Abstract
BACKGROUND: Data on the likelihood of left ventricle (LV) recovery in patients with severe LV dysfunction and severe aortic stenosis undergoing transcatheter aortic valve implantation (TAVI) and its prognostic value are limited.
AIMS: We aimed to assess the likelihood of LV recovery following TAVI, examine its association with midterm mortality, and identify independent predictors of LV function.
METHODS: In our multicentre registry of 17 TAVI centres in Western Europe and Israel, patients were stratified by baseline LV function (ejection fraction [EF] >/≤30%) and LV response: no LV recovery, LV recovery (EF increase ≥10%), and LV normalisation (EF ≥50% post-TAVI).
RESULTS: Our analysis included 10,872 patients; baseline EF was ≤30% in 914 (8.4%) patients and >30% in 9,958 (91.6%) patients. The LV recovered in 544 (59.5%) patients, including 244 (26.7%) patients whose LV function normalised completely (EF >50%). Three-year mortality for patients without severe LV dysfunction at baseline was 29.4%. Compared to this, no LV recovery was associated with a significant increase in mortality (adjusted hazard ratio 1.32; p<0.001). Patients with similar LV function post-TAVI had similar rates of 3-year mortality, regardless of their baseline LV function. Three variables were associated with a higher likelihood of LV recovery following TAVI: no previous myocardial infarction (MI), estimated glomerular filtration rate >60 mL/min, and mean aortic valve gradient (mAVG) (expressed either as a continuous variable or as a binary variable using the standard low-flow, low-gradient aortic stenosis [AS] definition).
CONCLUSIONS: LV recovery following TAVI and the extent of this recovery are major determinants of midterm mortality in patients with severe AS and severe LV dysfunction undergoing TAVI. Patients with no previous MI and those with an mAVG >40 mmHg show the best results following TAVI, which are at least equivalent to those for patients without severe LV dysfunction. (ClinicalTrials.gov: NCT04031274)
Transcatheter aortic valve implantation (TAVI) has revolutionised the treatment of patients suffering from severe symptomatic aortic stenosis (AS) and is now well established as a viable treatment across the entire spectrum of surgical risk according to both American1 and European2 practice guidelines. However, data on patients with reduced left ventricular (LV) systolic function undergoing TAVI are limited. Early studies reported that in patients with LV dysfunction, defined as an ejection fraction (EF) <40-50%, the EF improved by ≥10% post-TAVI in about 50% of patients; failure to improve following TAVI was associated with an increased risk of 1-year mortality or rehospitalisation3456. Recently, pooled data from the Placement of AoRTic TraNscathetER Valves (PARTNER) 1, 2, and S3 trials reported that in patients with an EF <50% at baseline (mean EF 38%), 33% demonstrated an improvement in EF of ≥10%, and this was associated with a significantly reduced risk for overall as well as cardiovascular mortality for up to 5 years of follow-up7. Data on TAVI outcomes in patients with severe LV dysfunction (≤30%) are scarce, as these patients were rarely included in the pivotal TAVI randomised trials (where the mean EF was >50%). The aim of this study was to describe the midterm results of patients with severe LV dysfunction (≤30%) undergoing TAVI − stratified by patterns of LV response following the procedure − and to identify predictors of LV recovery following TAVI.
Methods
STUDY POPULATION
The Aortic+Mitral TRAnsCatheter (AMTRAC) Registry (ClinicalTrials.gov: NCT04031274) is an investigator-Âinitiated, multicentre registry of 17 TAVI centres in Europe and Israel. Details of the design and data collection of the registry have been published previously89. For this study, we included consecutive cases of TAVI performed at the participating centres up to 31 December 2020.
INCLUSION CRITERIA
We included patients with available EF at baseline and at least one EF assessment post-procedure prior to hospital discharge.
EXCLUSION CRITERIA
Patients who had undergone TAVI using the transapical approach were excluded from our study.
ENDPOINTS
The primary endpoint was all-cause mortality at 3 years.
PATIENT GROUPING
Patients were grouped according to their baseline EF (>/≤30%), as assessed at each centre pre-TAVI, in line with local practice (no core lab analysis was performed). In addition, patients with an EF ≤30% at baseline were grouped according to their LV response to TAVI:
• no LV recovery – EF did not improve ≥10% within 60 days from TAVI
• LV recovery – EF improvement of ≥10% within 60 days from TAVI
• LV normalisation – EF recovery to ≥50% within 60 days from TAVI.
STATISTICAL METHODS
Baseline and procedural characteristics are presented as mean±standard deviation or median (interquartile range [IQR]), as appropriate, for continuous variables and as counts (%) for categorical variables. They were compared according to baseline EF (>/≤30%) and LV response to TAVI (no recovery/recovery/normalisation) using the independent sample t-test, Chi-square test and analysis of variance, as appropriate. Three-year cumulative all-cause mortality was compared according to baseline EF and LV response using Kaplan-Meier curves and the log-rank test. Adjusted hazard ratios (HRs) for mortality were calculated using a multiÂvariate adjusted Cox proportional hazards model which included age, sex, baseline New York Heart Association (NYHA) Class, Society of Thoracic Surgeons (STS) score, frailty and EF as covariates. To assess whether baseline LV dysfunction exerts a negative effect on post-TAVI survival even in the case of LV recoÂvery, the survival analysis was also performed for those with a baseline EF >30%, stratified to 30%
To account for survival bias, we performed a landmark analysis in which patients who did not survive 30 days post-TAVI were excluded.
