How does the reversal of aortic stenosis by transcatheter aortic valve implantation (TAVI) acutely alter myocardial physiology? Addressing this question – as was done in the recent collaborative manuscript from three centres in Italy and Belgium1 – allows us a timely opportunity to synthesise 30 years of data with a view to clinical application.
Components of resistance
While often written about in the singular form, the term “hyperaemic microvascular (or myocardial) resistance” actually contains two conceptual parts. First, how much flow (mL/min) results from a given coronary driving pressure (mmHg)? Second, at what coronary pressure does flow cease? The first notion corresponds to our intuition of resistance (a constant value during hyperaemia). However, the second notion reminds us that – unlike a classical electrical circuit – the system does not behave proportionally without accounting for what has been termed the zero-flow or wedge or back pressure (depending on the details of its assessment and with conceptual differences beyond the scope of this editorial). Visually, myocardial resistance can be depicted as shown in Figure 1A: a straight line linking coronary pressure and flow whose slope equals the inverse of resistance (steeper slope=less resistance).
How does aortic stenosis alter these two facets of resistance? Seminal animal work placed a supravalvular aortic band, creating 20-25 mmHg of peak systolic gradient2. Aortic banding reduced the slope (i.e., increased the resistance). A metric of left ventricular (LV) hypertrophy – the ratio of LV mass to body weight – demonstrated a significant, moderate, negative correlation with slope (higher resistance for more hypertrophy), explaining about a third of the variation. Additionally, LV filling pressures increased with aortic banding. A significant, strong, positive correlation of LV and zero-flow pressures explained almost two-thirds of the variation. Stated simply, aortic stenosis produced LV hypertrophy and higher filling pressures, shifting the myocardial resistance line to the right (due to higher zero-flow pressure) and flattening its slope (due to greater resistance).
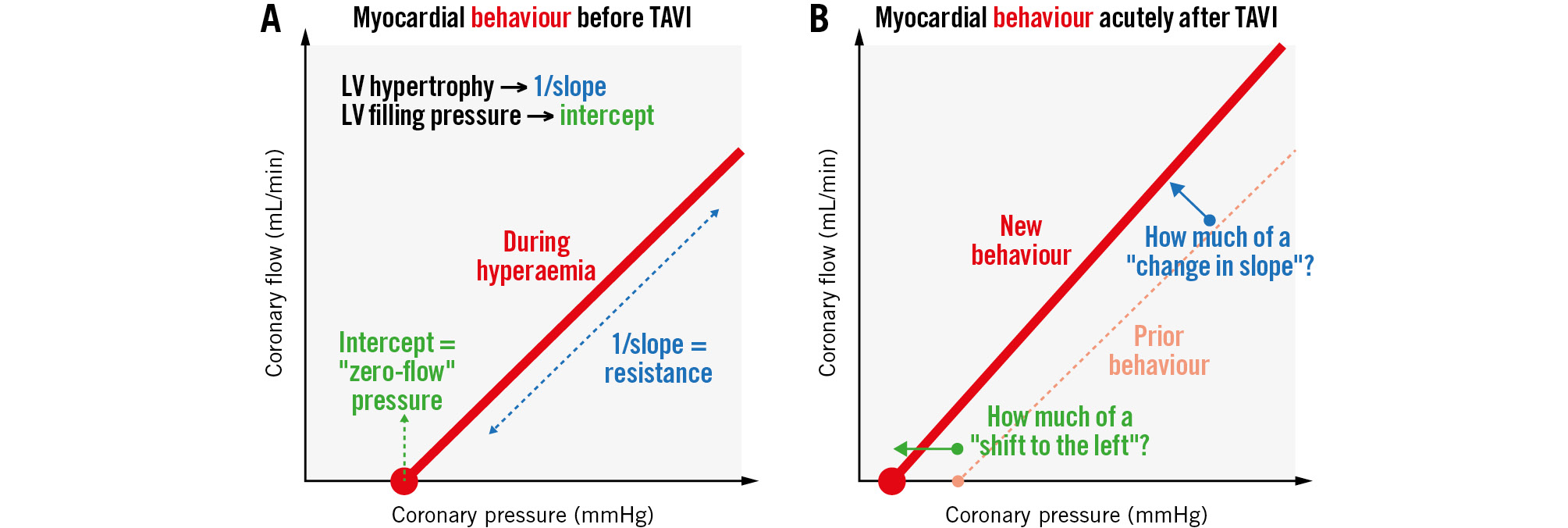
Figure 1. Hyperaemic microvascular resistance. Does transcatheter aortic valve implantation immediately “slide” the line to the left but maintain a similar slope? A) Shows myocardial behaviour before TAVI and (B) shows myocardial behaviour acutely after TAVI. LV: left ventricular; TAVI: transcatheter aortic valve implantation
Acute changes in resistance
In this issue of EuroIntervention, Scarsini et al present their study on coronary physiology immediately before and after TAVI using continuous thermodilution in 134 patients1. In keeping with a broad prior literature3, fractional flow reserve (FFR) fell from 0.90 to 0.88 (paired p-value=0.014). In contrast, coronary flow reserve (CFR; from 2.0 to 2.1; paired p-value=0.805) and microvascular resistance reserve (MRR; from 2.40 to 2.66; paired p-value=0.094) did not change significantly. Generally, other studies have also found a modest fall in FFR but no significant change in CFR acutely after TAVI3. Four prior comparisons of hyperaemic microvascular resistance before and immediately after TAVI produced heterogeneity (two significant decreases, two without significant change)3.
