consulting fees from Biotronik, TRiCares, Veryan, and Shockwave; speaker fees from Abbott, AstraZeneca, Biotronik, Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, ReCor Medical, and Shockwave Medical; participation on asteering committee at Biotronik and Edwards Lifesciences; and travel support from Boston Scientific, Cardiac Dimensions, Edwards Lifesciences, and SIS Medical. M. Barbanti is aconsultant for Boston Scientific, Edwards Lifesciences, and Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor is amember of the Data and Safety Monitoring Board for astudy by Venus MedTech and receives speaker honoraria (modest) from Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.