Independent predictors of LV recovery (a binary definition of EF improving 10% within 60 days from TAVI or not) were identified using a multivariate logistic regression model. For this analysis, the mean aortic valve gradient (mAVG) was examined both as a continuous variable as well as a binary variable using the standard definition of low-flow, low-gradient AS (LF-LG AS: mAVG <40 mmHg).
ETHICAL APPROVAL AND STUDY REGISTRATION
The registry protocol was approved by the local institutional review board as required at each participating centre. The registry is listed on ClinicalTrials.gov: NCT04031274.
Results
Our cohort included 11,029 patients. One hundred and fifty-seven patients were excluded (45 cases of transapical TAVI and 112 patients who did not have any postprocedural data on EF following TAVI). Of the remaining 10,872 patients, the baseline EF was ≤30% in 914 (8.4%) and >30% in 9,958 (91.6%) patients.
Baseline characteristics according to baseline EF category are presented in Supplementary Table 1.
Patients with severe LV dysfunction at baseline had a mean EF of 27.3%, compared to a mean of 58.9% in the EF >30% group. Patients in the EF ≤30% group were younger (79.8 years vs 81.2 years), more often male, had more comorbidities and a higher prevalence of previous coronary artery bypass graft (CABG), myocardial infarction (MI) and percutaneous coronary intervention (PCI). Also, these patients were more often frail, in NYHA Class III-IV and with higher STS scores. The mAVG at baseline was lower in the EF ≤30% group (34.9 mmHg vs 47.3 mmHg), but aortic valve area was not statistically different between the two groups. Use of the femoral approach was high in both groups, albeit slightly higher in the EF >30% group (94.7% vs 92.3%).
LEFT VENTRICLE RESPONSE TO TAVI
Of the 914 patients with a baseline EF ≤30% and with available data on EF following TAVI, LV recovery occurred in 544 (59.5%) patients. Of these, 300 (32.8%) patients showed an improvement in EF of ≥10%, although the EF remained under 50%; in the remaining 244 (26.7%) patients, EF normalised completely following TAVI (Central illustration). The median follow-up to the echocardiographic assessment showing LV recovery/normalisation was 34 (IQR 10-45) days. Baseline characteristics of patients with a baseline EF 30% according to the LV response to TAVI are presented in Table 1. Patients with no LV recovery were more often male (71.4% vs 62.3%), had a higher prevalence of previous CABG, MI, PCI and permanent pacemaker implantation. In terms of echocardiographic characteristics, those patients without LV recovery had lower baseline EF (26.4% vs 27.5%) and aortic valve gradients (31.1 mmHg vs 37.8 mmHg), a larger aortic valve area (0.79 cm² vs 0.63 cm2), and were more likely to meet the definition of LF-LG AS (79.1% vs 52.8%). All other baseline characteristics were similar between the groups, including NYHA Class and levels of N-terminal pro-brain natriuretic peptide (available for 319 patients).
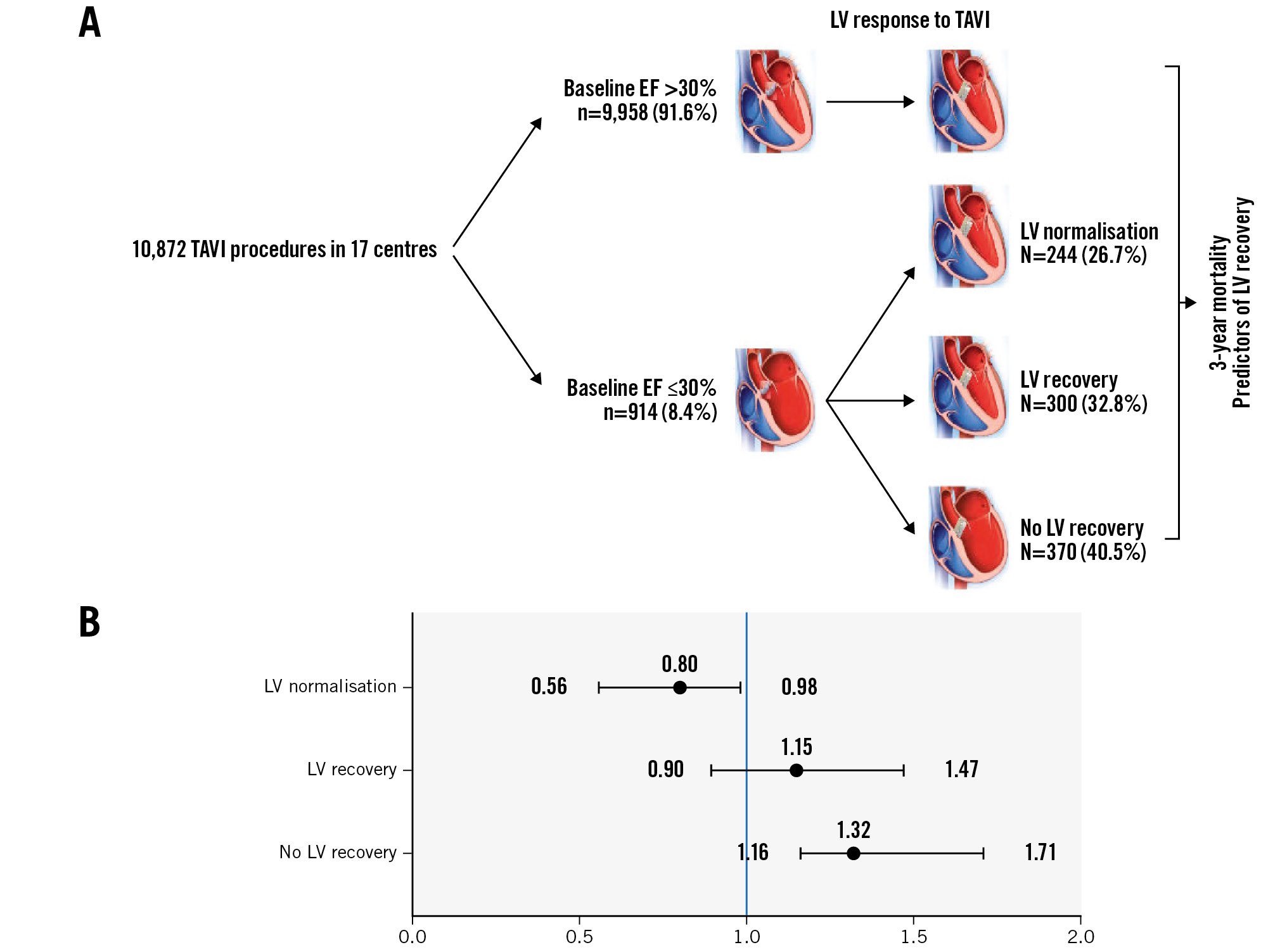
Central illustration. LV recovery in patients with severe LV dysfunction undergoing TAVI. A) Graphic presentation of the study design and outcomes. B) Forest plot of the adjusted hazard ratios for 3-year mortality according to LV response to TAVI. Reference is patients with baseline EF >30%. EF: ejection fraction; LV: left ventricle; TAVI: transcatheter aortic valve implantation
