Abstract
Aims: The aim of this study was to evaluate the presence, localisation and extent of myocardial injury as determined by late gadolinium enhancement (LGE) on cardiovascular magnetic resonance (CMR) imaging in patients undergoing transcatheter aortic valve implantation (TAVI).
Methods and results: A total of 37 patients, who underwent successful TAVI with a balloon-expandable valve (transapical [TA], n=11; non-TA, n=26), were included. Cardiac biomarker (CK-MB and cTnT) levels were determined at baseline and following TAVI. CMR was performed within a week before and within 30 days following TAVI. Some increase in cardiac biomarkers was detected in 97% of the patients as determined by a rise in cTnT, and in 49% of the patients as determined by a rise in CK-MB. Following TAVI, no new myocardial necrosis defects were observed with the non-TA approach. Nonetheless, all patients who underwent TAVI through the TA approach had new focal myocardial necrosis in the apex, with a median myocardial extent and necrotic mass of 5% [2.0-7.0] and 3.5 g [2.3-4.5], respectively.
Conclusions: Although some increase in cardiac biomarkers of myocardial injury was systematically detected following TAVI, new myocardial necrosis as evaluated by CMR was observed only in patients undergoing the procedure through the TA approach, involving ~5% of the myocardium in the apex.
Introduction
A mild rise in cardiac biomarkers of myocardial injury is frequently observed following transcatheter aortic valve implantation (TAVI)1-4, and a greater rise in these cardiac biomarkers has been associated with a negative effect on left ventricular ejection fraction (LVEF) and acute and midterm mortality1,2,4,5. However, the mechanisms associated with this increase in cardiac biomarkers of myocardial injury are not well understood and very few data exist on the presence, location and extent of new myocardial necrosis following TAVI.
Contrast-enhanced cardiovascular magnetic resonance (CMR) imaging permits the accurate detection and quantification of irreversible myocardial injury: it can detect very small areas of myocardial necrosis6,7, of which even minor necrosis of the order of 1.4% of LV myocardium is associated with a sevenfold increase in major cardiac events8. In the context of percutaneous coronary interventions, it has been shown that even mild increases in cardiac biomarkers were associated with new focal defects of myocardial necrosis7, but data in the context of TAVI are lacking. The objectives of the present study were therefore to evaluate the presence, location, and extent of myocardial injury following TAVI as determined by CMR.
Methods
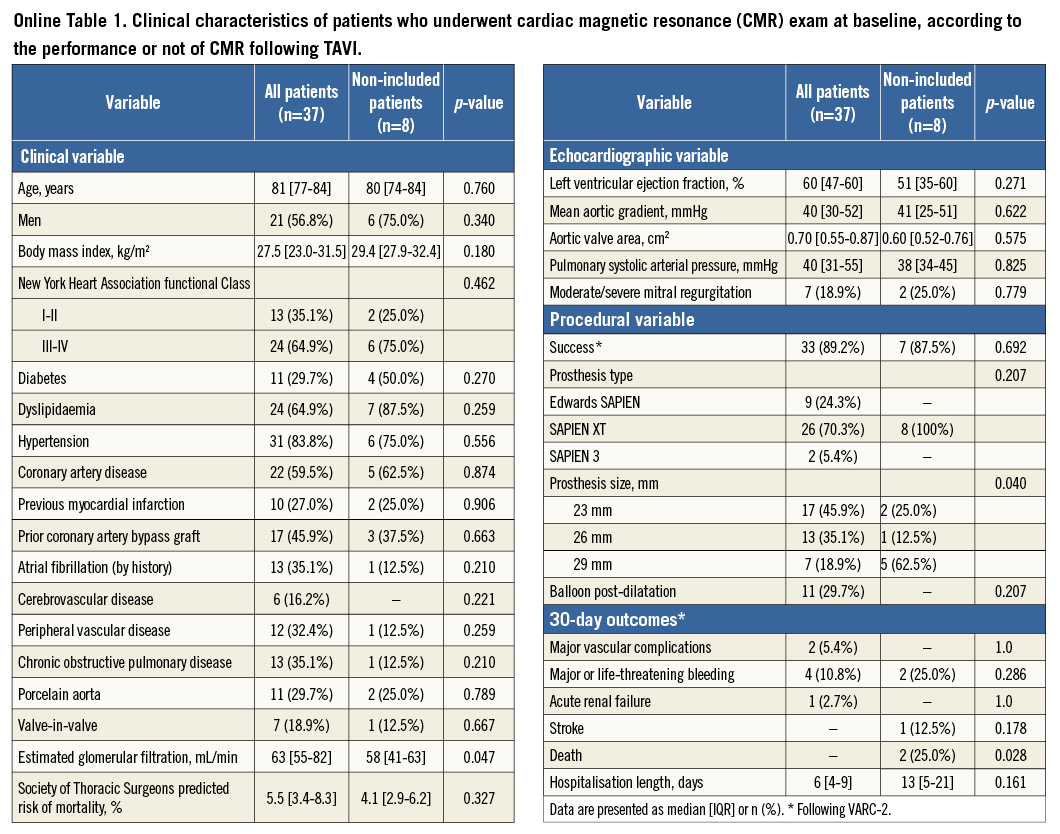
We prospectively screened 75 consecutive patients for the study, so that 45 patients diagnosed with severe symptomatic aortic stenosis who were accepted for a TAVI with a balloon-expandable valve were enrolled. A total of 30 patients were not included in the study due to the following reasons: critical state (n=12), previous pacemaker (n=5), and logistic reasons (n=13). Forty-five patients therefore had a CMR performed within seven days (median: 1 [1-2] days) before TAVI, and 37 of them had a repeat CMR exam performed within 30 days (median: 6 [3-27] days) following TAVI. The reasons for not repeating the CMR exam after TAVI were: pacemaker implantation post TAVI (n=4), death (n=2), and logistic reasons (n=2). The actual analysis included 37 patients submitted to uncomplicated TAVI (transfemoral [TF] approach: 22 patients; transaortic [TAo] approach: four patients; transapical [TA] approach: 11 patients). Patients undergoing TAVI through the retrograde approach (TF and TAo) were pooled together for analysis. The baseline characteristics and outcomes of the non-included patients are shown in Online Table 1.