However, no human study has separated the two components of microvascular resistance before and acutely after TAVI: slope and intercept. For example, paired measurements of LV filling pressures (or, although more complex, coronary wedge pressures) were not reported in the current series1. Its finding of unchanged CFR and MRR but a significant fall in FFR potentially suggests a change in the intercept rather than the slope of the hyperaemic resistance line. An ongoing longitudinal study of coronary physiology before, acutely after, and 6 months after TAVI incorporating repeated assessments of LV filling pressures and mass is due to be published soon4.
Two unexpected findings in the current report warrant discussion1. First, in the subgroup with high MRR >3 at baseline (and a corresponding median FFR of 0.88 and CFR of 3.22), while FFR fell to a median of 0.87, CFR also fell to 2.44 acutely after TAVI (resulting in a fall in MRR). Because greater hyperaemic flow produces more pressure loss, FFR and CFR generally move in opposite directions. Perhaps hyperaemic flow was indeed higher after TAVI but offset by a higher “baseline” flow, resulting in a lower CFR – highlighting the importance of reporting both components of the CFR ratio. Alternatively, or additionally, this subgroup with initial MRR >3 might have experienced a large change in LV filling pressures.
Second, the current manuscript1 found no significant relationship between MRR tertiles and LV mass index (p=0.836) or relative wall thickness (p=0.548), unlike in an animal model2 where there was significant correlation between hyperaemic conductance and LV hypertrophy (correlation coefficient 0.59; p<0.005). Does this discordance arise because of more numerous and complex factors in a human cohort (like diabetes and hypertension) compared to a uniform animal preparation? Or, does it arise from mixing slope and intercept into an aggregate single parameter?
Clinical translation
While modest correlations between MRR and morphological stages of cardiac dysfunction with aortic stenosis remain limited by substantial overlap and the need for invasive assessment1, perhaps quantitative assessment of microvascular resistance could improve patient selection. Following TAVI, approximately 25% to 40% of patients experience no improvement in 6-minute walk times or quality-of-life surveys5. Potentially, in patients with a reduced slope but no LV hypertrophy, a secondary intrinsic myocardial pathology that is unresponsive to TAVI might be identified – a hypothesis coupling microvascular physiology to aortic valve pathology.
Conflict of interest statement
N.P. Johnson has no direct conflicts of interest to declare, but outside of the present work, he receives internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis; he has patents pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology, and on methods to correct pressure tracings from fluid-filled catheters; and receives significant institutional research support from Neovasc and Shockwave Medical (PET core lab for COSIRA-II, ClinicalTrials.gov: NCT05102019). R. Eerdekens has no relevant conflicts of interest to declare.