Table 1. Baseline characteristics according to LV recovery following TAVI.
| No EF recoveryN=370 | EF recoveryN=300 | EF normalisationN=244 | p-value | |
|---|---|---|---|---|
| Age, years | 78.9±7.9 | 80.2±7.1 | 80.0±8.2 | 0.27 |
| Female | 106 (28.6) | 98 (32.6) | 107 (44.1) | <0.001 |
| BMI, kg/m2 | 26.2±4.5 | 26.2±4.8 | 25.1±5.3 | 0.461 |
| eGFR, mL/min | 51.3±25.1 | 55.1±25.5 | 54.0±27.1 | 0.232 |
| Haemoglobin, g/L | 12.4±2.4 | 12.3±2.4 | 11.9±2.3 | 0.349 |
| STS score | 7.0±1.6 | 7.1±1.8 | 7.9±1.9 | 0.151 |
| NT-proBNP, pg/mL* | 15,421±2,198 | 14,958±2,612 | 12,321±1,432 | 0.573 |
| Mean AVG, mmHg | 31.1±14.9 | 35.2±13.7 | 41.3±15.4 | <0.001 |
| AVA, cm2 | 0.79±0.18 | 0.66±0.24 | 0.60±0.21 | 0.022 |
| EF, % | 26.4±2.1 | 27.0±1.9 | 28.1±1.1 | 0.027 |
| LF-LG AS | 293 (79.1) | 172 (64.9) | 115 (47.2) | <0.001 |
| Baseline MR ≥moderate | 66 (17.8) | 50 (16.6) | 45 (18.4) | 0.458 |
| RV dysfunction ≥mild | 59 (15.9) | 57 (18.9) | 36 (14.6) | 0.614 |
| Previous CABG | 83 (22.5) | 57 (19.2) | 23 (9.6) | <0.001 |
| Previous PCI | 169 (45.6) | 101 (33.7) | 53 (21.7) | <0.001 |
| Previous MI | 162 (43.8) | 67 (22.3) | 36 (14.7) | <0.001 |
| Frailty | 99 (26.8) | 80 (26.5) | 75 (30.7) | 0.452 |
| AF | 130 (35.1) | 107 (35.6) | 80 (32.9) | 0.481 |
| PPM | 92 (24.9) | 65 (21.6) | 25 (10.2) | <0.001 |
| COPD | 84 (22.8) | 63 (21.0) | 56 (22.8) | 0.789 |
| DM | 133 (35.9) | 111 (37.0) | 84 (34.3) | 0.941 |
| Hypertension | 284 (76.7) | 229 (76.2) | 189 (77.4) | 0.987 |
| Valve-in-valve | 10 (2.7) | 14 (4.7) | 4 (1.8) | 0.143 |
| Femoral access | 344 (92.8) | 274 (91.2) | 223 (91.3) | 0.529 |
| NYHA III-IV | 311 (84.1) | 250 (83.3) | 191 (78.3) | 0.349 |
| Data are presented as mean±SD or n (%). *Available for 319 patients. AF: atrial fibrillation; AVA: aortic valve area; AVG: aortic valve gradient; BMI: body mass index; CABG: coronary artery bypass graft surgery; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; EF: ejection fraction; eGFR: estimated glomerular filtration rate; LF-LG AS: low-flow, low-gradient aortic stenosis; LV: left ventricle; MI: myocardial infarction; MR: mitral regurgitation; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; PPM: permanent pacemaker; RV: right ventricle; SD: standard deviation; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | ||||
SURVIVAL FOLLOWING TAVI
Kaplan-Meier curves for 3-year mortality are shown in Figure 1 and Supplementary Figure 1. The median follow-up was 596 days. Cumulative all-cause mortality rates at 3 years were 40.2% and 31.3% in the no LV recovery and LV recovery groups, respectively (p<0.001) (Supplementary Figure 1). When stratified according to the extent of LV recovery, mortality rates were 35.5% and 26.3% in the LV recovery and LV normalisation groups, respectively (p<0.001). In comparison, 3-year mortality for patients without severe LV dysfunction at baseline was 29.4% (Figure 1). Adjusted HRs for patients with an EF ≤30% at baseline, stratified according to LV response to TAVI, compared to those with a baseline EF >30% are presented in Table 2. No LV recovery was associated with a significant increase in mortality (HR 1.32; p<0.001), LV recovery was associated with a similar rate of mortality (HR 1.15; p=0.264), and LV normalisation was associated with improved survival (HR 0.80; p=0.041), compared to baseline EF >30%.