Selection of the approach was based on the appropriateness of the iliofemoral arteries as previously described9, and details about the TAVI procedure have been provided elsewhere9. In TA-TAVI cases, the technique used for apical closure consisted of two large pursestring sutures using Ethibond 2-0 large needle sutures with pledges. All baseline and procedural characteristics were prospectively collected on preset data collection forms. Baseline comorbidities were defined according to the Society of Thoracic Surgeons (STS) criteria, and periprocedural events according to the VARC (Valve Academic Research Consortium)-2 criteria5. Coronary artery disease was defined as the presence of a coronary lesion with a diameter stenosis ≥50% in vessels ≥2.0 mm, or prior coronary revascularisation. The procedures were performed under a compassionate clinical use programme approved by Health Canada, and all patients provided signed informed consent for the procedures.
All patients underwent a Doppler echocardiographic examination at baseline, before the intervention, and at six-month to one-year follow-up, and LVEF was calculated using the biplane Simpson method. Blood samples were collected at baseline, six to 12, 24, 48, and 72 hrs following the procedure. Creatine kinase-myocardial band (CK-MB) mass and cardiac troponin T (cTnT) levels were measured at each time point, by electrochemiluminescence immunoassay (Roche, Minneapolis, MN, USA). Based on the 99th percentile in a healthy population and the requirement of a ≤10% coefficient variation, the upper normal limits for CK-MB and cTnT levels in our institution were 10 and 0.05 μg/l, respectively. Myocardial injury was defined as a CK-MB level >10 μg/l or a cTnT level >0.05 μg/l. In those patients with elevated CK-MB or cTnT levels at baseline, myocardial injury was defined as any increase >20% after the procedure.
The CMR studies were performed using a 1.5 Tesla Philips Achieva scanner operating release 2.6 level 3, dedicated 32-channel phased-array cardiac coil, and vectorcardiographic gating during successive end-expiratory breath-holds (Philips Healthcare, Best, The Netherlands). Cine imaging of cardiac volumes and function was performed by steady-state free precession technique, at 30 phases per cardiac cycle, in 8-14 parallel short-axis (full coverage) and 2-chamber, 4-chamber, and two orthogonal left ventricular outflow tract (LVOT) planes (8 mm thickness, 0 mm gap). Typical parameters included TR/TE of 3.4/1.2 ms, flip angle 40°, NEX of 1, yielding in-plane spatial resolution of 1.6×2 mm.
Late gadolinium enhancement (LGE) images were acquired in 2D using an inversion recovery fast gradient echo sequence triggered every other heartbeat, 10 minutes after intravenous injection of 0.2 mmol/kg gadolinium diethyltriamine penta-acetic acid. The in-plane image resolution was typically 2.5 mm, and each imaging voxel represented approximately 42 μl of tissue. Volumetric coverage of the entire LV was achieved using two long-axis planes (2-chamber and 4-chamber) and the short-axis plane matching functional imaging to ensure precise co-registration between cine CMR and infarct measurements.
All CMR images were analysed in a central core laboratory by experienced technicians blinded to patient data and supervised by an experienced CMR reader cardiologist using commercially available software (QMass version 7.2; Medis, Leiden, The Netherlands). Briefly, LV volumes and ejection fraction were measured from semi-automated tracings of endocardium and epicardium performed on all 30 phases of the RR cycle in short-axis SSFP images. To determine infarct size, quantitative assessment of LGE volume was performed on short-axis inversion recovery images by semi-automated signal intensity analysis, using the full width at half-maximum technique on the 17-segment model.
Reproducibility on LGE data was evaluated in 10 patients and revealed excellent interobserver and intraobserver agreement with Lin’s concordance correlation coefficient of 0.92 (p<0.0001; Bland-Altman 95% CI: -2.43 to 2.48) and of 0.98 (p<0.0001; Bland-Altman 95% CI: -1.96 to 2.55), respectively.
Categorical variables are reported as n (%). Continuous variables are expressed as median (25th to 75th interquartile range [IQR]). Group comparisons were analysed using the Wilcoxon rank-sum test for continuous variables, and chi-square test for categorical variables. An analysis of variance for repeated measures was performed to test for equal means at different times (baseline, six to 12, 24, 48, and 72 hrs) for the cardiac enzyme values and LVEF. For the comparison of the continuous variables before and after TAVI (including LGE as determined by CMR) data were compared using the paired Student’s t-test or Wilcoxon signed-rank test, according to variable distribution. The results with p-values <0.05 were considered significant. All analyses were conducted using the statistical package SPSS, Version 19 (IBM Corp., Armonk, NY, USA).
Results
The main baseline and procedural characteristics of the study population are shown in Table 1. The TF/TAo patients presented baseline characteristics similar to those of the TA approach patients, except for increased age (p=0.040), and reduced incidence of coronary artery disease (p=0.001) and peripheral arterial disease (p=0.008). Additionally, in the TA group, the Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) was more frequently implanted, and there was an increased number of pace runs and an increased hospital stay (all with p<0.001).