A landmark analysis − restricted to patients who survived at least 30 days post-TAVI − yielded consistent results: 3-year mortality rates were 37.4%, 30.9%, 26.3% and 26.8% for patients with no LV recovery, LV recovery, LV normalisation and baseline EF >30%, respectively (p<0.001). Compared to patients with baseline EF >30% as reference, the HR for mortality was 1.39 (p<0.001) for no LV recovery, 1.10 (p=0.321) for LV recovery, and 0.93 (p=0.462) for LV normalisation (Figure 1, Table 2).
When comparing the outcomes of patients with severe LV dysfunction at baseline whose LV recovered following TAVI to their counterparts whose baseline EF was >30%, 3-year mortality was similar between those with baseline severe LV dysfunction, but LV recovery post-TAVI compared to those with a baseline EF of 30-50% (35.5% vs 33.7%, adjusted HR 1.03; p=0.783), and likewise, it was similar between those with baseline severe LV dysfunction, but LV normalisation post-TAVI compared to those whose baseline EF was >50% (26.3% vs 28.6%, adjusted HR 0.90; p=0.629) (Figure 2, Table 3). Results were consistent when including only those patients who survived 30 days or more post-TAVI: 3-year mortality was 30.9% versus 30.2% for those with LV recovery compared to those with a baseline EF of 30-50% (adjusted HR 1.01; p=0.865), and 26.3% versus 26.5% (adjusted HR 0.99; p=0.914) for patients with LV normalisation post-TAVI compared to those with a baseline EF >50% (Figure 2, Table 3).
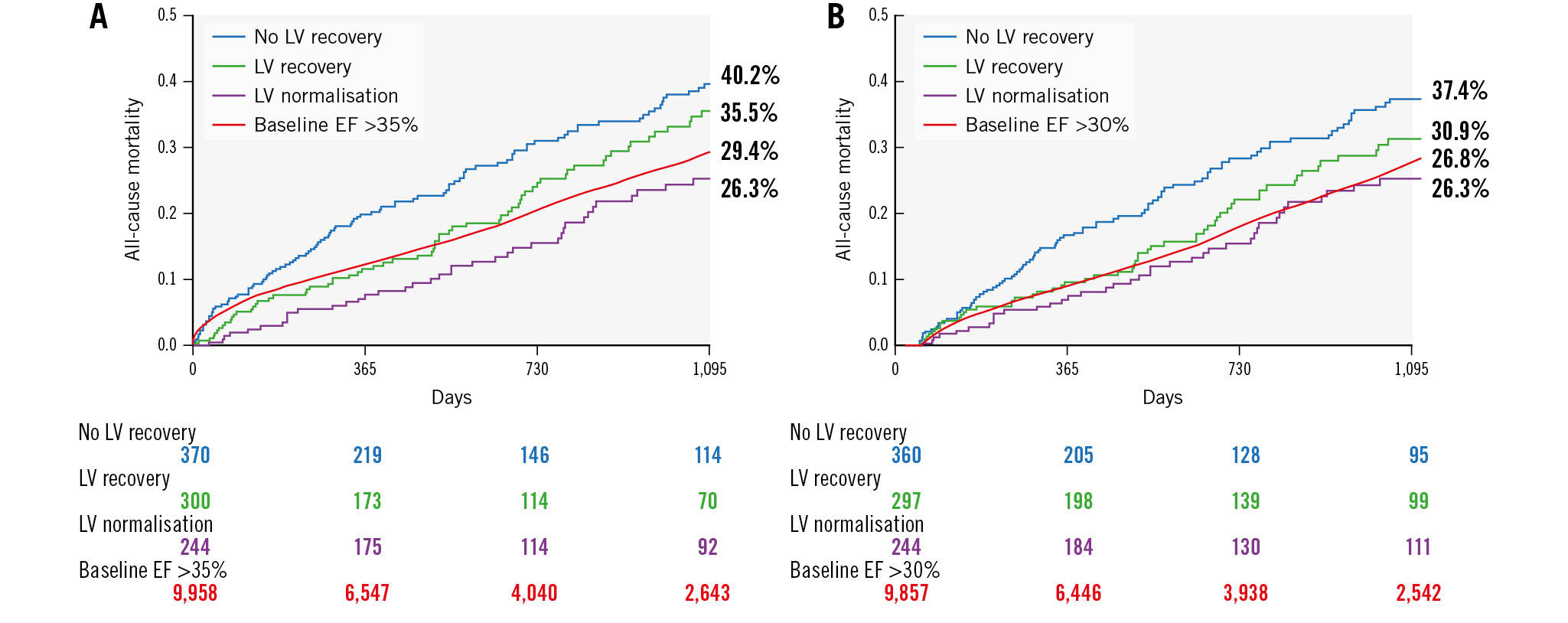
Figure 1. Three-year mortality following TAVI stratified by baseline LV function and LV response to TAVI. KM curves for 3-year cumulative mortality following TAVI stratified by baseline LV function and LV response to TAVI for the full cohort (A) and for a landmark analysis restricted to patients who survived at least 30 days post-TAVI (B). EF: ejection fraction; KM: Kaplan-Meier; LV: left ventricle; TAVI: transcatheter aortic valve implantation
Table 2. HR for mortality according to LV recovery following TAVI.
| Group | 3-year mortality | HR (95% CI)* | p-value |
|---|---|---|---|
| A: all patients | |||
| Baseline EF >30% | 29.4% | Reference | NA |
| No LV recovery | 40.2% | 1.32 (1.16-1.71) | <0.001 |
| LV recovery | 35.5% | 1.15 (0.90-1.47) | 0.264 |
| LV normalisation | 26.3% | 0.80 (0.56-0.98) | 0.041 |
| B: survived >30 days | |||
| Baseline EF >30% | 26.8% | Reference | NA |
| No LV recovery | 37.4% | 1.39 (1.11-1.78) | <0.001 |
| LV recovery | 30.9% | 1.10 (0.87-1.49) | 0.321 |
| LV normalisation | 26.3% | 0.93 (0.50-1.18) | 0.462 |
| *adjusted for age, sex, baseline NYHA Class, STS score, frailty and EF. CI: confidence interval; EF: ejection fraction; HR: hazard ratio; LV: left ventricle; NA: not applicable; NYHA: New York Heart Association; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | |||
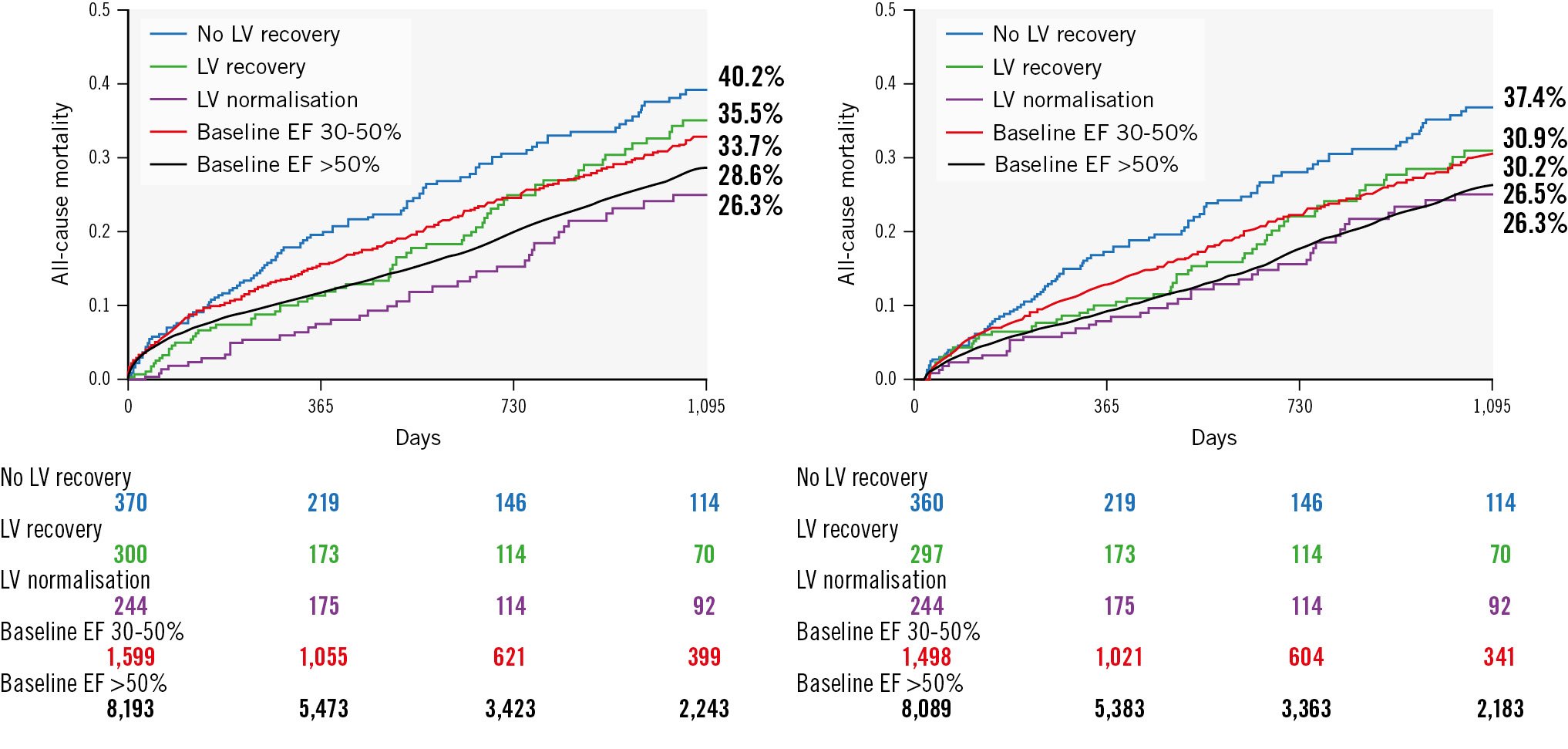
Figure 2. Three-year mortality following TAVI stratified by baseline LV function categories and LV response to TAVI. KM curves for 3-year cumulative mortality following TAVI stratified by baseline LV function categories and LV response to TAVI for the full cohort (A) and for a landmark analysis restricted to patients who survived at least 30 days post-TAVI (B). EF: ejection fraction; KM: Kaplan-Meier; LV: left ventricle; TAVI: transcatheter aortic valve
Table 3. HR for mortality for patients with recovered/normalised LV post-TAVI compared to those with similar EF at baseline.