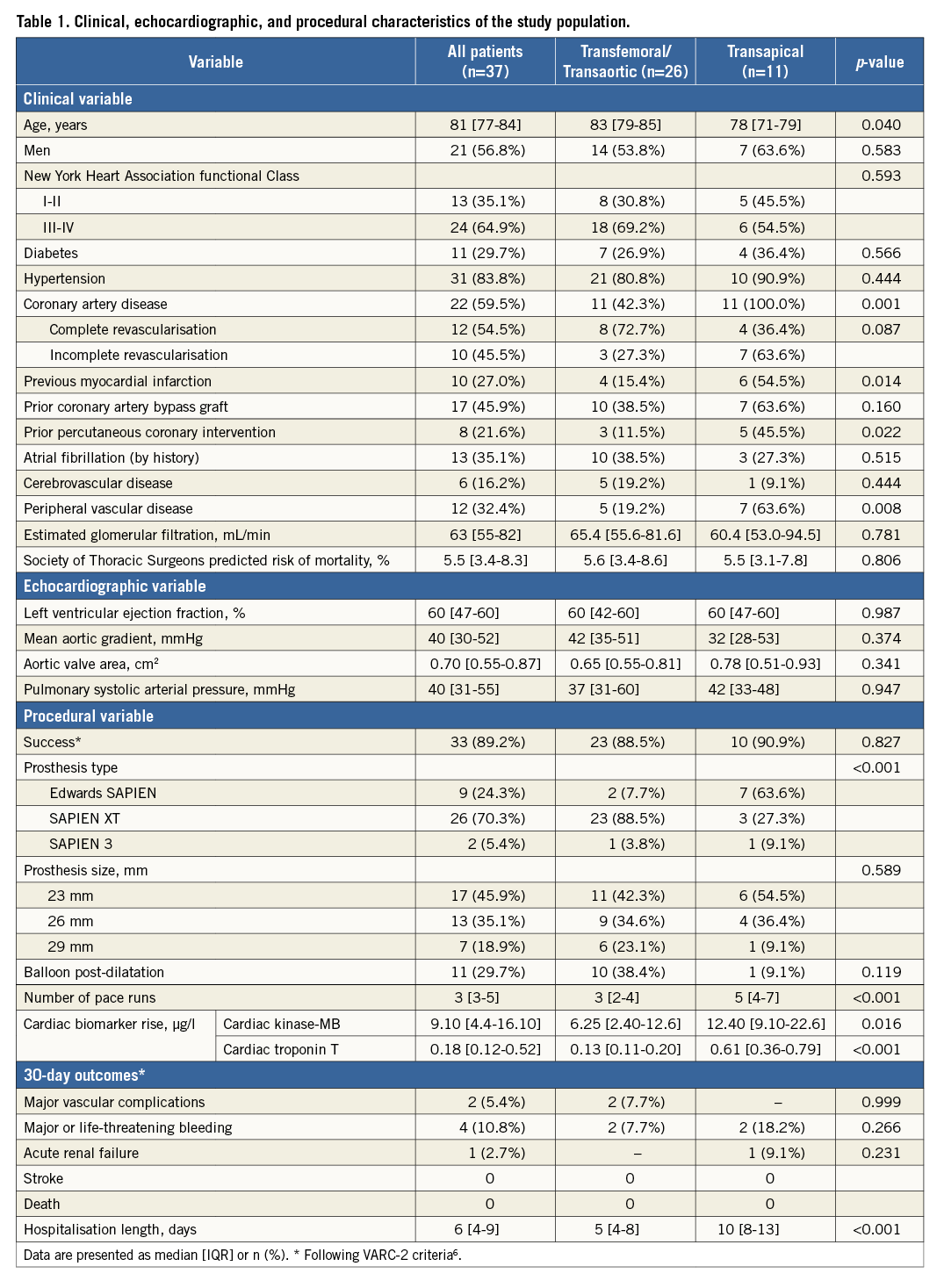
The mean values of CK-MB and cTnT at each time point within the 72 hrs following the procedure for the entire study population and for the TF/TAo and TA groups are shown in Figure 1. CK-MB levels were within normal limits in all patients at baseline and increased to above the upper normal limit in 49% of the patients with a median peak of 9.10 μg/l (4.4 to 16.10 μg/l) at 12-24 hrs following the procedure and returned to baseline values at 72 hrs after TAVI. In the TA group, the CK-MB levels were above the upper normal values in 73% of the patients compared to 35% of the patients in the TF/TAo group, with median peak values of 12.40 (9.10 to 22.6) μg/l and 6.25 (2.40 to 12.6) μg/l, respectively (p=0.023 vs. TF/TAo). The cTnT levels were within the normal limits at baseline in all patients and increased to above the upper normal limit in all patients but one (97.3%) following TAVI, with a median peak of 0.18 (0.12 to 0.52) μg/l at 48 hrs following the procedure (Figure 1A). The cTnT values continued to be above baseline values at 72 hrs following TAVI (Figure 1A). In the TF-TAVI group, peak cTnT was at six to 12 hrs after the procedure, whereas in the TA-TAVI group it occurred at 24 hrs after the procedure, and cTnT levels increased to above the upper normal values in all patients in the TA-TAVI group compared to 96% of the patients in the TF-TAVI group (p=0.518). The degree of cTnT increase was higher in the TA-TAVI group compared to the TF-TAVI group at all time points following the procedure (p<0.001) (Figure 1B). The median maximal cTnT value in the TF-TAVI group within the 72 hrs following the procedure was 0.13 (0.11 to 0.20) μg/l compared to 0.61 (0.36 to 0.79) μg/l in the TA-TAVI group (p<0.001).
Figure 1. Changes in creatine kinase-myocardial band (CK-MB) and cardiac troponin T (cTnT) levels within the 72 hrs following TAVI. A) In all patients. B) Grouped according to the approach (TF/TAo grouped and TA).