| Group | 3-year mortality | HR (95% CI)* | p-value |
|---|---|---|---|
| A: all patients | |||
| Baseline EF 30-50% | 33.7% | Reference | NA |
| LV recovery | 35.5% | 1.03 (0.75-1.36) | 0.783 |
| Baseline EF >50% | 28.6% | Reference | NA |
| LV normalisation | 26.3% | 0.90 (0.49-1.36) | 0.629 |
| B: survived >30 days | |||
| Baseline EF 30-50% | 30.2% | Reference | NA |
| LV recovery | 30.9% | 1.01 (0.72-1.38) | 0.865 |
| Baseline EF >50% | 26.5% | Reference | NA |
| LV normalisation | 26.3% | 0.99 (0.62-1.35) | 0.914 |
| *adjusted for age, sex, baseline NYHA Class, STS score, frailty and EF. CI: confidence interval; EF: ejection fraction; HR: hazard ratio; LV: left ventricle; NA: not applicable; NYHA: New York Heart Association; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | |||
PREDICTORS OF LV RECOVERY FOLLOWING TAVI
Four baseline variables: no previous MI, eGFR >60 mL/min and mAVG (expressed either as a continuous or binary variable) were associated with the higher likelihood of LV recovery following TAVI (Table 4, Figure 3).
Table 4. Predictors of LV recovery following TAVI.
| Variable | HR (95% CI)* | p-value |
|---|---|---|
| Previous MI | 0.45 (0.28-0.71) | <0.001 |
| eGFR <60 mL/min | 0.49 (0.32-0.77) | 0.002 |
| Mean AVG (per mmHg) | 1.02 (1.01-1.04) | 0.007 |
| LF-LG AS | 0.50 (0.29-0.84) | 0.009 |
| *adjusted for age, sex, EF and AVA. AVA: aortic valve area; AVG: aortic valve gradient; CI: confidence interval; EF: ejection fraction; eGFR: estimated glomerular filtration rate; HR: hazard ratio;LF-LG AS: low-flow, low-gradient aortic stenosis; LV: left ventricle; MI: myocardial infarction; TAVI: transcatheter aortic valve implantation | ||
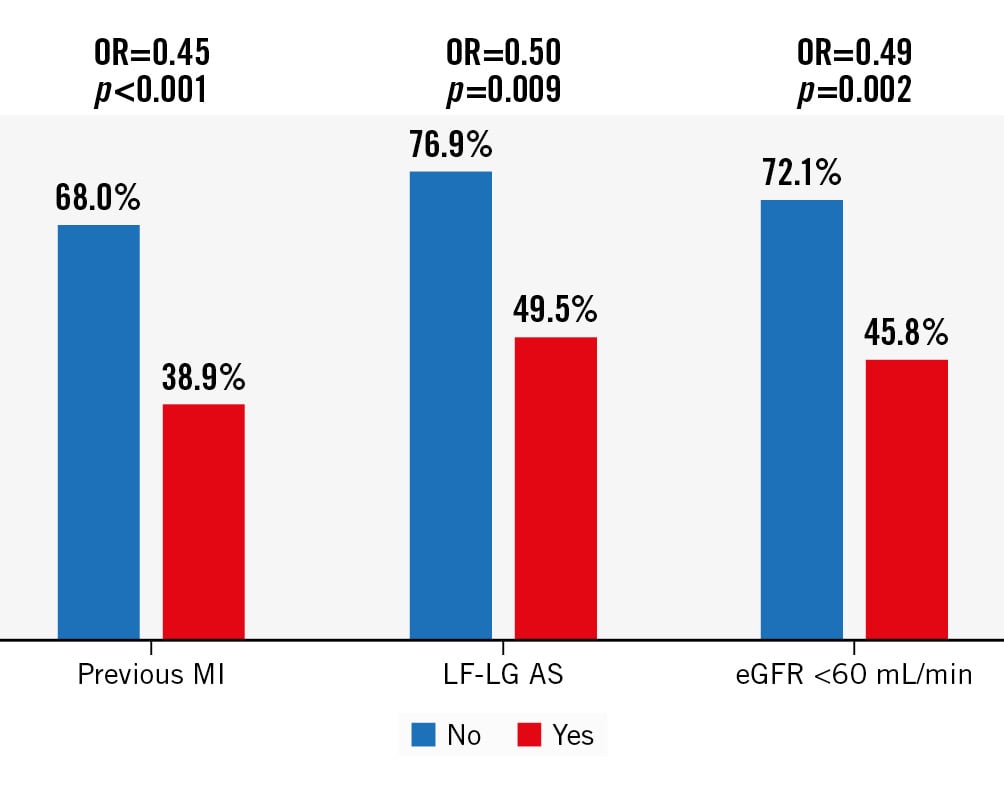
Figure 3. Independent predictors of LV recovery following TAVI. Likelihood of LV recovery for patients with (red bars) versus without (blue bars) baseline features found to be independent predictors of LV recovery following TAVI. OR for LV recovery for those patients with versus without each feature given at the top of each bar chart. eGFR: estimated glomerular filtration rate; LF-LG AS: low-flow, low-gradient aortic stenosis; MI: myocardial infarction; OR: odds ratio; TAVI: transcatheter aortic valve implantation
Discussion
Our results show that in patients with severe AS undergoing TAVI the prevalence of severe LV systolic dysfunction is 8.4%. Following TAVI, LV recovery occurs in almost 60% of cases, with approximately one-quarter achieving complete normalisation of LV function within 60 days post-TAVI. Patients experiencing LV recovery had similar midterm mortality to patients without severe LV dysfunction at baseline, and those achieving complete normalisation of LV function showed improved survival post-TAVI. We identified several predictors of LV recovery post-TAVI.