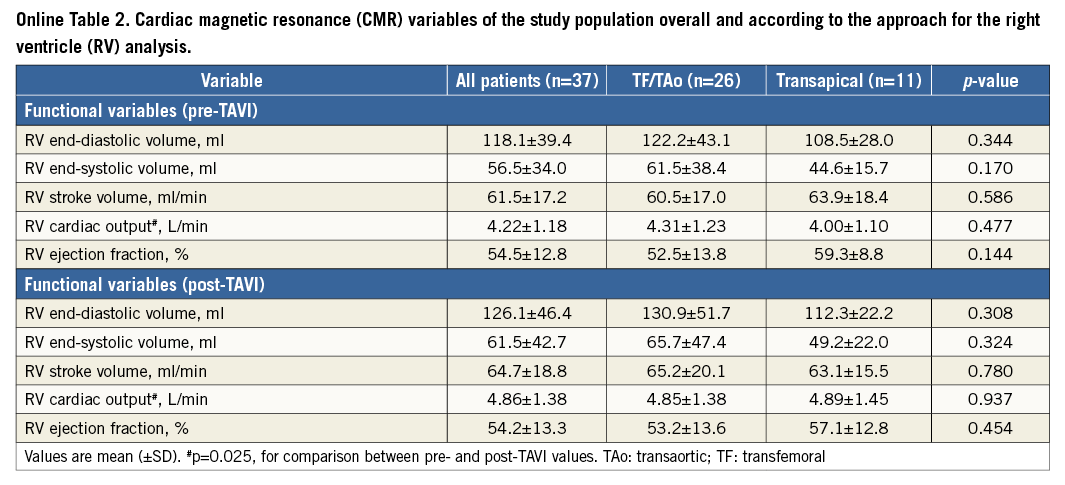
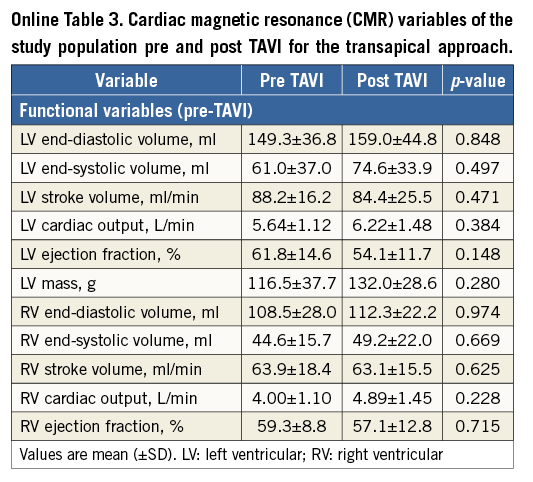
Baseline and post-TAVI CMR data are shown in Table 2, Online Table 2 and Online Table 3. There were no significant changes in left and right ventricle dimensions and function parameters following TAVI, except for an increase in left and right ventricular cardiac output, and a trend towards a reduced LVEF in the TA group after TAVI (61.8±14.8 vs. 54.1±11.7, respectively; p=0.148). There were no significant differences between the TF/TAo and TA approaches.
Echocardiography data at six to 12-month follow-up were available in 33 patients (89%), including nine of the 11 in the TA group (82%). Regarding the differences in LVEF by echocardiography at six to 12 months in relation to the baseline values (delta), there was an overall increase in LVEF: delta of +5 [0-19] (p=0.0072). However, when analysed according to the approach, this was only statistically significant for the TF/TAo group (delta LVEF: +5 [0-10], p=0.0374) as compared to TA (delta LVEF: 0 [0-10], p=0.0921).
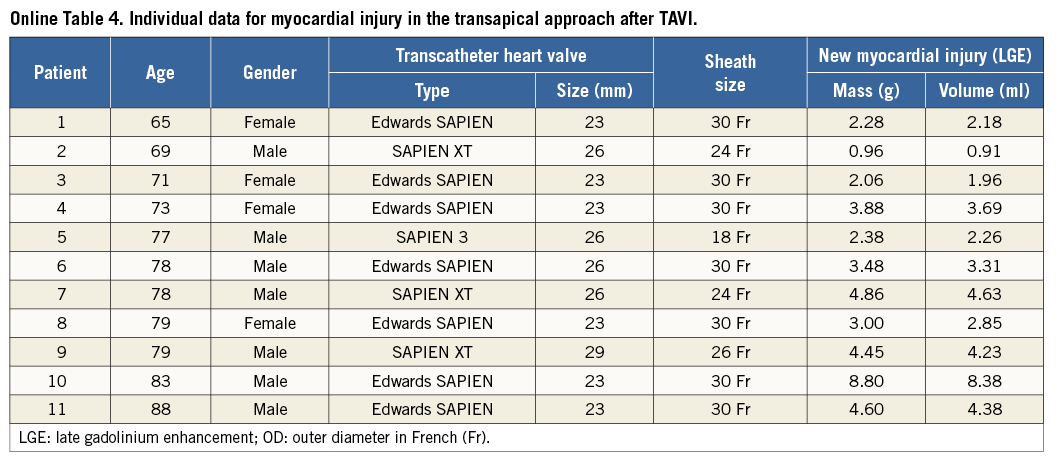
Myocardial necrosis was detected by LGE in 12 patients (32.4%) at baseline, with an ischaemic pattern in all of them (transmural in seven patients [58%] and subendocardial in five patients [42%]). The distribution and frequency (%) of myocardial necrosis defects at baseline is shown in Figure 2. LGE pre-TAVI was similar between TF/TAo and TA groups (median 0 g [0 to 1.5] versus 1.1 g [0 to 4.0], respectively; p=0.475), with an overall median of 0% (0 to 3.8) of the myocardium, and with a median of 0 g (0 to 3.3) of necrosis. After the TAVI procedure, new focal myocardial necrosis was detected only in the TA group: it was restricted to the apical segments in all patients, as shown in the examples in Figure 3 and Online Table 4 (individual data). The median extent of LGE after TAVI was of 5% (2.0 to 7.0) of the myocardium (versus 1.0% [0 to 5.0] before TAVI; p=0.031), and of 3.5 g (2.3 to 4.6) of necrosis (versus 1.1% [0 to 4.0] before TAVI; p=0.031) (Figure 4). All tracings were manually reviewed for accurate myocardial necrosis measurements in orthogonal axis, as short axis could be unsuitable to evaluate the apex, and showed 3.5 g (2.3 to 4.5) of new necrosis after TA-TAVI. No patient presented new focal defects in the LVOT septum at the level of the conduction system tract. The LGE distribution in the apex after TAVI is shown in Figure 3.