In patients suffering from severe AS, LV dysfunction can result from a multitude of aetiologies and pathophysiological mechanisms, either intrinsic to the valvular disease itself (such as pressure overload due to increased afterload of the left ventricle leading to reduced stroke volume and myocardial fibrosis secondary to LV hypertrophy) or secondary to various comorbidities that are prevalent in this elderly population (mainly ischaemic heart disease). When considering TAVI in such patients, the assessment of the expected risk/benefit profile is challenging. In terms of risk, a low EF is intuitively associated with worse outcomes. The expected benefit is harder to assess, compared to the TAVI population overall. When TAVI candidates present with normal LV function, their symptoms are primarily attributed to the valvular disease and therefore would be expected to significantly improve post-TAVI. In those with severe LV dysfunction, their symptoms may be the result of LV dysfunction and their clinical course may be less amenable to treatment of the valvular disease – leading to a futile procedure.
Baron et al, using data from the early period of commercial TAVI use (2011-2014), reported the prevalence of severe LV dysfunction in patients undergoing TAVI at 7.1%. Severe LV dysfunction was not associated with a significant increase in 1-year mortality or heart failure hospitalisation; however, patients with a low mAVG (<40 mmHg) experienced a 21% increase in mortality and a 52% increase in heart failure hospitalisation at 1 year, regardless of their baseline LV function5. Perhaps the more relevant question for these patients is on their LV response to TAVI. Intuitively, when LV dysfunction improves following TAVI, patients would be expected to show an additive benefit and a similar clinical course to those without severe LV dysfunction at baseline. Data on this issue are even more limited, as previous studies in TAVI populations focused on patients with any degree of LV dysfunction (EF <50%) and did not stratify those with severe LV dysfunction. Data from PARTNER Cohort A showed that in patients with a baseline EF <50% (n=93, mean EF 37.1%), EF improved by ≥10% in 51.6% of these by 30 days post-TAVI3. In a similar analysis from the Medtronic CoreValve U.S. Pivotal Trial focusing on patients with a baseline EF <40% (n=156, mean EF 32%), EF recovery occurred in 62% by 30 days post-TAVI4. More recently, Kolte et al7 reported that in the largest cohort to date of patients with LV dysfunction undergoing TAVI (a pooled cohort of patients included in the PARTNER 1, 2 and S3 trials and registries; n=659, mean EF 37.8%), LV recovery occurred in 32.8% by 30 days post-TAVI. In this cohort, LV recovery was associated with a 21% reduction in all-cause mortality up to 5-year follow-up; however, this was not statistically significant (p=0.06). In comparison, our cohort includes over 914 patients, all with baseline severe (EF ≤30%) LV dysfunction (mean EF 27.3%), and LV recovery occurred in 59.5% of patients. These rates are similar to the rates reported in PARTNER Cohort A and the CoreValve extreme- and high-risk cohorts, mentioned above, and significantly higher than the rates reported by Kolte et al. When examining the impact of LV recovery on survival, we found that both LV normalisation as well as LV recovery were associated with improved outcomes, compared to no LV recovery (HR 0.52 and 0.78, respectively). More importantly, both degrees of favourable LV response to TAVI were associated with either an improved (LV normalisation HR=0.80) or similar (LV recovery HR=1.15) risk for 3-year all-cause mortality post-TAVI. Overall, our results are aligned with those of Elmariah et al3 and Dauerman et al4 regarding the likelihood of LV recovery and with Kolte et al7 regarding the prognostic impact of LV recovery on overall mortality post-TAVI. The differences between our data and those of Kolte et al regarding the likelihood of LV recovery are probably explained by the higher EF threshold (50%) used to define LV dysfunction in the study by Kolte et al.