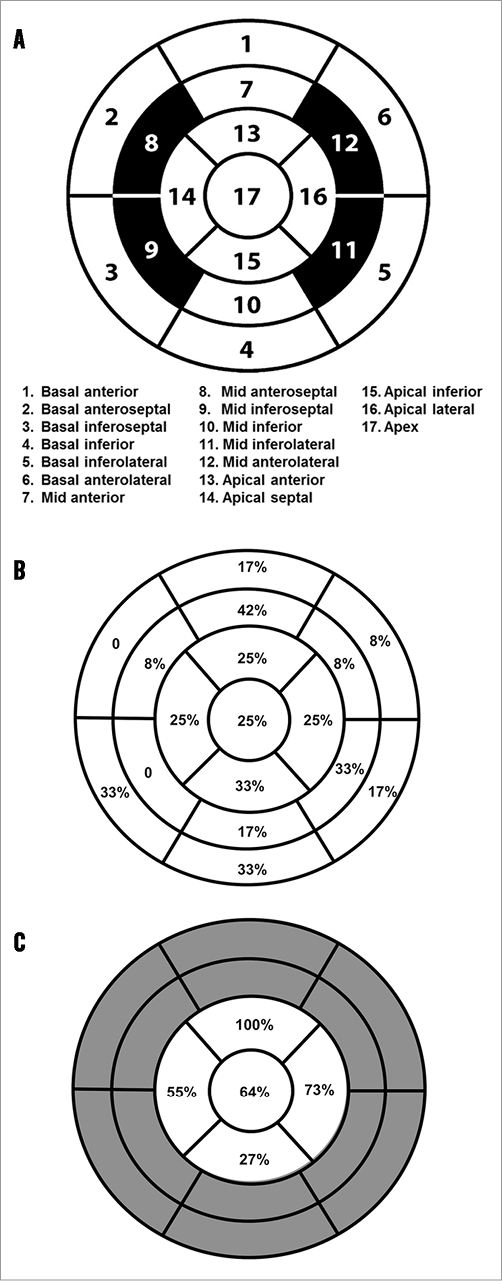
Figure 2. Schematic representations. A) 17-segment American Heart Association (AHA) model used to analyse myocardial necrosis distribution within the heart. B) Distribution and frequency (%) of focal myocardial necrosis before TAVI. C) Distribution and frequency (%) of focal myocardial necrosis after TAVI.
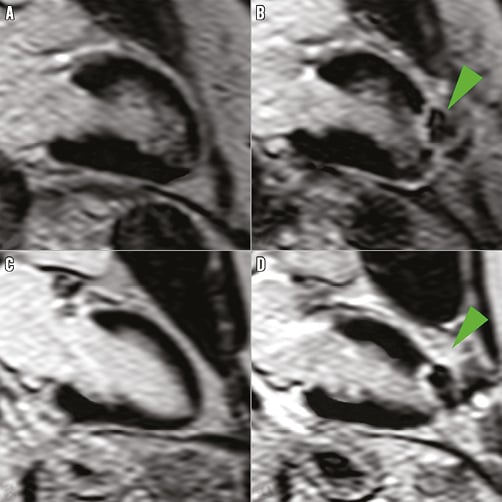
Figure 3. Representative cardiovascular magnetic resonance (CMR) imaging in two patients through the transapical approach. A) & C) Before TAVI. B) & D) After TAVI. Arrows show typical late gadolinium enhancement in the apex of the left ventricle.
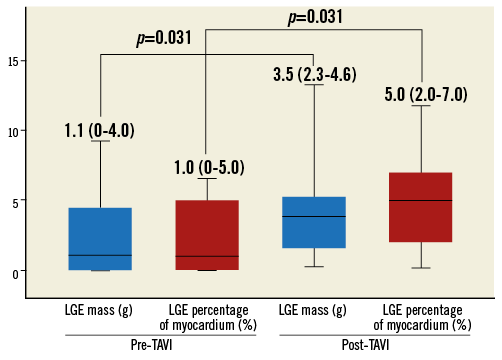
Figure 4. Degree and extent of myocardial necrosis at the apex before and after TAVI (transapical approach patients).
Discussion
Several previous studies have shown that TAVI is associated with some increase in the biomarkers of myocardial injury in most patients1-4. This rise in cardiac biomarkers has been observed following TAVI with both balloon-expandable and self-expandable valves, and, consistent with the results of the present study, a greater rise has been detected in those patients undergoing the procedure by the TA compared to the TF approach. Several mechanisms have been proposed to explain this systematic rise in cardiac biomarkers following TAVI. First, the lower (ventricular) part of the transcatheter valve usually sits within the LVOT, and mechanical compression of the myocardial septum at this level by the transcatheter valve can cause some myocardial injury. In fact, previous CMR studies have shown that myocardial necrosis can be detected in the LVOT septum at the level of the conduction tract system in patients undergoing TAVI with a self-expandable valve, which indeed may be responsible for some conduction disturbances after valve implantation10. In the present study, CMR studies failed to detect any focal myocardial necrosis at the level of the septum, suggesting that this mechanism is not responsible for the rise in cardiac biomarkers observed following TAVI with a balloon-expandable valve. Indeed, this could partially explain the lower incidence of conduction disturbances and need for pacemaker implantation associated with balloon-expandable compared to self-expandable valve implantation11. However, it must be borne in mind that as many as 9% of the patients were unable to undergo a repeated CMR in the days following TAVI due to pacemaker implantation, precluding ruling out the presence of new focal myocardial necrosis at the level of the septum in such patients.
It is well known that TAVI is associated with a high number of cerebral microemboli during the procedure, particularly during valve positioning and implantation9,12: up to 70% of patients undergoing TAVI with a dual carotid filter protection had some debris at the level of the filter at the end of the procedure12. Therefore, the occurrence of coronary emboli during the procedure may contribute to myocardial injury following TAVI. In the setting of PCI, irreversible myocardial injury as evaluated by CMR is related to either epicardial side branch occlusion in areas adjacent to the intervention site, or to microvascular circulation compromise, downstream to the intervened artery segment13. In the present study, the CMR data showing the absence of new focal myocardial necrosis in all TF/TAo patients do not support the coronary emboli hypothesis as a factor involved in myocardial injury in TAVI patients. Finally, TAVI is associated with episodes of severe hypotension and potential global myocardial ischaemia (rapid pacing runs, balloon valvuloplasty, valve implantation), which in turn can translate into diffuse myocardial injury. While no diffuse necrosis was detected in any patient in our study, the fact that the quantification of myocardial necrosis on LGE images was analysed using a semi-automatic, signal intensity threshold method, rather than the assessment of diffuse interstitial fibrosis accumulation as determined by myocardium T1 mapping14, may have been associated with an underdiagnosis of diffuse patterns of subendocardial myocardial necrosis, associated with episodes of severe hypotension or global ischaemia. The presence of diffuse myocardial necrosis as evaluated by myocardial T1 mapping will have to be evaluated in future studies.
The use of the TA approach involves the puncture and the introduction of a large catheter through the ventricular apex (≥24 Fr, with external diameter ≥7.9 mm). This has been associated with a greater increase in cardiac biomarkers of myocardial injury. The present study confirms the presence of significant myocardial necrosis at the level of the left ventricular apex in such patients. The CMR analysis also revealed that apical lesions extended beyond the puncture site in the apex, showing that both the puncture itself and also the pursestrings from the suture may explain the damage. This is also supported by a previous study in an experimental model showing that apical puncture closure with a device (without the sutures) did not cause LV myocardial fibrosis beyond the access site15. Importantly, the necrotic mass was ~3 g and represented ~5% of the left ventricular myocardial mass. This amount of necrosis is similar to that observed in the context of percutaneous coronary intervention (PCI)13, where new myocardial necrosis is detected in ~25% of cases, also extending to a mean of 5% of the LV mass13. However, this amount of myocardial injury by LGE is lower than that reported in patients undergoing open heart surgery, where a certain degree of cardiac biomarker elevation occurs almost invariably16, leading to irreversible myocardial injury as evaluated by CMR in more than one third of the patients16.