Apart from quantifying the decline in EF, the extent of cardiac damage in AS patients can be graded using the staging system devised by Généreux et al10. Data from the PARTNER 2 and 3 trials found that following aortic valve replacement, 15.6% of patients experienced an improvement in cardiac damage stage, and these patients fared better both in terms of 1-year survival11 and symptomatic improvement12, compared to those with an unchanged/deteriorated cardiac damage stage. In a cohort of consecutive Chinese patients undergoing TAVI, cardiac damage stage (per Généreux et al) improved by 30 days post-TAVI in 22% of patients, and this was associated with improved all-cause mortality at 2-year follow-up13. Our data, added to the previous studies mentioned above, reinforce the concept that when severe AS coincides with extra-valvular cardiac damage, TAVI has the potential to achieve improvement (and according to our data, complete regression) of cardiac damage in a significant percentage of patients, who subsequently enjoy a far better prognosis following TAVI.
Accepting this notion raises the next issue: the importance of identifying predictors of LV recovery in order to provide clinicians with the ability to estimate the likelihood of a specific patient achieving LV recovery; this will allow truly informed decisions to be made, guided by the expected benefit from TAVI. In our cohort, the baseline characteristics associated with LV recovery were no previous MI, eGFR >60 mL/min and a high mAVG (>40 mmHg; especially when using a binary definition for high-gradient AS). Previous MI was identified as an independent predictor of LV recovery by Kolte et al7, as well as by Dauerman et al4, with similar HRs to ours (0.65 and 0.44, respectively, compared to 0.45 in our cohort); mAVG as a continuous variable was an independent predictor of LV recovery in PARTNER Cohort A]3, again, with a similar HR to ours (1.03 compared to 1.02) and a mAVG >40 mmHg was identified by Dauerman et al4 as an independent predictor of LV recovery (HR 4.6 compared to 2 in our cohort). Interestingly, an unsupervised machine learning model trained with echocardiography images of patients with severe AS also identified a cluster of patients characterised by low EF and low AVG to be associated with higher rates of post-TAVI mortality – probably since these patients, as suggested from our data, are less likely to experience LV recovery post-TAVI14.
Renal function was not found to be an independent predictor of LV recovery in previous studies. This difference could be the result of our lower threshold of EF, the sample size, and the fact that previous studies only included patients enrolled in clinical trials compared to the all-comer patient population in our study. The consistent association of previous MI with lack of LV recovery raises the hypothesis that, broadly speaking, severe AS patients who concomitantly suffer from severe LV dysfunction can be placed on a spectrum which on one end represents those with established ischaemic cardiomyopathy, who subsequently develop severe AS, while on the other end it represents those with pure “valvular” cardiomyopathy. Our results suggest that the closer the patient is to the ischaemic end of the spectrum, the less likely they are to experience LV recovery and benefit from TAVI, and vice versa. The association between mAVG and LV recovery is also not surprising: patients with LF-LG AS have been shown to have increased risk for cardiac15 as well as overall16 1-year mortality following TAVI, which makes sense given their lower likelihood of achieving positive LV recovery following TAVI. The negative association of renal dysfunction with LV recovery can be explained by both the overall increased mortality risk for such patients following TAVI17 as well as the association of renal dysfunction with ischaemic heart disease.
Our study has several strengths. It is by far the largest cohort of patients with severe LV dysfunction undergoing TAVI and represents the outcomes of real-world all-comers patients treated under current clinical practice. Our follow-up period is longer compared to most of the previous studies, and we examined the prognostic benefit of different degrees of LV recovery (i.e., recovery/normalisation) following TAVI.
Limitations
Our study has several limitations which should be considered. The timing of the post-TAVI assessment of LV function was not standardised and was performed according to each centre’s local practice. The echocardiographic assessment was performed at each centre and not in a standardised manner at a dedicated core lab. We did not examine other relevant outcomes apart from mortality (e.g., Functional Class, quality of life, etc.), nor did we differentiate between cardiac and non-cardiac mortality. Our analysis may have introduced immortal time bias in favour of the LV recovery groups (although the results of a sensitivity analysis, which excluded patients who did not survive to 30 days post-TAVI, were consistent with the main analysis, some residual bias may remain due to the fact that the landmark analysis was performed at 30 days while repeat assessment of LV function was permitted for up to 60 days). Our data did not include echocardiographic parameters that may be associated with LV recovery (most notably, LV dimensions and a detailed assessment of RV function), and likewise, we did not have data on the presence of contractile reserve in cases of LF-LG AS, which is associated with improved outcomes following surgical aortic valve replacement1819 but not TAVI20. Likewise, very few patients underwent a pre-TAVI assessment with cardiac magnetic resonance imaging to quantify the extent of myocardial fibrosis, which may also be associated with the likelihood of recovery.
Conclusions
LV recovery and the extent of this recovery are major determinants of midterm mortality in patients with severe AS and severe LV dysfunction undergoing TAVI. Patients with no previous MI and those with an mAVG >40 mmHg show the best results following TAVI, which are at least equivalent to those for patients without severe LV dysfunction. We believe our results can promote informed, shared decision-making when discussing treatment options in this challenging patient subgroup. Better tools to predict the likelihood of LV recovery are needed.
Impact on daily practice
Data on the outcomes of transcatheter aortic valve implantation (TAVI) in patients with severe left ventricular (LV) dysfunction, specifically, the likelihood of LV recovery and its prognostic effect, are scarce. Teams caring for patients with severe aortic stenosis and severe LV dysfunction should assess the likelihood of LV recovery following TAVI; this information and its prognostic implications should be discussed with the patients. Larger-scale studies with longer follow-up are required. Studies focusing on specific subgroups of LV dysfunction (e.g., ischaemic vs valvular cardiomyopathy) will help better predict the likelihood of LV recovery following TAVI.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