Studies in the context of coronary artery disease have shown that even small amounts of myocardial necrosis (as low as 1 g) were associated with a 5% increase in major cardiac events17. Azevedo et al18 showed that new myocardial necrosis following surgical aortic valve replacement extending to ≥5% of the myocardium as determined by CMR was associated with increased mortality and decreased LVEF at two-year follow-up. Interestingly, in patients undergoing TAVI with a balloon-expandable valve, the degree and extent of cardiac biomarker elevation (also more frequent for the TA approach) have also been associated with less improvement in LVEF at one-year follow-up1. Also in accordance with these results, Barbash et al19 showed the presence of apical wall motion abnormalities in about one third of the patients treated through the TA approach, which translated into a lower LVEF at follow-up. While the poorer outcomes associated with the TA approach have been mainly related to the higher risk profile of the patients treated through this approach (usually patients with inadequate iliofemoral access)20, the TA approach was found to be an independent predictor of mortality in two large TAVI studies (FRANCE-2 and the UK TAVI Registry)21,22 as well as in a recent meta-analysis23. The present study showing that this approach is systematically associated with significant irreversible myocardial injury suggests that the loss of ~5% of the myocardium associated with this approach (>1 g of necrotic mass in all cases) may contribute to these poorer clinical outcomes. However, the small sample size of the present study precluded any evaluation of the correlation between the severity of myocardial necrosis as determined by CMR and clinical outcomes. This will have to be evaluated in future studies with a larger number of patients.
Limitations
This study had some limitations. The patients were not consecutive and a selection bias might have influenced the results. The limited number of patients and the lack of long-term follow-up do not allow us to determine a cut-off for the amount of myocardial necrosis associated with poorer clinical outcomes, to evaluate the changes in LV function, or to establish a correlation between cardiac biomarker elevation and new focal necrosis. The oedema-weighted T2 imaging was not analysed in the present study, and this would have helped to clarify further the effect of the TAVI procedure on myocardial damage in the LVOT septum. These aspects will have to be evaluated in future larger studies. The results of this study were obtained in patients undergoing TAVI with a balloon-expandable valve, and may not apply to those patients receiving a self-expandable valve.
Conclusions
In conclusion, while some increase in the biomarkers of myocardial injury was systematically detected in patients undergoing TAVI with a balloon-expandable valve, the presence of new myocardial necrosis as evaluated by CMR was detected only in patients undergoing TAVI through the TA approach. New myocardial necrosis was limited to the left ventricular apex, and affected about 5% of the ventricular mass. No other new myocardial necrosis defects were detected outside the ventricular apex. These results provide an important insight into the mechanisms of myocardial injury following TAVI and invite us to evaluate further the clinical impact of new myocardial necrosis on clinical outcomes.
| Impact on daily practice Transcatheter aortic valve implantation (TAVI) is systematically associated with some increase in cardiac biomarkers. In our study new myocardial necrosis as evaluated by cardiac magnetic resonance (CMR) imaging was observed only in patients undergoing the procedure through the transapical (TA) approach, involving ~5% of the myocardium in the apex. No patient presented new focal myocardial necrosis in the left ventricular septum at the level of the conduction system tract. The present results might explain in part the mechanisms of cardiac biomarker elevation following TAVI and should be confirmed in future larger studies. |
Funding
H. Ribeiro is supported by a research PhD grant from “CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico- Brasil”. E. Larose is a research scholar of the “Fonds de la recherche du Québec – Santé”. F. Le Ven is supported by a clinical and research fellowship from the “Fédération Française de Cardiologie”. L. Nombela-Franco has received funding via a research grant from the “Fundación Mutua Madrileña” (Spain). I. Amat-Santos was supported by the “Instituto de Salud Carlos III”, through a research contract at the Institute of Heart Sciences (Valladolid, Spain). M.A. Clavel is supported by a post-doctoral fellowship from CIHR. P. Pibarot holds the Canada Research Chair in Valvular Heart Disease supported by CIHR.
Conflict of interest statement
J. Rodés-Cabau has received research grants from Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Online data supplement